Abstract
Continuous discharge of untreated wastewater with high chromium contents from tanning industries causes severe environmental pollution in different aquatic systems as well as poses a variety of health risks. To reduce harmful chromium pollution in various water bodies, it is imperative to develop a method that is both affordable and environmentally benign. In the present study, chromium was successfully removed from tannery effluents using the roots of the water hyacinth (Eichhornia Crassipes). Chromium status in wastewater bodies before and after the biosorption processes was evaluated using standard spectrophotometric techniques such as UV–Vis and atomic absorption spectroscopy (AAS). The metal removal capacity of water hyacinth roots was determined by varying the biosorbent dosages, temperature, and starting chromium ion concentrations. The maximum removal of chromium was found 95% from dilute tannery wastewater and 72% of chromium was extracted directly from raw tannery effluents by using different quantities of water hyacinth. The Langmuir and Freundlich type isotherms were used to study the chromium biosorption process, and the results were found to be highly consistent with the Langmuir monolayer biosorption type isotherm. The results of kinetic investigation showed that the removal of chromium by water hyacinth roots followed a pseudo-second-order kinetic with a R2 of 0.99618. The biosorption of chromium on water hyacinth roots was exothermic and spontaneous which were confirmed from the evaluation of thermodynamic parameters such as ΔG°, ΔH°, and ΔS°. The present research findings revealed that this naturally available plant could be utilized as very promising, environmentally friendly, and cost-effective biosorbent for removing chromium species from raw as well as diluted industrial wastewater systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
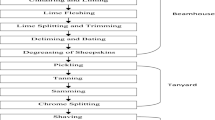
Trivalent basic chromium sulfate is commonly used in the processing of raw leathers in various tannery industries. Raw hides and skins are transformed into true leather species during the tanning process. The tanning of raw leathers involved with a variety of mechanical and chemical processes. During this procedure, the tanning industries generate a very large quantity of toxic complex wastewater. In most of the developing countries, these mixed toxic tannery wastewater streams are being discharged continuously without any prior treatment or with a partial treatment into the surrounding waterbodies and thus poses a potential threat of metal pollution in the respective aquatic systems. 34-56 m3 of water is used in the tanning procedure to process every ton of raw hides and skins [1,2,3]. The tannery industries in worldwide generate over 40 million tons of waste per year [4], which are continuously contaminating surrounding aquatic systems and thus posing severe threats to the respective environmental ecosystems. Chrome tanning method is broadly employed in tanning processes in various industries. This method offers some outstanding advantages in raw leather processing in various tanning industries such as it takes shorter time and produces thinner, softer, and more abundant leathers as well as its operation is very simple. Currently, almost 90% of the eighteen billion square feet of global leather production is being occurred through the chrome tanning processes [5, 6]. The tannery industries extensively utilized trivalent basic chromium sulfate, Cr(OH)SO4, solutions in tanning processes [3]. However, throughout the tanning process, only 60% of the initial chromium salt concentration is consumed by collagen fibers and permeated into the pickled pelt and 40% of this chromium salt remains unused which are being discharged along with the tannery wastewater [3]. After the manufacturing of wet blue leather, concentrations of chromium species in wastewater might be varied from 2656 to 5420 mg/L [7], 5001 to 5023 mg/L [8], 2500 to 8000 mg/L [9], 2000 to 5000 mg/L [6], 2000 to 5000 mg/L [10], and 3600 mg/L [11]. Every year, 1.5 × 104 ton of chromium salt is used in chrome tanning processes in various tannery industries in Bangladesh. These tanning industries continuously discharge a large quantity of wastewater enriched with toxic chromium species after a partial treatment or without any prior treatment into surrounding freshwater bodies and thus cause severe environmental pollution as well as pose a serious threat to the health of millions of people in Bangladesh [12]. The toxicity, carcinogenicity, mutagenicity, and teratogenicity of chromium in people, animals, and plants are highly known and well documented in literatures [4, 13].
The two chemical forms of chromium species that are typically found in tannery wastewater are chromium (III) and chromium (VI). After being disposed of from tannery industries, trivalent chromium present in tannery effluents can easily be transformed into highly toxic chromium (VI) species in the respective medium through the oxidation processes. Chromium (VI) is a hundred times more toxic than chromium (III), and it is also highly soluble into water [14]. Additionally, chromium (VI) works as a strong oxidizing agent which can produce free radicals and thus cause cancer in cells. Even at a very low concentration, hexavalent chromium is thought to be carcinogenic, and its presence in aquatic environments in larger quantities is highly alarming due to the drastic effects on various aquatic species [15]. Long-term exposure to some chromium compounds can cause acute tubular necrosis in the kidney, as well as diarrhea, heartburn, blindness, allergic responses, respiratory tract infections, and dermatitis in people [3, 16]. A study conducted by Kolomaznik et al. also reported the toxicity of chromium species which was found to be linked to kidney failure, high blood pressure, and lung cancer [17]. Therefore, the extraction and removal of any trace quantity of hazardous chromium pollutants from industrial wastewater streams with a suitable potential method is an urgent need to save aquatic ecosystems as well as to protect the health of humans and lives of aquatic species. Prior to release into fresh natural water bodies, hazardous metal pollutants in wastewater systems must be removed in an urgent basis. In most of the developing countries like Bangladesh, the removal of harmful chromium species from tannery effluents has become a major concern nowadays. Various approaches have been made previously to remove toxic chromium species from tannery wastewater which include electrocoagulation [18], chemical precipitation [9, 12], ion exchange [19], membrane processes [20], solvent extraction [21], and electro-dialysis [22]. Though these methods showed to be more or less effective to separate toxic chromium species from tannery wastewater bodies, they have been suffering with many limitations or drawbacks such as high operational costs, high energy requirements, and very large quantity of sludge production, and even some of the methods were observed to be difficult to control in large-scale practical applications [23]. In this perspective, a highly efficient and cost-effective alternative technology is an urgent need to effectively remove toxic chromium species from tannery effluents. In recent years, biosorption has become a viable alternative method for removing heavy metals from wastewater and this method was observed to be both affordable and environmentally acceptable [24]. Biosorption processes utilize the interesting and attractive properties of a natural plant and consider the invasive nature of weed as well as the environmental issues involved with its control [25]. Utilization of natural, household, and agricultural wastes has attracted a considerable attention in recent years to remove chromium and other heavy metals from wastewater due to their effectiveness, low cost, and availabilities [26,27,28,29,30]. The major advantage of this technique over the conventional methods is not only its relatively lower cost, but also its higher efficiency, the reduction of the formation of chemical or biological sludges, the capacity to replenish biosorbents, and the potential of metal recovery after the biosorption process [31].
Ligno-cellulosic agricultural waste products may be significant sources of possible metal-sorbing biomass [32]. The floating aquatic weed known as water hyacinth (Eichhornia Crassipes) typically thrives in tropical and subtropical areas of the world, particularly in Bangladesh. Due to its simple growth, rapid spread out, and high abundance in nearby waterbodies, it has gained the moniker “the world’s worst invasive aquatic plant,” which poses serious problems for navigation, irrigation, and power generation [33]. Only 12 days are needed for water hyacinth proliferation to multiply the population by 8% [34]. According to Zheng et al. [35], the numerous roots of the water hyacinth plants contain a good number of functional groups that may be able to bind with both cationic and anionic metal complexes [36]. According to a previous study, dried water hyacinth roots can function as stronger biosorbents than the biomass of the bacteria Mycobacterium Phlei, the yeast Candida parapsilosis, the fungi Rhizopus oryzae, and the Acacia confusa [37]. It was found that the biosorption mechanism is involved with the carboxylate group on the surface of the biomass.
Biosorbent materials can be technologically viable and highly adaptable. However, the applications of biosorbents in metal remediation technology greatly depend on their availability in vast quantities with relatively lower costs, high biosorption capability, and renewability. As a potential biosorbent, water hyacinth plants can effectively extract metal pollutants up to multiple elution cycles without any degradation in its biosorption capability [38]. Metal recovery and biomass regeneration are essential keys for the industrial applications of water hyacinth plants. Water hyacinth plant has garnered a lot of interest for the efficient removal of heavy metals from industrial wastewater streams due to their higher performance, relatively lower cost, and larger abundance in nature. Water hyacinth plants have already been employed successfully to remove dyes [37], heavy metals [39], lead [40], and arsenic [41] and to alleviate anthropogenic pollution [42] from various industrial wastewater sources. To the best of our knowledge, no research has yet been conducted and no data have been reported in the literatures that used water hyacinth plants to extract specific chromium species directly from tannery effluents. The present research work aimed to investigate the attractive and interesting properties of water hyacinth plant as a potential biosorbent to remove toxic chromium species directly from raw tannery effluents and examine the efficiency of water hyacinth roots to effectively extract the chromium species from tannery wastewater streams. The study also evaluates the optimum capacity of water hyacinth roots in chromium remediation technology in both raw tannery effluents and diluted tannery wastewater bodies and identifies water hyacinth roots as highly efficient biomass and cheaper naturally available potential biosorbent.
Water hyacinth is a rapidly growing, floating aquatic plant which is readily available in the countryside in Bangladesh as well as in many other countries. The roots of water hyacinth are endowed with elongated treads, freely dangling purple-black, and grow and spread out just 2.54 cm below water surface. The ideal water temperature for their proper growth in the underneath of water surface is about 28 °C. Under ideal conditions, the surface of water bodies can be covered by the mat which can be doubled within only within 4–7 days [43]. Three water hyacinth plants can produce up to 3000 young plants in as little as 50 days, whereas only two parents can produce 30 offspring in as little as 23 days. The water hyacinth plant’s highest reproductive rate is 54.4 g dry weight/m2/day [44]. Instead of on the mat, seedlings of water hyacinth plants appear from the moist soils in the surrounding area. These plants typically grow more swiftly in the summer and daily cover more than 15% of the water’s surface.
Materials and Methods
Tannery Wastewater Collection
Wastewater released after the wet blue manufacture of leather was collected from a tannery industry located in the Tannery Industrial Estate Dhaka (TIED), Savar, Bangladesh, using pre-sterilized polyethylene bottles. All polyethylene bottles were adequately cleaned with a nitric acid solution, rinsed several times with distilled and deionized water, and dried before being used for sample collection. The polyethylene bottles containing the tannery effluent samples were directly delivered to the lab, where the pH of each sample was assessed using a calibrated pH meter and then preserved all samples at a suitable temperature in a refrigerator until further analysis.
Biosorbent Preparation
Freshwater hyacinth plants were collected randomly from “Buribhadra,” a small river near Jessore district in Bangladesh. The whole plant bodies were carried out to the lab. Plant parts such as shoots and roots were separated using a knife. First, tap water was used to properly wash the roots, and then, distilled and deionized water was used. After proper cleaning, the water hyacinth roots were dried in air under sunlight for a week. The roots were further dried up in an oven at 85–90 °C for about 12 h to remove all the moisture contents and finally meshed them with a crushing machine (FRITSCH, PSDFS 90L2, Germany) to transform them into powder form. The procedure was repeated to obtain the powdered form of water hyacinth roots. This homogenized powdered form of water hyacinth roots was used in all biosorption experiments without determining the particle sizes in them. All the biosorption experiments were repeated three times and the average results have been reported here.
Chromium extraction and removal study has been carried out by biosorption method. To investigate the effectiveness of water hyacinth roots as a potential biosorbent to remove chromium species from tannery wastewater samples, a series of batch studies were carried out. The various amounts of dried, homogenized, and powdered water hyacinth roots were used in the batch studies. In batch A, 5, 10, 20, and 50 g of biosorbent were added into each 500 mL of raw tannery wastewater in a 1-L beaker separately. Each beaker with biosorbent material and raw tannery effluent was covered with cellophane, stirred, and agitated properly. Similar batch experiments were carried out with diluted tannery wastewater samples. For this purpose, each raw tannery wastewater sample was diluted to ten times to decrease the original concentration of chromium ion present in raw tannery effluents collected from the point sources. In batch B, 5, 10, and 20 g of the biosorbent were added into each of the diluted tannery wastewater samples. The sorption experiments under batch C were carried out in a different way in which 5 g of the biosorbent material was applied separately into three tannery wastewater samples in different 1-L beaker where the concentrations of chromium were 100, 300, and 500 ppm respectively. The purpose of the biosorption experiments in batch C was to determine the maximum chromium removal efficiency of water hyacinth roots through the biosorption processes. During the biosorption experiments, the mixtures of the biosorbent material and tannery wastewater were agitated at 150 rpm for 3.5 h at ambient temperature in a thermostat incubator with shaker (Model ZPH-100). After 3 h of shaking, each mixture of finely crushed water hyacinth root biosorbent and tannery wastewater was left for 1–2 h without any disturbance. After filtering the mixture with Whatman no. 42 filter paper, the filtrate was split into two portions. Major part of the filtrate was saved in a refrigerator at approximately 4 °C for metal analysis by AAS, while a minor portion was analyzed by UV–Vis spectrophotometer.
Digestion of Tannery Wastewater Samples for AAS Analysis
One hundred milliliters of raw tannery wastewater was transferred into a 250-mL conical flask, and 10 mL of concentrated nitric acid solution was added. The mixture was gradually heated until the volume of liquid mixture was reduced to 40 mL. After cooling, the mixture was filtered using Whatman no. 42 filter paper and the filtrate was diluted to 100 mL with distilled and deionized water [45]. All tannery wastewater samples were digested following the similar procedures [45]. For metal analysis, the digested wastewater samples were kept in a refrigerator at around 4 °C. The blank sample was prepared following the same procedure by using distilled and deionized water only.
Two spectrophotometric methods such as UV–Vis and AAS were utilized to identify and assay the chromium contents in the starting raw tannery wastewater samples as well as in the filtrates obtained after the biosorption studies. A double-beam UV–visible spectrophotometer (UV-1650A, Shimadzu, Japan) was employed to identify the chromium species in both the filtrates and raw tannery wastewater samples. All UV–Vis spectroscopic measurements were repeated three times and the average result was reported. The extents of total chromium in all acid-digested filtrates after the biosorption studies as well as in acid-treated raw tannery wastewater samples were measured by atomic absorption spectrophotometer (Model AA-7000 Shimadzu). A chromium hollow cathode lamp with a 0.7-nm slit width and 25-mA lamp current was used at a wavelength of 357.9 nm. A deuterium lamp was used to measure the absorbance of blank samples. All measurements were carried out in triplicate. The analytical procedures were checked and confirmed against a known concentration of the standard reference material purchased from CPAchem, Bulgaria. All chemicals (Merck, Germany) used in the study were in analytical grades, including the standard stock solutions and chromium solution with different concentrations employed in the biosorption experiments. The blank samples were run after every seven samples. The precision and accuracy of all measurements were checked and confirmed by analyzing the standard reference material (CPAchem, Bulgaria).
Following the biosorption studies, the extents of chromium removal were calculated using the simple concentration difference method. The percentage of chromium removal by water hyacinth roots was calculated using the following Eq. 1 [46].
where \({C}_{0}\) and \({C}_{f}\) are the initial and final concentrations (mg/L) of chromium respectively. The biosorption capacity of water hyacinth roots was calculated by using the following Eq. 2 [47].
Biosorption capacity (mg/g)
where V is the volume of solution taken,\({C}_{0}\) is the initial concentration, \({C}_{e}\) is the equilibrium concentration, and W is the amount of biosorbent utilized.
Results and Discussion
Structural Analysis of Water Hyacinth Plant by FT-IR spectroscopy
Many chemical species may exist within the roots of water hyacinth plants and they are highly endowed with polyfunctional metal-binding sites which have a great affinity for both cationic and anionic metal complexes. FT-IR analysis on water hyacinth roots before and after the biosorption study showed the characteristic resonances indicating the presence of different functional groups which can potentially bind chromium species on surfaces of the biosorbent.
On the surfaces of the water hyacinth roots, O–H functional group is present, as shown by the broad IR resonance at 3332 cm−1. The C-H vibration is shown by the emergence of distinctive absorption bands at 2921 cm−1. Significant bands at 1635, 1419, and 1372 cm−1 demonstrate the presence of the aromatic skeletal -HC = CH- bond, whereas a very high peak at 1004 cm−1 was caused by the typical C-O stretching. In Fig. 1, the spectrum of the water hyacinth root after biosorption (S-2) showed that many absorption peaks shifted slightly into lower frequencies at 3314, 2898, 1638, 1422, and 1003 cm−1, in comparison to the respective resonances in the spectrum S-1 indicating the possible interaction of the those functional groups with chromium ion. It is clear that the characteristic IR resonance for -OH functionality, which typically occurs at roughly 3332 cm−1, was moved to a lower frequency of 3314 cm−1 with relatively modest intensity, indicating the possibility of chromium species interacting with the surfaces of the adsorbent. The FT-IR resonances obtained from water hyacinth roots were highly comparable as well as consistent with the data reported previously in literature from similar studies [35, 48].
pH and Point of Zero Charge (pHpzc) on the Surfaces of Water Hyacinth Root
The pH of water hyacinth root was measured by a pH meter (Ohaus Bench-Top, Parsippany, NJ 07054 USA). The biosorbent was mixed with distilled and deionized water at a ratio of 1:10 (w/w) and the mixture was agitated at a speed of 200 r/min for 24 h at ambient temperature [16]. The mixture was filtered with Whatman no. 40 filter paper and the pH of the filtrate was measured. The pH values of the water hyacinth root were found 4.0.
The biosorbent’s point of zero charge is a valuable parameter for figuring out how well metal ions adhere to surfaces. Point of zero charge (pHpzc) on the surfaces of water hyacinth roots was determined by using the instrument, Anton Paar (Model-Litesizer 500, Austria), in the pH range from 1.0 to 5.0 following the established procedure [49, 50]. The results of point zero charge analysis are shown in Fig. 2.
Ten milliliters of the resultant filtrate was transferred into another small glass container and 0.1 M HCl was added into the filtrate and the pH of the solution of filtrate was adjusted to 1 with acid. The mixture was settled to reach to the equilibrium point. The pH was determined in the resultant solution by using a pH meter. Similarly, four different individual solutions of the filtrate were prepared by adding 0.1 M HCl or NaOH solution and the pH of the resulting filtrate solutions with acid or alkali were adjusted at 2, 3, 4, and 5 respectively. A syringe was used to inject each of the corresponding filtrate solutions separately into the sample cell. The zeta potential was then measured accordingly, and a curve was found from the plot of zeta potential versus pH which showed the change of zeta potential with the variation of pH. The pH at the zeta potential of zero was determined from the curve which corresponds to the expected pH where the point of zero charge was found on the biosorbent. Thus, the point of zero charge was attained by plotting mean zeta potential values (mV) measured from within the pH 0 to pH 5 versus initial pH (pHi). At pH 1.6, the biosorbent’s surface charge density (pHpzc) was found to be zero. At this point, the ratio of positively and negatively charged sites was balanced. The biosorbent surface will display negative charge at the magnitudes of pH higher than pHpzc, and in that case, positively charged species will preferably be adsorbed onto the surfaces of biosorbent. On the other hand, the surface of the biosorbent will be endowed with positive charge at the extents of pH lower than pHpzc, favoring the adsorption of contaminants which are negatively charged. A similar agreement was found in the FT-IR absorption spectra of the water hyacinth root. As a result, the roots of the water hyacinth efficiently work as an ideal potential solid biosorbent for removal of trivalent chromium ions through the biosorption process [50].
X-Ray Diffraction Analysis
To comprehend the structural alterations in the water hyacinth roots’ surfaces before and after the biosorption tests, X-ray diffraction analysis was done. Figure 2 shows the characteristic structural features in the surfaces of the water hyacinth biosorbent before and after the conduction of chromium extraction studies. The pattern of the appearances of characteristic signals was observed to be similar in both the biosorbent samples obtained before and after the biosorption experiments. However, most of the peaks were found to be shifted slightly to the lower θ values with significantly decrease in intensity which might be due to the attachment of chromium species on the surfaces of water hyacinth roots (Fig. 3: S-2).
Raw tannery effluent samples were taken directly from point sources and carefully evaluated in the current investigation to determine the level of chromium present. The pH of the wastewater sample was first examined; the average pH value observed was 3.2; this pH was used for all studies. Chromium contents in the initial raw tannery effluents as well as in diluted tannery wastewater samples were determined by using atomic absorption spectroscopic method. The results revealed that both types of tannery effluent samples contained extremely high levels of chromium (Table 1). Chromium extraction and removal study in the tannery wastewater samples were carried out by the biosorption method using a series of batch experiments with varied quantities of dried crushed finely divided homogenized water hyacinth roots. Estimation of the extents of chromium elimination from raw and diluted tannery effluent has been accomplished through the precise determination of chromium concentrations in the respective wastewater samples before and after the adsorption processes during the batch experiments. The optimum chromium removal efficiency of the dried finely divided crushed roots of water hyacinth in tannery wastewater was finally investigated. The biosorption studies were performed in three sets of batch experiments such as batch A, batch B, and batch C. In batch A, all biosorption experiments were conducted with raw tannery wastewater by varying the quantities of water hyacinth roots from 5 to 50 g (Table 1). Diluted tannery wastewater samples were treated with the water hyacinth roots in batch B using the similar quantities of the adsorbent materials for the effective deduction of chromium species (Table 1). The amount of biosorbent material remained the same in all sorption experiments in batch C which was 5 g. However, all biosorption experiments in batches A, B, and C were carried out with the same quantity of chromium-contaminated raw tannery wastewater and diluted tannery wastewater (Table 1). The purpose of using the same quantity of tannery wastewater in all biosorption experiments was to examine and contrast the application of various masses of the biosorbent material with the effectiveness of water hyacinth roots in removing chromium.
UV–Visible Spectrometric Analysis
UV–visible spectrophotometric analysis in raw and diluted tannery effluents showed the characteristic absorbance peaks for chromium species within the range of 496 to 657 nm. Figures 4 and 5 display the UV–Vis spectra of raw and dilute tannery effluents before and after the biosorption experiments with various quantities of the respective biosorbent material—water hyacinth roots. UV–visible spectrophotometric method is one of the standard techniques which has been used in different wastewater treatment technologies to evaluate the progress of industrial wastewater treatment processes [51]. In the raw tannery wastewater without any treatment with water hyacinth roots, the maximum absorption peak was appeared at 574 nm (λmax) and the corresponding absorbance was found 3.4. With the application of different quantities of the water hyacinth roots such as 5, 10, 20, and 50 g into 500 mL of raw tannery wastewater sample separately, the UV–Vis absorbances were observed to be decreased to 3.0, 2.8, 2.6, and 1.5 respectively at 574 nm (Fig. 4). It is highly apparent that the raw tannery wastewater is extremely contaminated with very concentration of toxic chromium (Table 1). However, the results from UV–Vis study clearly demonstrate that after the biosorption experiments with varied quantities of water hyacinth roots, the concentration of chromium species in tannery wastewater has been decreased significantly which is evidenced from the reduction of the absorbance values from 3.4 to 1.5 (Table 1). This is also confirmed by the sharp decrease in the intensities of the characteristic peak for chromium in the UV–Vis spectra obtained from the raw tannery wastewater and the treated wastewater samples after the biosorption studies (Figs. 4 and 5). Chromium contents have been observed to be decreased significantly in the treated diluted tannery wastewater when the masses of the biosorbent have been increased from 5 to 20 g (Table 1, Fig. 5). Similar results were obtained from a UV–Vis spectrophotometric analysis of raw tannery effluent samples before and after treatment with different concentrations of water hyacinth roots, as shown in the corresponding spectra (Fig. 4). Due to the increase in adsorbent doses, more surface area can be exposed in which there are an increasing number of readily available binding sites for the biosorption process that results the potential rise in the percent removal of the metal ion. On the other hand, biosorption capacity in mg/g can be decreased as biosorbent doses are increased. Biosorbent doses also affect the removal efficiency at different contact times. For all contact times, higher biosorption was found at 20 g/L, but in this case, the percent removal of chromium was varied depending on the contact times. With increasing in the biosorbent masses, the chromium absorbance in dilute tannery effluents at 574 nm was observed to be reduced from 0.3 to 0.07 (Fig. 5).
To optimize the maximum chromium removal capacity of finely crushed water hyacinth roots as a potential biosorbent, 5 g of water hyacinth roots was applied into 500, 300, and 100 ppm chromium solutions separately. Each mixture was stirred and shaken for 3 h. The mixture of the biosorbent and chromium solution was then filtered, and the filtrate was run into the UV spectrophotometer. The UV–Vis spectrum from the filtrates certainly indicates that chromium absorbance is being decreased when the dilution in tannery wastewater is increased and finally the chromium absorbance is found to be almost zero in the case of more diluted (100 ppm) wastewater solution (Fig. 6).
Assessment of Chromium Concentrations in Raw Tannery Wastewater and in Filtrates by AAS
The mass of biosorbent is a major parameter in the biosorption experiments to effectively remove chromium species from tannery wastewater. Atomic absorption spectrophotometric analysis on tannery effluents before and after the biosorption studies has provided significant information on how the different quantities of water hyacinth roots as a biosorbent have significantly impacted the chromium extraction and removal from different tannery effluents following the respective mass transfer kinetics. From the AAS study, the initial total chromium concentration in raw tannery effluent was found 4633 mg/L, and in diluted tannery wastewater, the initial total chromium contents were 500, 463.3, 300, and 100 mg/L (Table 1). Five hundred milliliters of raw tannery effluent containing 4633 mg/L of chromium concentration was treated separately with 5, 10, 20, and 50 g water hyacinth roots for the biosorption tests, and the resulting residual chromium concentrations were measured as 4226.3, 4083, 3387, and 1262.2 mg/L, respectively (Table 1). Treatment of 500 mL of ten times diluted tannery wastewater sample with similar masses of the biosorbent such as 5, 10, and 20 g separately resulted in a dramatic decrease in chromium concentrations from 463.3 to 49.5 mg/L and 36.6 and 19.5 mg/L respectively which is truly significant with regard to the efficiency of water hyacinth roots for effective deduction of chromium from tannery wastewater systems. These results also indicate the excellent chromium reducing capacity of water hyacinth roots as evidenced by 95.79% removal of chromium from tannery wastewater samples (Table 1). Application of relatively lower quantity of the biosorbent such as 5 g in the sorption experiment in batch C with diluted tannery wastewater samples also showed the reduction of toxic chromium from 88.13 to 95.4% (Table 1) which is quite impressive and represents the remarkable performance of water hyacinth roots as a potential biosorbent for effective extraction and elimination of chromium from tannery effluents. The results of all biosorption experiments with initial and remaining concentrations of chromium in tannery wastewater samples as well as the respective percentage of chromium removal have been illustrated Table 1.
From Table 1, it is highly apparent that when the extents of the biosorbent material are being increased, the extraction and removal of chromium from tannery effluents are enhanced dramatically through the biosorption processes. Figures 4, 5, and 6 also illustrated that the toxic chromium contents in raw and dilute tannery wastewater samples are significantly reduced as the mass of the respective biosorbent material increases. In the case of raw tannery effluent, the removal of chromium was found to be increased from 8.78 to 72.76% when the quantity of biosorbent material was improved from 5 to 50 g (Table 1). However, the percent chromium removal from diluted tannery wastewater was found very higher 89.32% even with the application of only 5 g of the adsorbent material and chromium removal efficiency was changed from 89.32 to 95.79% when the amount of water hyacinth roots was changed from 5 to 20 g during the biosorption processes. The increase in chromium removal through the biosorption process followed by the elevated quantities of water hyacinth roots might be due to the abundance of biosorbent particles with the potential active sites which provided additional surface areas for chromium binding. The increase in surface area effectively exposes more available additional binding sites for the potential biosorption of chromium which resulted in a higher percentage of chromium removal. The results from the present study revealed that 95% of chromium could be extracted and removed from diluted tannery wastewater by the application of appropriate quantities of water hyacinth roots. Chromium removal capacity of this biosorbent material can be varied based on the applications of different quantities of water hyacinth roots (Table 1). Previous studies reported in literatures showed the similar results regarding the impact of adsorbent dosages on the efficacy of Cr biosorption [30, 48].
Potential binding of chromium with the active sites on surfaces of finely crushed powdered water hyacinth roots might have occurred through the biosorption processes. The electrostatic interactions between the positively charged chromium species and the accessible negatively charged functional groups on the surfaces of the adsorbent material, as well as the hydrogen bonding contact, may lead to these biosorption interactions [52]. In the chromium removal process, in some cases, chemisorption process might also probably be involved. The presence of C = C bond in the structure of water hyacinth roots was revealed by the FT-IR spectrum of the biomaterial. This suggests that an electrostatic force of attraction between the delocalized pi electrons in aromatic ring moiety available in water hyacinth roots and the positively charged chromium ion might possibly be occurred. Nucleophilic substitution of O–H group in water hyacinth roots might be possible with Cr3+ species as well as a strong force of attraction could exist between the negatively polar parts of chemical species available in water hyacinth roots and the positively charged chromium ions. The delocalized pi electrons in aromatic ring moiety in water hyacinth roots could work as a Lewis base to coordinate with the chromium ion by sharing the pi electron densities with the empty d orbitals of chromium ion. The adsorption of Cr3+ ions on the surfaces of biosorbent materials with the presence of various polar functional groups, such as -OH and -COOH, was also supported by earlier literature investigations [53].

Effect of the Biosorbent Masses on the Chromium Removal Efficiency
Application of different masses of water hyacinth roots in the biosorption processes showed tremendous impacts on the extraction and removal of chromium from both raw and diluted tannery effluents and the results have been illustrated in Figs. 7 and 8. The percent removal of chromium was 8.78, 11.87, 26.89, and 72.76 from each 500 mL of raw tannery wastewater samples with the application of 5, 10, 20, and 50 g of the water hyacinth roots respectively. After ten-fold dilution of the respective raw tannery wastewater samples, the percent chromium removal was found as 89.32, 92.10, and 95.79 with the addition of 5, 10, and 20 g of the biosorbent, respectively. As the quantities of the biosorbent are being increased, the removal percentage of chromium by finely crushed water hyacinth roots found to be improved dramatically reaching 72.76% biosorption of chromium from the raw tannery wastewater and 95.78% in dilute tannery wastewater. Water hyacinth roots showed excellent chromium removal capacity in dilute tannery wastewater solution, 89.32 to 95.79%, whereas in the case of raw tannery effluents, the removal of chromium was varied from 8.78 to 72.76%. Larger quantities of the biosorbent provide relatively more surface area for the extraction of metal ion since there are more spaces readily available for the attachment of metal ion to the biosorbent. Generally, the sorption capacity of a suitable material depends on the pH, contact time, temperature, and biosorbent doses. At room temperature, pH of tannery chrome wastewater was 3.2 and the maximum chromium removal efficiency was found 95.79%. pH is one of the significant factors in the biosorption studies. At pH 3, the optimum chromium biosorption was realized as 57.71 mg/g was and there was a declining trend of metal biosorption at about pH 5 due to the decrease in protonation of the binding sites resulting in the reduction of metal biosorption [54].
The chromium removal capacity of water hyacinth roots was quite remarkable to effectively extract toxic chromium species directly from raw tannery effluents, and the results from the present investigation are highly comparable to the data reported previously on chromium separation studies from different sources [55,56,57]. A study showed that by employing dried and grounded roots of water hyacinth, 85% of Cr6+ species could be removed from synthetic chromium solutions [55]. In a different study, Agave Lechuguilla biomass was used as a biosorbent to extract chromium from aqueous solution with a maximum effectiveness of 94% [54]. Only 1 g of dried water hyacinth leaves could effectively remove 46% of chromium from 50 mL of 1000 ppm chromium solution [57]. The present research’s findings demonstrated the excellent efficacy of water hyacinth roots as a potential biosorbent to effectively remove toxic chromium up to 95.79% and have opened up an intriguing possibility for using water hyacinth for the extraction and removal of chromium species directly from tannery effluents.
Langmuir Adsorption Isotherm
The Langmuir equation is constructed under the assumptions that there is no biosorbate transmigration in the plane of the surface, the energy of biosorption is constant, and the maximum biosorption corresponds to a saturated monolayer of sorbate molecules on the biosorbent surface [58]. A plot of 1/qe versus 1/Ce has been drawn (Fig. 9) using the Langmuir model as depicted in Eq. 3:
where Ce stands for the equilibrium concentration (mg/L); qe is the equilibrium biosorbate concentration in solution; qmax is the maximum adsorption capacity (mg/g), which is calculated from the slope; and KL is the Langmuir constant connected to the binding sites and determined from the intercept.
The magnitudes of Langmuir parameters and correlation coefficients R2 are summarized in Table 2. The biosorption isotherms for Cr(III) removal were studied using the initial concentrations of metal ions at 100, 300, and 500 ppm respectively with the biosorbent mass of 5 g. From the perspective of predicting whether the biosorption system is “favorable” or “unfavorable,” the isotherm shape has been determined. Hall et al. proposed a dimensionless separation factor or equilibrium parameter, RL, as an essential feature of the Langmuir isotherm to predict if a biosorption system is “favorable” or “unfavorable, and it was deified by the following relationship [59]:
\({C}_{\mathrm{o}}\) is often the maximum fluid-phase concentration found in a single biosorption system. For chromium, the separation factor (RL) value from the current study is 0.05410, indicating that the isotherm’s form is good.
Freundlich Adsorption Isotherm
The exponential distribution of active sites and their energies, as well as surface heterogeneity, can be demonstrated by the Freundlich sorption isotherm. The following equation illustrates the Freundlich model and a linear plot of log qe versus log Ce was found following Eq. 5 [60]:
where Ce is the equilibrium concentration (mg/L), 1/n is the adsorption intensity, and Kf roughly represents an indicator of the adsorption capacity (mg/g). The constants Kf and 1/n are produced via the Freundlich formulation in a linear form. A non-ideal adsorption on heterogeneous surfaces is assumed by the Freundlich isotherm model, which implies that stronger binding sites are occupied first, followed by weaker binding sites. The plot of log qe vs log Ce following the Freundlich isotherm model is shown in Fig. 10.
The Freundlich parameters together with correlation coefficients R are summarized in Table 3. The slope l/n was in the range of 0 to 1 which demonstrated the measure of adsorption intensity or heterogeneity and the surfaces of biosorbent became more heterogeneous as the slope values turned in very close to zero. The magnitudes of 1/n below 1 indicates a normal isotherm, while the value of 1/n above 1 is indicative of cooperative adsorption. The magnitude of l/n was found 0.59612 which was in between 0 and l indicating the water hyacinth root biosorbent was highly favorable for biosorption of chromium ions under the conditions used in the present study. Freundlich constant (Kf) provides information on adsorption capacity of a biosorbent. It also demonstrates the strength of the relationship between adsorbent and adsorbate. The magnitude of Kf obtained from water hyacinth roots for chromium removal was 4.26226 which showed the strong favorability for the biosorption process. The correlation coefficients (R2) of Langmuir isotherm and Freundlich isotherm were found 0.99618 and 0.91347 respectively. Thus, both isotherms could be potentially fitted with the results of chromium biosorption process occurred on water hyacinth roots, and chromium removal capacity of the biosorbent was strongly supported by them. However, the appearance of slightly higher magnitude of R2 from Langmuir isotherm in comparison to the value obtained from the Freundlich adsorption isotherm suggests that the biosorption of chromium ions on the surfaces of water hyacinth roots is likely to be more aligned with the Langmuir type isotherm.
Kinetic Investigation on the Adsorption of Chromium by Water Hyacinth Roots
Kinetic study was accomplished by conducting a series of experiments at different time intervals. It described the effects of contact time of wastewater sample solutions with biosorbent for the removal of chromium (III) ions. In this study, a particular amount of biosorbent was added into a chromium (III) solution with concentration of 500 ppm. The biosorption experiments were carried out by properly shaking the mixture of the biosorbent and chromium solution separately at different time intervals such as 90, 120, 150, 180, 210, and 240 min respectively. However, pH, biosorbent dose, and chromium ion concentration (500 ppm) in the resulting mixtures remained unchanged in each of the experiments. During all experiments, the mixtures of biosorbent and chromium solution were maintained with a constant pH (3.2) and agitated at 150 rpm at ambient temperature. Chromium concentrations were determined separately in the resulting mixtures after agitation for 90, 120, 150, 180, 210, and 240 min and the percentage of chromium removal was obtained as 85.4, 89.7, 92.0, 94.5, 95.4, and 95.4% respectively. The maximum percentage of chromium removal was found at 210 min and this contact time was considered standard for all the next consecutive experiments. The experimental results showed that the agitation of wastewater samples with water hyacinth root for 240 min did not increase the percentage of chromium removal. No significant differences in the removal of chromium was observed at 240-min contact time and more which revealed that all surface area of biosorbent was saturated at 210-min contact time. The rate, nature, and type of biosorption that occurred on the surfaces of water hyacinth roots were investigated using the pseudo-first-order and pseudo-second-order kinetic models [61]. Equation 6 shown below is Lagergren’s 1898 formulation of the pseudo-first-order kinetic model [62]:
where k1 is the rate constant of pseudo-first-order kinetics and qe and qt (mg/g) are the amounts of chromium adsorbed per unit weight of the biosorbent at equilibrium with time t (min), respectively. The experimental data are entered into the pseudo-first-order kinetic model developed by Lagergren. The plot of ln(qe-qt) vs. time in Fig. 11 illustrates the pseudo-first-order kinetic model best fit with the biosorption data.
The value of correlation coefficient, R2, of the pseudo-first-order model is 0.96214 which is presented in Table 4.
The pseudo-second-order kinetic model can be expressed by the following Eq. 7:
The rate constant, k2, and equilibrium biosorption capacity were determined from the slope and intercept of the graph. The chromium biosorption data were best fitted in the pseudo-second-order kinetic model, and the plot of t/qt versus t was found to be straight line (Fig. 12). However, the plot of t/qt versus t obtained from the experimental results of chromium biosorption on water hyacinth roots showed very good agreement with the pseudo-second-order kinetics with the interesting magnitude of R2. The correlation coefficients (R2) from pseudo-second-order kinetic model were found 0.99574 in Table 5 which was very closer to unity in comparison to those obtained from the pseudo-first-order kinetic model (0.96214) of chromium (III) ion extraction by water hyacinth roots. Though the magnitudes of correlation coefficients (R2) obtained from both kinetic models are closer to each other, in the case of pseudo-second-order kinetic, the R2 value was much closer to 1 which indicated that the process of chromium biosorption on the surfaces of water hyacinth roots followed the pseudo-second-order kinetic.
Effect of Temperatures on the Extraction of Chromium by Water Hyacinth Roots
Chromium adsorption capacity of water hyacinth roots was examined at different temperatures to understand the effect of heating on the potential ability of the adsorbent to extract chromium from tannery waste at various conditions. The adsorption experiments were carried out separately at different temperatures such as 303, 313, 323, and 333 K respectively for 210 min at a pH 3.2. The results showed the decrease in adsorption efficiency of water hyacinth roots from 95.6 to 88.2% with the increase in temperatures from 303 to 333 K. Similar reduction (23.9 to 21.5 mg/g) in adsorption capacities of other biosorbents with increasing in temperatures from 30 to 60 °C was realized in some previous studies reported in literature [63]. The successive decrease in chromium (III) uptake on the surfaces of water hyacinth roots with rising in temperatures might be due to the occurrence of desorption caused by an increase in thermal energy available in the system. The mass transfer kinetics between adsorbate and adsorbent might also have played a significant role in the adsorption processes occurred at higher temperatures [63]. The reduction in adsorption capacity of water hyacinth roots with increasing in temperatures indicates that metal adsorption process was exothermic in nature. The value of correlation coefficient, R2, and other parameters are shown in Table 6 and the plot of lnKL vs 1/T is shown in Fig. 13.
Different thermodynamic parameters such as ΔG°, ΔH°, and ΔS° were determined to investigate the nature and efficiency of chromium biosorption process on the surfaces of water hyacinth roots from tannery wastewater streams. The results of thermodynamic analysis have been presented in Table 7. The thermodynamic data showed that the extents of ΔG° turned into more positive with the increase in temperatures in the system. The negative magnitudes of Gibbs free energy change (ΔG°) confirmed the feasibility of the biosorption of Cr(III) ion on surfaces of the biosorbent as well as it indicated that the chromium removal process was spontaneous in nature whereas the lower value of Gibbs free energy suggested that the method was favorable at a lower temperature. The metal adsorption process on the surfaces of adsorbents also depends on the magnitudes of ΔH° as positive or negative numbers. The ΔH° value was found negative in the present study which indicated that the adsorption process of chromium ion onto the surface of water hyacinth was exothermic in nature (Table 6). The magnitude of enthalpy change (ΔH°) was found − 30.86 kJ mol−1, which indicated the presence of strong electrostatic interactions between the Cr(III) ions and available functional groups on the biosorbent surfaces. The negative entropy change (ΔS°) suggested that adsorption process on the surfaces of water hyacinth roots was highly favorable for the potential extraction and separation of chromium from tannery wastewater bodies.
Comparison of Removal Percentage of Current Study with Other Biosorbents
The significant findings of the present study on chromium removal from tannery wastewater using water hyacinth roots were highly comparable with the chromium separation data reported previously in literatures from other research groups. Table 8 shows the comparison of the results of maximum percentage of chromium removal from tannery wastewater by water hyacinth roots with the studies conducted previously by other natural and agricultural adsorbents. The comparison studies showed that water hyacinth root could remove much higher percentage of toxic chromium from tannery wastewater and this naturally available plant root has higher chromium removal capacity than other biosorbents except the chemically modified synthetic sorbent materials. Thus, water hyacinth roots can be utilized as an effective low cost biosorbent to remove toxic chromium species from tannery wastewater bodies.
Conclusion
The presence of high concentrations of non-biodegradable chromium in tannery wastewater is a major environmental concern nowadays. Herein, water hyacinth, a common natural floating aquatic weed, was applied as a cost-effective potential biosorbent to remove toxic chromium species from raw tannery effluents as well as from diluted tannery wastewater bodies. The samples were treated with crushed, homogenized, and powdered water hyacinth root in a series of batch experiments to effectively remove chromium from tannery wastewater through the biosorption mechanism. Water hyacinth root showed excellent chromium removal capacities to extract chromium species from tannery wastewater bodies. The percent removal of chromium from tannery wastewater was found to be dependent on the dosages of biosorbent as well as the respective metal concentrations in the samples. The maximum chromium removal efficiency was 96% in diluted tannery wastewater, whereas 73% chromium was extracted directly from raw tannery effluent by water hyacinth root powder which is truly significant considering the presence of very higher chromium contents in tannery wastewater in Bangladesh. The biosorption data were justified by Langmuir and Freundlich adsorption isotherms and the R2 values were observed to be 0.99618 and 0.91347 respectively suggesting that the results of chromium biosorption study were best fitted to the Langmuir adsorption isotherm. Both pseudo-first-order and pseudo-second-order kinetic models were employed to evaluate the chromium biosorption kinetics and the results revealed that the biosorption of chromium on the surfaces of water hyacinth roots followed the pseudo-second-order kinetics. No significant differences were observed between the magnitudes of correlation coefficients (R2: 0.99574 and 0.96214) obtained from both kinetic models. However, the magnitude of R2 0.99574 was much closer to 1 which indicated that the chromium biosorption data were ideally suited to the pseudo-second-order kinetic model. Thermodynamic study with the measurements of ΔG°, ΔH°, and ΔS° suggested the spontaneous nature of chromium biosorption process on water hyacinth root powder as evidenced from the negative magnitudes of ΔG° and ΔS°. The appearance of negative enthalpy change (ΔH°) indicated that chromium removal process was exothermic. The traditional methods for removing toxic chromium species from tannery effluents have many drawbacks such as higher investment and operational costs to treat tannery effluents which make them unsuitable and incompatible to practical applications. This method of toxic chromium removal directly from tannery effluents by water hyacinth root powder was found very cost-effective and highly efficient in terms of chromium extraction efficiency, energy use, labor, and investment. Proper applications of water hyacinth roots can potentially build up an effective alternative method of chromium removal from tannery wastewater streams in Bangladesh and other countries.
Data Availability
All data generated or analyzed during this study are included in this article.
Change history
31 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s41101-023-00203-1
References
Lofrano G, Meriç S, Zengin GE, Orhon D (2013) Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci Total Environ 461-462:265–281. https://doi.org/10.1016/j.scitotenv.2013.05.004
Desta AF, Nzioki J, Maina S, Stomeo F (2017) Molecular biomonitoring of microbial communities in tannery wastewater treatment plant for the removal retaining chemicals. Biological Wastewater Treatment and Resource Recovery.:141–155 https://www.intechopen.com/chapters/54166
Adeel SS, Wajid A, Hussain S, Malik F, Sami Z, Haq IU, Hameed A, Channa RA (2012) Recovery of chromium from the tannery wastewater by use of Bacillus Subtilis in Gujranwala, Pakistan. IOSR J Pharm Biol Sci 2(2):36–45
Jobby R, Jha P, Yadav AK, Desai N (2018) Biosorption and biotransformation of hexavalent chromium [Cr(VI)]: A comprehensive review. Chemosphere 207:255–266. https://doi.org/10.1016/j.chemosphere.2018.05.050
Islam BI, Musa AE, Ibrahim EH, Sharafa SAA, Elfaki BM (2014) Evaluation and characterization of tannery wastewater. J For Prod Ind 3(1):141–150
Belay AA (2010) Impacts of chromium from tannery effluent and evaluation of alternative treatment options. J Environ Prot 1:53–58. https://doi.org/10.4236/jep.2010.11007
Faghihian H, Bowman RS (2005) Adsorption of chromate by clinoptilolite exchanged with various metal cations. Water Res 39(6):1099–1104. https://doi.org/10.1016/j.watres.2004.12.010
Hashem MA, Islam A, Mohsin S, Nur-A-Tomal MS (2015) Green environment suffers by discharging of high-chromium-containing wastewater from the tanneries at Hazaribagh, Bangladesh. Sustain Water Resour Manag 1(4):343–347. https://doi.org/10.1007/s40899-015-0033-4
Minas F, Chandravanshi BS, Leta S (2017) Chemical precipitation method for chromium removal and its recovery from tannery wastewater in Ethiopia. Chem Int 3(4):392–405
Hafez AI, El-Manharawy MS, Khedr MA (2002) RO membrane removal of unreacted chromium from spent tanning effluent. A pilot scale study, Part 2. Desalination 14:237–242. https://doi.org/10.1016/S0011-9164(02)00318-1
Chaudry MA, Ahmad S, Malik MT (1998) Supported liquid membrane technique applicability for removal of chromium from tannery wastes. Waste Manage 17(4):211–218. https://doi.org/10.1016/s0956-053x(97)10007-1
Mottalib MA, Somoal SH, Saiful MI, Alam MN, Abser MN (2015) Removal of chromium from tannery wastewater by tannery lime liquor: A very cost-effective method. Int J Curr Res 7(6):16795–16798
Saranraj P, Sujitha D (2013) Microbial bioremediation of chromium in tannery effluent: A review. Int J Microbiol Res 4(3):305–320. https://doi.org/10.5829/idosi.ijmr.2013.4.3.81228
Saha R, Nandi R, Saha B (2011) Sources and toxicity of hexavalent chromium. J Coord Chem 64(10):1782–1806. https://doi.org/10.1080/00958972.2011.583646
Wang W (1986) The effect of river water on phytotoxicity of Ba, Cd, and Cr. Environ Pollut B 11(3):193–204. https://doi.org/10.1016/0143-148X(86)90023-6
Wang D, He S, Shan C, Zhang W, Pan B, Ma H, Zhang X (2016) Chromium speciation in tannery effluent after alkaline precipitation: Isolation and characterization. J Hazard Mater 316:169–177. https://doi.org/10.1016/j.jhazmat.2016.05.021
Kolomaznik K, Mladek M, Langmaier F, Janacova D, Taylor MM (2000) Experience in industrial practice of enzymatic dechromation of chrome shavings. J Am Leather Chem Assoc XCV(2):55–63
Genawi NM, Ibrahim MH, El-Naas MH, Alshaik AE (2020) Chromium removal from tannery wastewater by electrocoagulation: Optimization and sludge characterization. Water 12(5):1374
Ahmad T, Mustafa S, Naeem A, Anwar F, Mehmood T, Shah KH (2014) Ion exchange removal of chromium (iii) from tannery wastes by using a strong acid cation exchange resin amberlite IR-120 H+ and its hybrids. J Chem Soc Pak 36(5):818–828
Vinodhini PA, Sudha PN (2016) Removal of heavy metal chromium from tannery effluent using ultrafiltration membrane. Text Cloth Sustain 2:5. https://doi.org/10.1186/s40689-016-0016-3
Wionczyk B, Cierpiszewski R, Mól A, Prochaska K (2011) Studies on the kinetics and equilibrium of the solvent extraction of chromium (III) from alkaline aqueous solutions of different composition in the system with Aliquat 336. J Hazard Mater 198:257–268. https://doi.org/10.1016/j.jhazmat.2011.10.038
Moura RCDA, Bertuol DA, Ferreira CA, Amado FDR (2012) Study of chromium removal by the electrodialysis of tannery and metal finishing effluents. Int J Chem Eng:1–7. https://doi.org/10.1155/2012/179312
Sabur MA, Rahman MM, Safiullah S (2013) Treatment of Tannery Effluent by Locally Available Commercial Grade Lime. J Sci Res 5(1):143–150. https://doi.org/10.3329/jsr.v5i1.12557
Tchobanoglus G, Burton F, Stensel HD (2003) Wastewater engineering: Treatment and reuse. J Am Water Works Assoc 95(5):201
Aksu Z (2005) Application of biosorption for the removal of organic pollutants: A review. Process Biochem 40(3-4):997–1026. https://doi.org/10.1016/j.procbio.2004.04.008
Bayuo J, Rwiza M, Abukari MA, PeligBa KB (2022) Modeling and optimization of independent factors influencing lead (II) biosorption from aqueous systems: A statistical approach. Sci Afr 16:e01270. https://doi.org/10.1016/j.sciaf.2022.e01270
Bayuo J, Rwiza M, Mtei K (2022) A comprehensive review on the decontamination of lead (II) from water and wastewater by low-cost biosorbents. 18. https://doi.org/10.1039/D2RA00796G
Bayuo J (2021) An extensive review on chromium (VI) removal using natural and agricultural wastes materials as alternative biosorbents. J Environ Health Sci Eng 19:1193–1207. https://doi.org/10.1007/s40201-021-00641-w
Bayuo J, Abukari MA, Pelig-Ba KB (2020) Optimization using central composite design (CCD) of response surface methodology (RSM) for biosorption of hexavalent chromium from aqueous media. Appl Water Sci 10:135. https://doi.org/10.1007/s13201-020-01213-3
Bayuo J, Pelig-Ba KB, Abukari MA (2019) Adsorptive removal of chromium (VI) from aqueous solution unto groundnut shell. Appl Water Sci 9:107. https://doi.org/10.1007/s13201-019-0987-8
Abbas SH, Ismail IM, Mostafa TM, Sulaymon AH (2014) Biosorption of heavy metals: A review. J Chem Sci Technol 3(4):74–102
Sud D, Mahajan G, Kaur MP (2008) Agricultural waste material as potential adsorbent for sequestering heavy metal ions from aqueous solution-A review. Bioresour Technol 99(14):6017–6027. https://doi.org/10.1016/j.biortech.2007.11.064
Malik A (2007) Environmental challenge vis a vis opportunity: The case of water hyacinth. Environ Int 33(1):122–138. https://doi.org/10.1016/j.envint.2006.08.004
APIRIS (2005) Invasive nonindigenous plants in Florida. Florida Exotic Pest Plant Council
Zheng JC, Feng HM, Lam MHW, Lam PKS, Ding YW, Yu HQ (2009) Removal of Cu(II) in aqueous media by biosorption using water hyacinth roots as a biosorbent material. J Hazard Mater 171(1-3):780–785. https://doi.org/10.1016/j.jhazmat.2009.06.078
Mahamadi C (2011) Water hyacinth as a biosorbent: A review. Afr J Environ Sci Technol 5(13):1137–1145. https://doi.org/10.5897/AJESTX11.007
Priya ES, Selvan PS (2017) Water hyacinth (Eichhornia Crassipes)- An efficient and economic adsorbent for textile effluent treatment- A review. Arab J Chem 10:3548–3558. https://doi.org/10.1016/j.arabjc.2014.03.002
Schneider IAH, Rubio J, Smith RW (2001) Biosorption of metals onto plant biomass: exchange adsorption or surface precipitation. Int J Miner Process 62(1):111–120. https://doi.org/10.1016/S0301-7516(00)00047-8
Huynh AT, Chen YC, Tran BNT (2021) Small-scale study on removal of heavy metals from contaminated water using water hyacinth. Processes 9:1802–1811. https://doi.org/10.3390/pr9101802
Le TP, Le TD (2016) Studying on absorbing possibility of lead in aquatic environments of Hyacinth (In Vietnamese: Nghiên cứu hấp thụ Pb2+ trong nước của cây lục bình). Sci J TDMU 3:42–49
Le HQ, Chen YC, Huynh TA, Thai VL (2019) A study on removing arsenic contamination in soil by phytoremediation. Key Eng Mater 818:113–117. https://doi.org/10.4028/www.scientific.net/KEM.818.113
Häder DP, Banaszak AT, Villafañe VE, Narvarte MA, González RA, Helbling WE (2020) Anthropogenic pollution of aquatic ecosystems: Emerging problems with global implications. Sci Total Environ 713:136586. https://doi.org/10.1016/j.scitotenv.2020.136586
Mahmood T, Malik SA, Hussain ST (2010) Biosorption and recovery of heavy metals from aqueous solutions by Eichhornia Crassipes (Water Hyacinth) ash. Bio Resources 5(2):1244–1256
Trivedy RK (1998) Water hyacinth-based systems for waste treatment: advanced wastewater treatment technologies. Glob Sci 1:463–486
Allen SE, Grimshaw HM, Rowland AP (1986) Chemical analysis. In: Moore PD, Chapman SB (eds) Methods in plant ecology. Blackwell Scientific Publication, Oxford, pp 285–344
Lu D, Cao Q, Li X, Cao X, Luo F, Shao W (2009) Kinetics and equilibrium of Cu(II) adsorption onto chemically modified orange peel cellulose biosorbents. Hydrometallurgy 95:145–152. https://doi.org/10.1016/j.hydromet.2008.05.008
Santhi T, Manonmani S, Smitha T (2010) Removal of methyl red from aqueous solution by activated carbon prepared from the annona squmosa seed by adsorption. Chem Eng Res Bull 14:11–18. https://doi.org/10.3329/cerb.v14i1.3767
Bayisa YM, Bullo TA, Akuma DA (2021) Chromium removal from tannery effluents by adsorption process via activated carbon chat stems (Cayha Edulis) using response surface methodology. BMC Res Notes 14:431–437. https://doi.org/10.1186/s13104-021-05855-7
Munagapati VS, Wen HY, Vijaya Y, Wen JC, Garcia JR et al (2021) Removal of anionic (Acid Yellow 17 and Amaranth) dyes using aminated avocado (Persea americana) seed powder: adsorption/desorption, kinetics, isotherms, thermodynamics, and recycling studies. Int J Phytoremediation 23(9):911–923. https://doi.org/10.1080/15226514.2020.1866491
Melliti A, Srivastava V, Kheriji J, Sillanpa M, Hamroun B (2021) Date Palm Fiber as a novel precursor for porous activated carbon: Optimization, characterization and its application as Tylosin antibiotic scavenger from aqueous solution. Surf Interfaces 24:10104. https://doi.org/10.1016/j.surfin.2021.101047
Thomas O, Baures E, Pouet MF (2005) UV spectrophotometry as a non-parametric measurement of water and wastewater quality variability. Water Qual Res J Canada 40(1):51–58. https://doi.org/10.2166/wqrj.2005.004
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interf Sci 209:172–184. https://doi.org/10.1016/j.cis.2014.04.002
Baniamerian MJ, Moradi SE, Noori A, Salahi H (2009) The effect of surface modification on heavy metal ion removal from water by carbon nanoporous adsorbent. Appl Surf Sci 256:1347–1354. https://doi.org/10.1016/j.apsusc
Louarrat M, Rahman AN, Bacaoui A, Yaacoubi A (2017) Removal of chromium Cr (VI) of tanning effluent with activated carbon from tannery solid wastes. Am J Phys Chem 6(6):103–109. https://doi.org/10.11648/j.ajpc.20170606.11
Najem AM (2015) Evaluation the biosorption capacity of water hyacinth (Eichhornia crassipes) root for some heavy metals. Iraqi J Sci 56(4):2846–2852
Romero-González J, Gardea-Torresdey JL, Peralta-Videa JR, Rodríguez E (2005) Determination of equilibrium and kinetic parameters of the adsorption of Cr(III) and Cr(VI) from aqueous solutions to Agave Lechuguilla biomass. Bioinorg Chem Appl 3(1-2):55–68. https://doi.org/10.1155/BCA.2005.55
Mohammed AK, Ali SA, Najem AM, Ghaima KK (2013) Effect of some factors on biosorption of lead by dried leaves of water hyacinth (Eichhornia Crassipes). Int J Pure Appl Sci Technol 17(2):72–78
Badillo-Almaraz V, Trocellier P, Dávila-Rangel I (2003) Adsorption of aqueous Zn (II) species on synthetic zeolites. Nucl Instrum Methods Phys Res B: Beam Interact Mater At 210:424–428. https://doi.org/10.1016/S0168-583X(03)01063-2
Hall KR, Eagleton LC, Acrivos A, Vermeulen T (1966) Pore-and solid-diffusion kinetics in fixed-bed adsorption under constant-pattern conditions. Ind Eng Chem Fundamen 5(2):212–223. https://doi.org/10.1021/i160018a011
Jumean F, Khamis M, Sara Z, Rich AM (2015) Concurrent removal and reduction of Cr(VI) by wool: short and long term equilibration studies. Am J Analyt Chem 6:47–57. https://doi.org/10.4236/ajac.2015.61005
Ho YS, McKay G, Wase D, Foster CF (2000) Study of the sorption of divalent metal ions on to peat. Adsorpt Sci Technol 18:639–650
Lagergren S (1898) Zurtheorie der sogenannten adsorption gelosterstofe. Kungliga Svenska Vetenskapsakademiens Handlingar 24:1–39
Badessa TS, Wakuma E, Yimer AM (2020) Bio-sorption for effective removal of chromium (VI) from wastewater using Moringa stenopetala seed powder (MSSP) and banana peel powder (BPP). BMC Chem 14:71. https://doi.org/10.1186/s13065-020-00724-Z
Afzaal M, Hameed S, Abbasi NA, Liaqat I, Rasheed R, Khan AAA, Manan HA (2022) Removal of Cr (III) from wastewater by using raw and chemically modified sawdust and corn husk. Water Pract Technol 17(9):1937–1958. https://doi.org/10.2166/wpt.2022.093
Kaźmierczak B, Molenda J, Swat M (2021) The adsorption of chromium (III) ions from water solutions on biocarbons obtained from plant waste. Environ Technol Innov 23:101737. https://doi.org/10.1016/j.eti.2021.101737
Bukhari A, Hassan Z, Atta M, Nazir A, Aslam F, Naouar A, Al-Fawzan FF, Alissa SA, Iqbal M, Ahmad N (2022) Removal of Cr(III) from aqueous solution using labeo rohita chitosan-based composite. Adsorpt Sci Technol, Article ID:5395720. https://doi.org/10.1155/2022/5395720
Pietrelli L, Francolini I, Piozzi A, Sighicelli M, Silvestro I, Vocciante M (2020) Chromium(III) removal from wastewater by chitosan flakes. Appl Sci 10(6):1925. https://doi.org/10.3390/app10061925
Samaraweera APGMV, Priyantha N, Gunathilake WSS, Kotabewatta PA, Kulasooriya TPK (2020) Biosorption of Cr(III) and Cr(VI) species on NaOH-modified peel of Artocarpus nobilis fruit. Investigation of Kinetics 10:115. https://doi.org/10.1007/s13201-020-01187-2
Irfan M, Rashid U, Ibrahim M, Nisa Z, Aal-muhtaseb A, Ali S (2013) Optimization of Cr(III) removal from wastewater using thespesia populnea particles by response surface methodology. Asian J Chem 25(16):9315–9320. https://doi.org/10.14233/ajchem.2013.15493
Giri DD, Shah M, Srivastava N, Hashem A, Abd Allah EF, Pal DB (2021) Sustainable chromium recovery from wastewater using mango and jackfruit seed kernel bio-adsorbents. Front Microbiol 12:717848. https://doi.org/10.3389/fmicb.2021.717848
Author information
Authors and Affiliations
Contributions
Nure Jannat, Hijmun-Nahar, and Nadia Sultana Khan: experimental work, methodology, data collection, and original draft manuscript preparation. Mostak Ahamed Tanmoy: experimental work, data generation. Md. Abdul Mottalib: conceptualization, design, methodology, review and editing. Md. Abdul Goni: review and editing. Mala Khan: instrumental help and sample analysis. Muhammed Shah Miran: instrumental help, sample analysis, editing.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: The original version of this article unfortunately contained mistakes.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jannat, N., Nahar, H., Khan, N.S. et al. Potential Removal of Chromium from Tannery Wastewater by Water Hyacinth Roots. Water Conserv Sci Eng 8, 21 (2023). https://doi.org/10.1007/s41101-023-00196-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s41101-023-00196-x