Abstract
We aimed to investigate the potential association between urinary albumin-to-creatinine ratio (UACR) and estimated glomerular filtration rate (eGFR) and diabetic peripheral neuropathy (DPN). We were especially interested in the relationship between normal or mildly abnormal UACR and eGFR with DPN. A retrospective study was performed in 1059 patients with type 2 diabetes patients from Fuzhou, China, who were seen between 2010 and 2015. The DPN population demonstrated higher UACR and lower eGFR than the non-DPN population. Nerve conduction velocities (NCVs) were negatively correlated with UACR and were positively correlated with eGFR. UACR and eGFR were associated with the risk of DPN. Even in the UACR < 30 mg/g and eGFR ≥ 60 ml/min/1.73 m2 groups, the relationship above still existed and patients in the highest tertiles of UACR and lowest tertiles of eGFR demonstrated a greater risk of DPN (OR = 2.456, 95% CI 1.461–4.127; OR = 2.021, 95% CI 1.276–3.203). Receiver operating characteristic (ROC) analysis revealed that the area under curve (AUC) of UACR, eGFR, and joints indicates that DPN was 0.749, 0.662, and 0.731, respectively. Lower eGFR and higher UACR may be associated with the risk of DPN, even though normal or mildly abnormal UACR and eGFR have already been found to be predictive factors of DPN. Further, UACR is more sensitive than eGFR. Separately, UACR was a moderate indication of DPN, and combining it with eGFR did not increase its effect of indication to DPN.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Diabetic peripheral neuropathy (DPN) is one of the most significant complications associated with diabetes [1]. Previous studies have shown the prevalence of DPN in patients with diabetes to be about 20–30%; of these, 30 to 40% do not have subjective symptoms [2, 3]. DPN seriously affects the quality of life in patients with diabetes. Nerve conduction velocity (NCV) is the gold standard for diagnosing peripheral neuropathy [1]. However, few patients receive appropriate neurological examination in a timely manner. It is therefore necessary to research early warning signs of DPN in order to improve early detection of DPN.
Researchers have suggested various etiologies and pathogeneses of DPN, including vascular lesions, metabolic disorders [4], oxidative stress [5], and lack of neurotrophic factors [6]; among these, vascular factors play an important role in DPN occurrence and development [4]. DPN is closely related to a variety of vascular complications, including microangiopathy and macroangiopathy inpatients with diabetes [7,8,9]. As microvascular complications, DPN and diabetic nephropathy (DN) are major contributors to the morbidity associated with diabetes, and they affect a large number of patients [1, 10]. A gene/network-level comparison of DN and DPN reveals that they share common pathogenic mechanisms but also have distinct characteristics [11]. Studies have shown that there are common risk markers for DPN including diabetic retinopathy (DR) and DN [9, 12]. Research has also shown that albuminuria and estimated glomerular filtration rate (eGFR) are not the only indicators of renal function impairment in DN and that they are also associated with DR, DPN, cardiac autonomic neuropathy (CAN), and other diabetes-related complications [13, 14]. Early cohort studies with small sample populations have demonstrated that the urinary albumin-to-creatinine ratio (UACR) is independently associated with DPN in patients with diabetes over the course of 10 years [15]. Studies of eGFR have reached conflicting conclusions. Some studies have found that even though serum cystatin C is independently associated with DPN and may be a potential biomarker of DPN, GFR is not an independent risk marker of DPN [16]. However, Yang et al. [17] found that low levels of eGFR (<60 ml/min/1.73 m2) were associated with DPN. However, there are limited reports on the correlation between DPN with normal or mild abnormal UACR and eGFR. Additionally, there are few reports on UACR combined with eGFR for evaluating the risk of DPN. Our study aims to investigate the correlation between UACR, eGFR, and DPN, including the correlation between normal or mildly abnormal UACR and eGFR with DPN, and the feasibility of using these factors to evaluate DPN. We have also looked for potential associations between UACR, eGFR, and DPN, in order to find early warning signs that would indicate a need for DPN screening.
Material and methods
Study population
Between May 1, 2010 and June 30, 2015, 1059 patients with type 2 diabetes (average age 60.29 years) were recruited from the Endocrinology and Neurology Department at the First Affiliated Hospital of Fujian Medical University. The population included 589 males and 470 females. No patients enrolled in the study had a history of neurotrophic drug use. The American Diabetes Association standards for type 2 diabetes mellitus diagnosis were used [18]. Patients with the following conditions were excluded: (i) type 1 diabetes, gestational diabetes, and other types of diabetes, diabetic ketoacidosis, hyperosmolar nonketotic comas; (ii) other neuropathic conditions including chronic inflammatory demyelinating polyradiculoneuropathy (CIPD), mononeuropathy, or conditions caused by vitamin B deficiency and thyroid dysfunction; (iii) other diseases including Guillain-Barre syndrome, cerebral infarction, complicated with degenerative changes in cervical and lumbar vertebra, severe arteriovenous vascular disease (venous embolism, lymphatic inflammation); and (iv) other nerve lesions caused by drugs, including chemotherapy drugs. This study was approved by the Ethics Committee of Biomedical Research of the First Affiliated Hospital of Fujian Medical University, and all of the participants provided signed informed consent.
Clinical measurements
Information regarding patient demographic characteristics, disease duration, lifestyle, medical history, and drug use history was obtained from medical records. Symptoms of somatic neuropathy were documented, including numbness, burning, deep aching, and unsteadiness in walking. First, all patients had a neurological symptom history taken and were given a physical examination that included height, weight, blood pressure, and a neurological examination. During the neurological examination, touch sensation was tested using a 10 g monofilament, pain sensation was tested using a pin, reflexes were tested using a tendon hammer, and vibration sensation was tested using a standard 128 Hz tuning fork. Blood pressure was measured after 15 min of rest. Body weight and height were measured with the patient barefoot and wearing light clothes. Body mass index (BMI, kg/m2) was determined by dividing weight (in kilograms) by height (in meters squared).
Biochemical measurements
After a 10-h overnight fast, venous blood samples were collected for measuring fasting plasma glucose, lipids, creatinine (Scr), uric acid, routine hematological and biochemical parameters, and glycated hemoglobin A1c (HbA1c). UACR was calculated by dividing urine albumin by urine creatinine during morning urine collection (mg/g). Glomerular filtration rate (GFR) was estimated based on serum creatinine concentration using the modification of diet in renal disease formula: estimated GFR = eGFR (ml/min) = 186 × [SCr mmol/l) / 88.41]−1.154 × age−0.203 (×0.724 for female).
We define eGFR and UACR categories based on the Kidney Disease: Improving Global Outcomes standards [19]: G1: normal or high (eGFR ≥ 90 ml/min/1.73 m2), G2: mildly decreased (eGFR 60–89 ml/min/1.73 m2), G3: mildly to severely decreased (eGFR 30–59 ml/min/1.73 m2), G4: severely decreased (eGFR 15–29 ml/min/1.73 m2), G5: kidney failure (eGFR < 15 ml/min/1.73 m2), A1: normal to mildly increased (UACR < 30 mg/g), A2: moderately increased (UACR 30–300 mg/g), and A3: severely increased (UACR > 300 mg/g).
Neuropathy assessment
Testing was performed on each participant by the same experienced physicians using standard procedures. The results were evaluated using age-related reference values.
Nerve conduction velocity tests
Electromyography (EMG) (key point, Alpine Biomed ApS, Denmark) was performed on each subject to assess ulnar, median motor nerve conduction velocity (MCV), sensory nerve conduction velocity (SCV), tibial, common peroneal nerve MCV and their sensory branches, and superficial peroneal, sural SCV. The normal reference values are based on the results of an epidemiological survey conducted in the Chinese population in 1984 by Tang et al. [20]. The threshold for the slowed NCV was set at <20% of the control NCV. When two or more nerves tested as abnormal, nerve conduction (NC) was considered abnormal. There were no interference sources in the examination room, and the environment was kept quiet. The examination room temperature was kept at 18–25 °C, and local skin temperature was kept constant (28–30 °C).
Diagnosis of DPN
The diagnosis of DPN was based on the criteria proposed by an International European and North American Expert Committee [1]. DPN is defined as patients with diabetes (having or not having clinical symptoms and signs) who have abnormal nerve conduction velocity, including both diagnosis and subclinical DPN (when there are no signs or symptoms of neuropathy, DPN is confirmed with abnormal nerve conductions).
Statistical analysis
We used SPSS version 18.0 for Windows (Chicago, IL, USA) to perform statistical analysis. The data were expressed as the median with the 25th and 75th quartiles for skewed data or as the mean (SD) for normally distributed data. Differences between groups were evaluated by the non-parametric test or the Student’s t test. ANOVA was used to assess multiple comparisons among groups. Percentages were compared using the chi-squared test. Spearman’s correlation analysis was performed to explore the interrelationship between UACR, eGFR, and NCV. Multivariate logistic regression analysis was performed to identify independent factors for DPN. Forest plots were drawn using a GraphPad Prism. Receiver operating characteristic (ROC), drawn by SPSS version 18.0, was performed to identify the optimal cutoff point of UACR and eGFR for indicating DPN. Statistical significance was determined with P < 0.05.
Results
Study population characteristics
A total of 1059 patients (mean age, 60.29 ± 11.52 years; male/female, 589/470; and mean diabetes duration, 8.20 ± 6.81 years) were enrolled in this study. Among these patients, 642 patients did not have DPN and 417 patients had DPN confirmed by EMG. Of the patients with DPN, 352 were diagnosed by clinical evidence and an EMG confirmed and 65 had subclinical DPN. The clinical characteristics of the patients with DPN (clinical DPN and subclinical DPN) and without DPN are given in Table 1. The two groups (DPN and non-DPN) had a similar constituent ratio of age and gender. The medications being used by the two groups were not found to be statistically significant, with the exception of differences between insulin and renin-angiotensin-aldosterone system (RAAS) blockers. Compared with patients who did not have DPN, a significant number of patients with DPN had a longer duration of diabetes and higher prevalence rates of hypertension, retinopathy, and diabetic foot (both P < 0.001). Systolic blood pressure and HbA1c, BUN, Cr, UA, and UACR levels were all higher (both P < 0.001) for patients with DPN. EGFR was lower in patients with DPN (P < 0.001). No significant differences were observed in BMI, FPG, TC, TG, HDL cholesterol (HDL-C), and LDL cholesterol (LDL-C) levels. Further analysis was performed among the three groups of clinical DPN, subclinical DPN, and non-DPN. The three groups also had a similar constituent ratio of age and gender. The insulin and RAAS blockers being used by the three groups were found to be statistically significant. A longer duration of diabetes, higher prevalence of retinopathy, higher prevalence of diabetic foot, higher systolic blood pressure, and higher HbA1c, BUN, Cr, UA, and UACR while EGFR was lower were found in clinical DPN and subclinical DPN compared with non-DPN T2DM patients (all P < 0.05). Data are shown in Table 1. In the next section, clinical DPN and subclinical DPN are integrated into DPN for analysis.
DPN detection rate and changes in NCV by varying UACR and eGFR
In stratifications of the entire group, increasing UACR paralleled a gradual increase in the detection rate of DPN (26.3, 50.5, and 86.0%, respectively, P < 0.001), while decreasing eGFR paralleled a gradual increase in the detection rate of DPN (30.1, 44.4, and 78.9%, respectively, P < 0.001). Furthermore, in subgroups with UACR < 30 mg/g and eGFR ≥ 60 ml/min/1.73 m2, patients were stratified into tertiles (lowest tertile, UACR < 5.9 mg/g; highest tertile, UACR ≥ 11.22 mg/g; lowest tertile, eGFR < 93.72 ml/min/1.73 m2; highest tertile, eGFR > 114.49 ml/min/1.73 m2). Statistical differences in the detection rate of DPN were observed across different UACR and eGFR subgroups spanning the lowest to highest ACR and eGFR tertiles. A significant increase in DPN was observed in the middle and highest tertiles compared with the lowest tertile in the UACR subgroups; a significant decrease was observed in the eGFR subgroups (Table 2). Odds ratios (ORs), calculated for the comparison of detection rates among groups of UACR and eGFR, are shown in Table 2.
Both MCV and SCV decreased with the increase of UACR and the decrease of eGFR. This relationship was present in the stratification of UACR < 30 mg/g. However, in the stratification of eGFR ≥ 60 ml/min/1.73 m2, this trend was only observed in the SCV of the median nerve and the superficial peroneal nerve, and there was no significant difference in the changes of MCV of these nerves (data not shown.)
The correlation of UACR, eGFR, and DPN
In whole group analysis, UACR was inversely correlated to MCV and SCV, and eGFR was positively correlated to MCV and SCV of all nerves detected when adjusting for age, gender, diabetes duration, systolic blood pressure, BMI, HbA1c, FPG, UA, history of smoking and drinking, use of insulin and RAAS blockers, retinopathy, and diabetic foot. Additionally, in subgroup analysis, UACR level was closely associated with MCV of the ulnar, tibial, and peroneal nerves and SCV of the superficial peroneal sural nerve (UACR < 30 mg/g). Meanwhile, eGFR level was only associated with MCV of the median peroneal nerve and SCV of the superficial peroneal sural nerve in the eGFR ≥ 60 ml/min/1.73 m2 subgroups (Table 3).
In whole group analysis, patients were divided into three groups according to ACR and eGFR levels (<30, 30–300, and >300 mg/g; ≥90, 60–89.99, <60 ml/min/1.73 m2). Multiple logistic regression analyses showed that higher UACR and lower eGFR were associated with DPN after adjusting for age, gender, diabetes duration, systolic blood pressure, BMI, HbA1c, FPG, UA, history of smoking and drinking, use of insulin and RAAS blockers, retinopathy, and diabetic foot (OR = 3.143, 95% CI = 2.427–4.069, P < 0.001; OR = 2.616, 95% CI = 2.000–3.423, P < 0.001, respectively) (Fig. 1a).
Figure 1b illustrates multivariate-adjusted ORs of DPN with an UACR tertile increment and eGFR tertile decrement in different subgroups (UACR < 30 mg/g, eGFR ≥ 60 ml/min/1.73 m2). In the unadjusted model 1, diabetic patients in the highest tertile (Q3) of UACR demonstrated a significantly higher risk of DPN compared to patients in the lowest tertile (Q1, referent) (P < 0.01). The risk of DPN in the second tertile (Q2) did not increase significantly in comparison to Q1. After adjusting for age, gender, diabetes duration, BMI, SBP, DBP, FPG, HbA1C, UA, smoking, drinking, drug use, and history of retinopathy and diabetic foot, a fully adjusted logistic regression (model 3) demonstrated that diabetic patients in Q3 were 2.456 times more likely to experience DPN than those in Q1 (OR = 2.456, 95% CI 1.461–4.127, P = 0.001). Further, after adjusting for the above variables, a fully adjusted logistic regression (model 3) demonstrated that diabetic patients in the lowest eGFR tertile (Q3) were 2.021 times more likely to experience DPN than those in Q1 (OR = 2.021, 95% CI 1.276–3.203, P = 0.003).
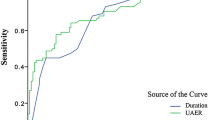
ROC analysis of UACR and eGFR to indicate DPN (Fig. 2)
ROC analysis revealed that the optimal cutoff point for UACR that indicated DPN was 22.31 mg/g (AUC = 0.749; 95% CI, 0.717–0.780; sensitivity, 62.00%; specificity, 75.0%; negative predictive value, 75.24%; positive predictive value, 65.70%), while 34.04 mg/g for clinical DPN (AUC = 0.732; 95% CI, 0.699–0.766; sensitivity, 57.4%; specificity, 76.4%; negative predictive value, 78.3%; positive predictive value, 54.7%). The optimal cutoff point for eGFR that indicated DPN was 87.21 ml/min/1.73 m2 (AUC = 0.662; 95% CI, 0.627–0.697; sensitivity, 47.4%; specificity, 77.3%; negative predictive value, 69.34%; positive predictive value, 57.56%), while 77.93 ml/min/1.73 m2 for clinical DPN (AUC = 0.712; 95% CI, 0.607–0.681; sensitivity, 38.1%; specificity, 83.7%; negative predictive value, 73.1%; positive predictive value, 53.8%). The AUC of combined UACR and eGFR that indicated DPN was 0.731 (95% CI, 0.698–0.764; sensitivity, 55.30%; specificity, 81.5%; negative predictive value, 73.73%; positive predictive value, 66.01%), while 0.712 of AUC for clinical DPN (95% CI, 0.676–0.747; sensitivity, 50.9%; specificity, 82.5%; negative predictive value, 77.1%; positive predictive value, 59.1%) (Fig. 2a, b).
Discussion
Our study revealed a close relationship between both UACR levels and DPN and eGFR levels and DPN in patients with type 2 diabetes. When UACR increased and eGFR decreased (including the UACR < 30 mg/g and eGFR ≥ 60 ml/min/1.73 m2 groups), MCV and SCV decreased, and the detection rate of DPN increased. Correlation analysis revealed that UACR and eGFR were correlated with SCV and MCV in each nerve. In addition, independent of covariables, an elevated UACR level and a depressed eGFR level were both associated with DPN. These associations were present in both the normal and mildly abnormal UACR and eGFR groups. UACR showed a moderate indication to DPN; combined with eGFR, it did not increase the effect of DPN indication.
Previous studies have shown that diabetic neuropathy is associated with renal function [15, 17]. We sought to determine the potential association between DPN and UACR and eGFR, in order to provide early clues or warning signals to improve DPN screening. Our results showed that patients with DPN had higher UACR levels and lower eGFR levels, and with an increase of UACR and decrease of eGFR, the detection rate of DNP increased. Previous studies support our results, and studies have shown that diabetic cardiac autonomic neuropathy (CAN) and DPN patients have lower eGFR levels and higher urine microalbumin levels [13, 16, 21]. Both our study and previous studies suggest that eGFR and UACR may be associated with DPN in patients with diabetes.
Therefore, we further analyzed the correlation between UACR and eGFR with DPN and found that eGFR and UACR were not only significantly correlated with CV of the peripheral nerve but they were also related risk markers for DPN. Previous studies have found that UACR was significantly correlated with DPN [15], but for eGFR, there are differing views in various studies. Studies have shown that GFR estimated by serum cystatin C was more sensitive than GFR estimated by serum creatinine [22] and was closely related to DPN. However, another study has shown that eGFR (<60 ml/min/1.73 m2) is one of the most important risk markers of DPN [17]. We found that the correlation in eGFR and NCV was weaker than the correlation between UACR and NCV, especially in the eGFR ≥ 60 ml/min/1.73 m2 subgroup. One possible reason for this is that during the progression of diabetic nephropathy, the increase of UACR and the decrease of eGFR were not completely parallel; patients who had large amounts of albumin also did not necessarily have low levels of eGFR [19]. This may also be related to high kidney filtration rates in some cases, and the high filtration rate itself is also a sign of kidney damage, which can lead to the increase of urinary albumin excretion [23]. Some authors have demonstrated that low eGFR is a frequent finding in patients with type 2 diabetes, even in the absence of albuminuria. It has been suggested that low GFR and increased UACR have different meanings and different predictive capabilities toward different end points [24, 25]. Our study also found that the correlations between NCV of lower limbs with UACR and eGFR were stronger than those between NCV of upper limbs with UACR and eGFR. Moreover, the correlation between SCV and UACR and eGFR is stronger than the correlation between MCV and UACR and eGFR. This may be related to the fact that abnormal CV in the peripheral nerve in lower limbs appeared earlier than in the upper limbs and the abnormal SCV appeared earlier than MCV in patients with DPN [26].
Currently, many studies show that normal or mildly abnormal UACR and eGFR were associated with arteriosclerosis [27], hypertension [28], congestive heart failure [29], and other heart defects. However, there are few reports on the relationship between normal or mildly abnormal ranges of UACR (UACR < 30 mg/g) and eGFR (eGFR ≥60 ml/min/1.73 m) with DPN. Our study showed that even in the UACR < 30 mg/g groups, the detection rate of DPN increased and the CV of each nerve decreased with the increase of UACR, and UACR and NCV had different degrees of correlation after controlling for confounding factors. Similar results also appeared in the eGFR ≥ 60 ml/min/1.73 m2 groups. Our results suggest that the increase of UACR and the decrease of eGFR may be associated with an increased risk of DPN; even though they were in the normal or mildly abnormal ranges, they may also indicate the risk of DPN.
Previous evidence supports our findings, and studies have reported that albumin in urine may indicate a wide range of vascular endothelial dysfunction, while the damming of vascular endothelial cells can lead to a decrease of blood flow, increase of blood viscosity, ischemia, and hypoxia of endoneurium, damming of the epineurium vessel, degeneration of nerve fiber, and conduction dysfunction [30]. Moreover, the decline of GFR can also lead to an increase in the level of advanced glycationend products (AGEs) [31] and a chronic low-grade inflammatory state [32, 33], which can lead to DPN. Tahrani et al. [14] found that patients with CAN had lower eGFR and higher prevalence of albuminuria. As previously discussed, the relationship between DPN and diabetic nephropathy might be bidirectional and mediated by CAN. CAN has proved to be a possible progression promoter of diabetic nephropathy [34], even in type 2 diabetes and among Asian populations [35]. It has been suggested that this could be mediated by CAN-induced changes in glomerular hemodynamics and in the circadian rhythms of blood pressure and albuminuria. The unified mechanism theory of diabetic complications proposed by Brownlee may provide an explanation for this. This theory suggests that diabetes selectively damages cells and causes damage to a particular subset of cell types: capillary endothelial cells in the retina, mesangial cells in the renal glomerulus, and neurons and Schwann cells in peripheral nerves. A consistent differentiating feature common to all cell types that are damaged by hyperglycemia is an increased production of reactive oxygen species which then induces oxidative stress in tissue cells and eventually leads to the occurrence of chronic complications of diabetes [5]. Therefore, we have reason to believe that DN and DPN have a common pathogenesis; the increase of UACR and decrease of eGFR may also indicate the increased risk of DPN.
Both albuminuria and eGFR are independent risk markers for the endpoint events of cardiovascular disease (CVD) and kidney disease and are the evaluation index of CVD risk [36]. It is also recommended to use a combination of albumin and eGFR to evaluate the prognosis of diabetic kidney disease (DKD) [19]. However, there are few reports addressing a united evaluation of UACR and eGFR for DPN so far. Our study showed that a separate evaluation of UACR had a moderate indication for DPN, and combination with eGFR did not increase the effect of indication of DPN. A possible reason for this is that the correlation between eGFR and DPN is weak, and the etiological agents of DPN are not due to a single factor.
Conclusion
In summary, our study found that in the diabetic population, elevated UACR and decreased eGFR can be predictive factors of DPN. Even when variations of UACR and eGFR are in the normal or mildly abnormal range, it is a dangerous signal of DPN; moreover, UACR is a more sensitive indicator of DPN than eGFR. Separately, UACR is a moderate indicator of DPN, and combined with eGFR, there is no increase in the effect of the indication of DPN. Patients with diabetes who have even mildly abnormal UACR and eGFR need to be alert to the presence of other microvascular lesions and should be screened for peripheral neuropathy, in order to detect DPN early.
Study limitations
Although there is potential value in our findings, several limitations of the study should be noted. The main limitation concerns the retrospective study design that does not allow us to derive any cause–effect relationship, even though our data clearly indicate an association of evaluated DPN with renal function. In addition, our study indicates that a correlation exists between DPN and renal function, but a high UACR level and a low eGFR level are not necessarily the exclusive risk markers for DPN. This study was also lacking in nerve conduction velocity data for non-diabetic patients for use as a healthy control cohort and also lacked data concerning physical activity. Less physical activity [37] and poor level of glycemic control may increase the risk of DPN. Most of our patients were from the Han ethnicity. A low prevalence of cardiometabolic risk factors in that population possibly affected the natural history of both DPN and diabetic nephropathy [38]. Moreover, variability in laboratory measurements might have influenced the results. We will design a prospective follow-up study to obtain further results.
Reference
Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, Lauria G et al (2010) Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33(10):2285–2293. doi:10.2337/dc10-1303
Tesfaye S, Stevens LK, Stephenson JM, Fuller JH, Plater M, Ionescu-Tirgoviste C, Nuber A, Pozza G, Ward JD (1996) Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: the EURODIAB IDDM Complications Study. Diabetologia 39(11):1377–1384
Cabezas-Cerrato J (1998) The prevalence of clinical diabetic polyneuropathy in Spain: a study in primary care and hospital clinic groups. Neuropathy Spanish Study Group of the Spanish Diabetes Society (SDS). Diabetologia 41(11):1263–1269
Cameron NE, Eaton SE, Cotter MA, Tesfaye S (2001) Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetologia 44(11):1973–1988. doi:10.1007/s001250100001
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54(6):1615–1625. doi:10.2337/diabetes.54.6.1615
Chiarelli F, Santilli F, Mohn A (2000) Role of growth factors in the development of diabetic complications. Horm Res 53(2):53–67
Yokoyama H, Yokota Y, Tada J, Kanno S (2007) Diabetic neuropathy is closely associated with arterial stiffening and thickness in type 2 diabetes. Diabet Med 24(12):1329–1335. doi:10.1111/j.1464-5491.2007.02278.x
Brownrigg JR, de Lusignan S, McGovern A, Hughes C, Thompson MM, Ray KK, Hinchliffe RJ (2014) Peripheral neuropathy and the risk of cardiovascular events in type 2 diabetes mellitus. Heart 100(23):1837–1843. doi:10.1136/heartjnl-2014-305657
Kärvestedt L, Mårtensson E, Grill V, Elofsson S, von Wendt G, Hamsten A, Brismar K (2009) Peripheral sensory neuropathy associates with micro- or macroangiopathy: results from a population-based study of type 2 diabetic patients in Sweden. Diabetes Care 32(2):317–322. doi:10.2337/dc08-1250
Edwards JL, Vincent AM, Cheng HT, Feldman EL (2008) Diabetic neuropathy: mechanisms to management. Pharmacol Ther 120(1):1–34. doi:10.1016/j.pharmthera.2008.05. 005
Hur J, O’Brien PD, Nair V, Hinder LM, McGregor BA, Jagadish HV, Kretzler M, Brosius FC 3rd, Feldman EL (2016) Transcriptional networks of murine diabetic peripheral neuropathy and nephropathy: common and distinct gene expression patterns. Diabetologia 59(6):1297–1306. doi:10.1007/s00125-016-3913-8
Pradeepa R, Anjana RM, Unnikrishnan R, Ganesan A, Mohan V, Rema M (2010) Risk factors for microvascular complications of diabetes among South Indian subjects with type 2 diabetes—the Chennai Urban Rural Epidemiology Study (CURES) Eye Study-5. Diabetes Technol Ther 12(10):755–761. doi:10.1089/dia.2010.0069
Parving HH, Mogensen CE, Thomas MC, Brenner BM, Cooper ME (2005) Poor prognosis in proteinuric type 2 diabetic patients with retinopathy: insights from the RENAAL study. QJM 98(2):119–126. doi:10.1093/qjmed/hci017
Tahrani AA, Dubb K, Raymond NT, Begum S, Altaf QA, Sadiqi H, Piya MK, Stevens MJ (2014) Cardiac autonomic neuropathy predicts renal function decline in patients with type 2 diabetes: a cohort study. Diabetologia 57(6):1249–1256. doi:10.1007/s00125-014-3211-2
Bell DS, Ketchum CH, Robinson CA, Wagenknecht LE, Williams BT (1992) Microalbuminuria associated with diabetic neuropathy. Diabetes Care 15(4):528–531
Hu Y, Liu F, Shen J, Zeng H, Li L, Zhao J, Zhao J, Lu F, Jia W (2014) Association between serum cystatin C and diabetic peripheral neuropathy: a cross-sectional study of a Chinese type 2 diabetic population. Eur J Endocrinol 171(5):641–648. doi:10.1530/EJE-14-0381
Yang CP, Lin CC, Li CI, Liu CS, Lin WY, Hwang KL, Yang SY, Chen HJ, Li TC (2015) Cardiovascular risk factors increase the risks of diabetic peripheral neuropathy in patients with type 2 diabetes mellitus: the Taiwan diabetes study. Medicine (Baltimore) 94(42):e1783. doi:10.1097/MD.0000000000001783
American Diabetes Association (2014) Executive summary: standards of medical care in diabetes—2014. Diabetes Care 37(Suppl 1):S5–13. doi:10.2337/dc14-S005
Andrassy KM (2013) Comments on ‘KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease’. Kidney Int 84(3):622–623. doi:10.1038/ki.2013.243
Tang XF, Yang T, Yang BX, Liu XZ, Rong ZP (1984) Electromyographic findings in normal Chinese. Analysis of 310 subjects. Chin Med J 97(8):613–622
Russo GT, Giandalia A, Romeo EL, Scarcella C, Gambadoro N, Zingale R, Forte F, Perdichizzi G, Alibrandi A, Cucinotta D (2016) Diabetic neuropathy is not associated with homocysteine, folate, vitamin B12 levels, and MTHFR C677T mutation in type 2 diabetic outpatients taking metformin. J Endocrinol Investig 39(3):305–314. doi:10.1007/s40618-015-0365-9
Mussap M, Dalla Vestra M, Fioretto P, Saller A, Varagnolo M, Nosadini R, Plebani M (2002) Cystatin C is a more sensitive marker than creatinine for the estimation of GFR in type 2 diabetic patients. Kidney Int 61(4):1453–1461. doi:10.1046/j.1523-1755.2002.00253.x
Smithies O (2003) Why the kidney glomerulus does not clog: a gel permeation/diffusion hypothesis of renal function. Proc Natl Acad Sci U S A 100(7):4108–4113. doi:10.1073/pnas.0730776100
De Cosmo S, Viazzi F, Pacilli A, Giorda C, Ceriello A, Gentile S, Russo G, Rossi MC, Nicolucci A, Guida P, Feig D, Johnson RJ, Pontremoli R (2015) AMD-Annals Study Group. Serum uric acid and risk of CKD in type 2 diabetes. Clin J Am Soc Nephrol 10(11):1921–1929. doi:10.2215/CJN.03140315
Penno G, Solini A, Bonora E, Fondelli C, Orsi E, Zerbini G, Trevisan R, Vedovato M, Gruden G, Cavalot F, Cignarelli M, Laviola L, Morano S, Nicolucci A, Pugliese G (2011) Renal Insufficiency And Cardiovascular Events (RIACE) Study Group. Clinical significance of nonalbuminuric renal impairment in type 2 diabetes. J Hypertens 29(9):1802–1809. doi:10.1097/HJH.0b013e3283495cd6
Felsenthal G, McIvor ME (1984) Reappraisal of the electroneurographic and electromyographic diagnosis of diabetic peripheral neuropathy. Am J Phys Med 63(6):278–288
Huang L, Yang L, Zhang S, Liu D, Yan X, Yan S (2015) Low-grade albuminuria associated with brachial-ankle pulse wave velocity in young adults with type 2 diabetes mellitus in China. Diabetes Metab Res Rev 31(3):262–268. doi:10.1002/dmrr.2598
Takase H, Sugiura T, Ohte N, Dohi Y (2015) Urinary albumin as a marker of future blood pressure and hypertension in the general population. Medicine (Baltimore) 94(6):e511. doi:10.1097/MD.0000000000000511
Blecker S, Matsushita K, Köttgen A, Loehr LR, Bertoni AG, Boulware LE, Coresh J (2011) High-normal albuminuria and risk of heart failure in the community. Am J Kidney Dis 58(1):47–55. doi:10.1053/j.ajkd.2011.02.391
Yasuda H (2009) Pathophysiology and treatment for diabetic neuropathy. Rinsho Shinkeigaku 49(4):149–157
Wada R, Yagihashi S (2005) Role of advanced glycation end products and their receptors in development of diabetic neuropathy. Ann N Y Acad Sci 1043:598–604. doi:10.1196/annals.1338.067
Kajitani N, Shikata K, Nakamura A, Nakatou T, Hiramatsu M, Makino H (2010) Microinflammation is a common risk factor for progression of nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 88(2):171–176. doi:10.1016/j.diabres.2010.01.012
Vallance P, Collier J, Bhagat K (1997) Infection, inflammation, and infarction: does acute endothelial dysfunction provide a link. Lancet 349(9062):1391–1392. doi:10.1016/S0140-6736(96)09424-X
Spallone V, Ziegler D, Freeman R, Bernardi L, Frontoni S, Pop-Busui R, Stevens M, Kempler P, Hilsted J, Tesfaye S, Low P, Valensi P (2011) Toronto Consensus Panel on Diabetic Neuropathy. Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 27(7):639–653. doi:10.1002/dmrr.1239
Yun JS, Ahn YB, Song KH, Yoo KD, Kim HW, Park YM, Ko SH (2015) The association between abnormal heart rate variability and new onset of chronic kidney disease in patients with type 2 diabetes: a ten-year follow-up study. Diabetes Res Clin Pract 108(1):31–37. doi:10.1016/j.diabres.2015.01.031
Goff DC Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D (2014) 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 129(25 Suppl 2):S49–S73. doi:10.1161/01.cir.0000437741.48606.98
Chiang SS, Lee CL, Liu HC, Wang JS, Lee IT, Song YM, Fu CP, Li YF, Sheu WH, Lin SY (2016) Physical activity and albuminuria were associated with painful diabetic polyneuropathy in type 2 diabetes in an ethnic Chinese population. Clin Chim Acta 462:55–59. doi:10.1016/j.cca.2016.08.022
Gong H, Pa L, Wang K, Mu H, Dong F, Ya S, Xu G, Tao N, Pan L, Wang B, Shan G (2015) Prevalence of diabetes and associated factors in the Uyghur and Han population in Xinjiang, China. Int J Environ Res Public Health 12(10):12792–12802. doi:10.3390/ijerph121012792
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Sunjie Yan conducted the design of the study and reviewed/edited the drafts, and is guarantor. Yongze Zhang researched data and edited the drafts and contributed to discussion. Ying Jiang wrote manuscript. Ximei Shen cowrote the final draft.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Electronic supplementary material
Table S1
(DOC 53 kb).
Rights and permissions
About this article
Cite this article
Zhang, Y., Jiang, Y., Shen, X. et al. Can both normal and mildly abnormal albuminuria and glomerular filtration rate be a danger signal for diabetic peripheral neuropathy in type 2 diabetes mellitus?. Neurol Sci 38, 1381–1390 (2017). https://doi.org/10.1007/s10072-017-2946-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-2946-1