Abstract
Aims/hypothesis
The aim of this work was to assess the impact of cardiac autonomic neuropathy (CAN) on the development and progression of chronic kidney disease (CKD) in patients with type 2 diabetes.
Methods
We conducted a cohort study in adults with type 2 diabetes. Patients with end-stage renal disease were excluded. CKD was defined as the presence of albuminuria (albumin/creatinine ratio GFR > 3.4 mg/mmol) or an estimated (eGFR) < 60 ml min−1 1.73 m−2. CKD progression was based on repeated eGFR measurements and/or the development of albuminuria. CAN was assessed using heart rate variability.
Results
Two hundred and four patients were included in the analysis. At baseline, the prevalence of CKD and CAN was 40% and 42%, respectively. Patients with CAN had lower eGFR and higher prevalence of albuminuria and CKD. Spectral analysis variables were independently associated with eGFR, albuminuria and CKD at baseline. After a follow-up of 2.5 years, eGFR declined to a greater extent in patients with CAN than in those without CAN (−9.0 ± 17.8% vs −3.3 ± 10.3%, p = 0.009). After adjustment for baseline eGFR and baseline differences, CAN remained an independent predictor of eGFR decline over the follow-up period (β = −3.5, p = 0.03). Spectral analysis variables were also independent predictors of eGFR decline.
Conclusions/interpretation
CAN was independently associated with CKD, albuminuria and eGFR in patients with type 2 diabetes. In addition, CAN was an independent predictor of the decline in eGFR over the follow-up period. CAN could be used to identify patients with type 2 diabetes who are at increased risk of rapid decline in eGFR, so that preventative therapies might be intensified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic kidney disease (CKD) in diabetes is the most common cause of end-stage renal disease (ESRD) and is associated with increased morbidity and mortality [1, 2]. The speed at which CKD progresses is variable and largely dependent on BP, obesity, metabolic control and other factors such male sex and ethnicity [3, 4]. Despite attempts to improve metabolic control and inhibition of the renin–angiotensin–aldosterone system (RAAS), CKD in diabetes remain very common and many patients ultimately require renal replacement therapy (RRT). Hence, better understanding of the pathogenesis of CKD complicating diabetes is needed so that more effective treatments can be developed.
Cardiac autonomic neuropathy (CAN) is common in patients with type 2 diabetes, with a prevalence of 21–73% depending on the population studied and the methods and criteria used for its diagnosis [5–8]. Sympathetic overactivity has been shown to cause renal and tubular dysfunction in animal models of diabetes via indirect (hypertension and angiotensin II) and direct (vascular smooth muscle proliferation, vasoconstriction, podocyte injury) insults [9]. CAN is associated with increased cardiovascular disease morbidity and mortality [10, 11] and also with haemodynamic changes such as lack of nocturnal BP dipping (causing increased intraglomerular pressure resulting in albuminuria) [12], diurnal postural falls in BP (resulting in lower intraglomerular pressure) [13] and endothelial dysfunction [14] in humans. In rats with streptozotocin-induced diabetes, sympathetic overactivation has been shown to be involved in the pathogenesis of CKD [15] and renal denervation can prevent glomerular hyperfiltration [16]. In addition, CAN may occur very early in the course of diabetes and subclinical abnormalities in cardiac sympathetic innervation (as shown by PET [11C]HED retention studies) are associated with the presence of microvascular complications in patients with type 1 diabetes [7, 17]. Hence it is plausible that CAN is involved in the development and progression of CKD.
Several studies have examined the association between CAN and albuminuria and/or GFR [18–23]. These studies were of cross-sectional design, hence causation could not be proven, particularly since factors thought to contribute to the pathogenesis of CAN are similar to those proposed for other microvascular complications including CKD. Longitudinal studies are scarce and are limited to a small number of patients with type 1 diabetes [13, 24]; data regarding the longitudinal impact of CAN on CKD in patients with type 2 diabetes are lacking.
The primary aim of this study was therefore to assess the impact of CAN on estimated GFR (eGFR) progression longitudinally in patients with type 2 diabetes. Secondary aims included assessing the impact of CAN on the progression and development of CKD and albuminuria in these patients.
Methods
Participants
We conducted a prospective observational cohort study in White European and South Asian adults with type 2 diabetes. Patients were recruited in 2009 or 2010 and were followed until the end of 2012. Patients with ESRD receiving RRT were excluded. Patients were recruited from the diabetes clinic of a UK-based hospital and were approached consecutively in the waiting area by the investigator or a research nurse without any prior knowledge of their medical condition. Consent was obtained from the study participants and ethnicity was determined in accordance with the UK decennial census. The project was approved by the Warwickshire Research Ethics Committee (REC number 08/H1211/145).
CAN
CAN was assessed using heart rate variability (HRV) and was analysed using the continuous wavelet transform method. Numerical and graphical data were generated using ANX-3.0 software (ANSAR, Philadelphia, PA, USA) as described previously [25]. The R–R intervals were recorded and the HRV was plotted in the frequency domain to separate high (respiratory)-frequency (RFa, 0.15–0.4 Hz) from the low-frequency (LFa, 0.04–0.15 Hz) components by spectral analysis. HRV and BP were recorded with the patient in a sitting position during resting, deep breathing and the Valsalva manoeuvre and also in a standing position [26]. Data recorded included the expiratory/inspiratory (E/I) ratio, Valsalva ratio, 30:15 ratio, frequency domain analysis with respiratory adjustment (LFa, RFa and LFa/RFa) and time domain analysis including standard deviation of normal RR intervals (SDNN), square root of the mean squared differences of successive RR intervals (RMSSD) and percentage of adjacent R–R intervals that varied by more than 50 ms (pNN50). CAN was diagnosed when two or more of the following tests were abnormal: E/I ratio, Valsalva ratio, 30:15 ratio and postural drop in BP (drop of 20 mmHg in systolic or drop of 10 mmHg in diastolic BP) [27]. CAN assessment was performed in accordance with guidelines from the recent CAN Subcommittee of the Toronto Consensus Panel on Diabetic Neuropathy [28, 29]. Age-related normative values were defined as previously reported [30]. For more details about the protocol please see the electronic supplementary material (ESM). Further information about the ANX technology used in this manuscript can be found at http://www.ans-hrv.com/introduction.htm.
CKD and diabetic nephropathy
CKD and diabetic nephropathy (DN) were assessed using eGFR, which was calculated using the four-variable MDRD equation [31] and the urinary albumin/creatinine ratio (ACR) of a single early-morning urine measurement as we have described previously [32]. Microalbuminuria was defined as ACR > 3.4 mg/mmol and macroalbuminuria was defined as ACR ≥ 30 mg/mmol [33–35]. If a urine sample showed evidence of urinary tract infection it was repeated once the patient was free from infection. CKD was defined as the presence of albuminuria (micro or macro) or an eGFR < 60 ml min−1 1.73 m−2 [36]. DN was defined as eGFR < 60 ml min−1 1.73 m−2 and albuminuria. ACR and eGFR were measured at baseline and the end of the study. Rapid eGFR decline was defined as 4% decline in eGFR per year [37]. Study-end measurements were taken during patient visits to the follow-up appointments of the diabetes clinic. eGFR and ACR measurements during acute illness or following imaging using contrast, or from patients with significant haematuria or proteinuria without albuminuria, were excluded.
All assessments in the study (renal function and biochemical profiles) were conducted by personnel who were blinded to CAN status.
Outcome measures and analyses
Data analysis was performed using SPSS 21.0 software (SPSS, Chicago, IL, USA). Data distribution was examined using histograms and the Shapiro–Wilk test. Data are presented as mean (SD). Independent continuous variables were compared using Student’s t test or the Mann–Whitney test. Categorical variables were compared using the χ 2 test. Correlations between continuous variables were performed using the Pearson or Spearman’s tests depending on data distribution. Skewed data was normalised by log transformation when used in the regression models to comply with the regression assumptions. A subgroup univariable analysis by ethnicity, eGFR status (below and above 60 ml min−1 1.73 m−2) and albuminuria was performed.
Baseline analysis
To assess univariable correlations between continuous variables, the Pearson’s and Spearman’s tests were used. To assess whether CAN was independently associated with eGFR, CKD, DN and albuminuria, multiple linear regression (for eGFR) and logistic regression (for CKD/DN/albuminuria) were used. Proportion of variation explained by models (R 2), plus statistical significance and effect size of CAN are reported. Variables included in both the logistic and linear regression models were based on variables that differed between patients with and without CAN.
Longitudinal analysis
To assess the impact of CAN on CKD progression (i.e. eGFR), only patients with eGFR measurements at baseline and study-end were used. To account for baseline differences, linear regression was used with eGFR at study end and eGFR change from baseline as the outcome measures, and CAN status, baseline eGFR and other confounders as the predictors. To assess progression to albuminuria (micro and macro), only patients with normal ACR at baseline were included and logistic regression was used.
Residuals and colinearity were considered in assessing fit of models to data. Sequentially removing variables involved in multicolinearity had limited impact on model estimates for the main exposure. Hence, the final models presented include variables based on the variables that differed between patients with and without CAN, regardless of the presence of colinearity. A p value of <0.05 was considered significant in all statistical testing.
Results
Baseline cross-sectional analysis
Two hundred and four patients were included in the analysis. Of the 204 patients with baseline eGFR measurements, 192 patients had baseline ACR measurements and an assessment of CKD status was possible in 193 patients.
The prevalence of CKD, DN, albuminuria and macroalbuminuria was 40.4% (78/193), 7.3% (14/192), 33.3% (64/192) and 9.9% (19/192), respectively. The prevalence of eGFR ≥ 90, 60–89, 30–59, 15–29 and <15 ml min−1 1.73 m−2 was 49.0% (100/204), 36.8% (75/204), 13.2% (27/204), 1.0% (2/204) and 0.0% (0), respectively. The prevalence of CAN was 42.2% (86/204). The proportion of patients that had abnormal E/I, Valsalva and 30:15 ratios was 64.2%, 39.7% and 18.1%, respectively. A postural drop in BP was evident in 10.3% of patients. There was no difference in the prevalence of CAN or CKD between ethnicities, but white Europeans had lower eGFR at baseline and a higher prevalence of DN (ESM).
Patients with CAN were older, had longer diabetes duration, lower diastolic BP and a greater proportion received insulin treatment (Table 1), otherwise the variables did not differ between patients with and without CAN.
CAN, CKD and DN: cross-sectional univariable analysis
Patients with CAN had lower eGFR and higher prevalence of CKD, albuminuria, macroalbuminuria, DN and eGFR < 60 ml min−1 1.73 m−2 compared with those without CAN (Table 2). A subgroup analysis by ethnicity and albuminuria status showed similar findings to the analysis in the total cohort (ESM). Patients with CKD also had lower E/I, Valsalva and 30:15 ratios (ESM).
Baseline eGFR correlated positively with the majority of HRV and spectral analysis variables (Table 3). These correlations suggest that better autonomic function is associated with higher eGFR.
CAN, CKD and DN: cross-sectional multivariable analysis
After adjustment for age, diabetes duration, diastolic BP and insulin use using logistic regression, CAN was not associated with CKD (OR 1.4, 95% CI 0.7, 2.7, p = 0.3). However, spectral analysis variables remained independently associated with CKD after adjustment using the same logistic regression model (Nagelkerke R 2 for the different models 0.19–0.21). CKD was associated with baseline LFa (OR 0.5, 95% CI 0.3, 0.9, p = 0.02), deep breathing RFa (OR 0.5, 95% CI 0.3, 0.8, p = 0.006), Valsalva LFa (OR 0.6, 95% CI 0.4, 0.9, p = 0.009), standing LFa (OR 0.4, 95% CI 0.3, 0.8, p = 0.004) and Valsalva SDNN (OR 0.4, 95% CI 0.1, 0.9, p = 0.03). The OR and 95% CI were <1, suggesting that lower values for spectral analysis variables (i.e. worse autonomic function) are associated with the presence of CKD.
CAN and albuminuria: cross-sectional multivariable analysis
Similarly to CKD, CAN was not associated with albuminuria (OR 1.4, 95% CI 0.7, 2.7, p = 0.3) after adjustment and the associations between spectral analysis and albuminuria were similar to those reported with CKD.
CAN and eGFR: cross-sectional multivariable analysis
After adjusting for variables that differed between patients with and without CAN (i.e. age, diabetes duration, diastolic BP and insulin use), using linear regression the association between CAN and baseline eGFR became non-significant (β = −4.4, p = 0.2, R for the model = 0.6). After similar adjustments using HRV and spectral analysis variables instead of CAN in the model, the following remained independently associated with eGFR (Nagelkerke R 2 for the different models 0.25–0.36): E/I ratio (β = 93.7, p = 0.04), baseline LFa (β = 7.6, p = 0.01), baseline RFa (β = 6.4, p = 0.04), deep breathing LFa (β = 7.6, p = 0.002), deep breathing RFa (β = 7.6, p = 0.001), standing LFa (β = 8.6, p = 0.001), standing RFa (β = 7.1, p = 0.01), baseline SDNN (β = 15.7, p = 0.02), deep breathing SDNN (β = 16.6, p = 0.003), deep breathing RMSSD (β = 14.5, p = 0.003) and deep breathing pNN50 (β = 6.7, p = 0.009). These results suggest that better autonomic function variables are associated with higher eGFR.
Study-end longitudinal analysis
Out of the 204 patients with eGFR measurements at baseline, 200 had study-end eGFR measurements (four patients were lost to follow-up) and out of the 192 patients with baseline urinary ACR measurements, 162 had study-end ACR results available (30 patients did not provide urine samples). It was possible to make a diagnosis of CKD in 167 patients with baseline and study-end data. The follow-up duration was similar in patients with and without CAN (2.4 ± 0.7 vs 2.6 ± 0.6 years, p = 0.06 for no CAN vs CAN, respectively). The decline in eGFR was similar in South Asians and white Europeans over the follow-up period (ESM). There was no difference in the use of antihypertensive medications between patients with and without CAN by study-end.
CAN and decline in eGFR
Study-end eGFR was lower and the decline in eGFR was greater in patients with CAN than in those without (Table 4, Fig. 1). These findings were true for both South Asians and white Europeans and those with and without albuminuria, but the impact of CAN was much greater in those with albuminuria at baseline (ESM). Similarly, although CAN had an impact on decline in eGFR in patients with baseline eGFR above and below 60 ml min−1 1.73 m−2, its impact was greater in those with baseline eGFR < 60 ml min−1 1.73 m−2 (data not shown)
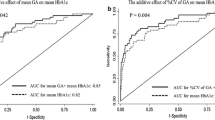
After adjustment for eGFR and other variables that differed between patients with and without CAN at baseline (including, diabetes duration, age, diastolic BP and insulin use), CAN was an independent predictor of study-end eGFR (β = −3.5, p = 0.03, R 2 = 0.85). Replacing study-end eGFR as the outcome measure with the change in eGFR in absolute terms, or as a proportion of baseline eGFR, demonstrated that CAN remained an independent predictor of decline in eGFR both in absolute terms (β = −3.5, p = 0.03, R 2 = 0.1) and as percentage of baseline eGFR (β = −0.05, p = 0.03, R 2 = 0.1). Using logistic regression, CAN was also an independent predictor of rapid decline in eGFR (OR 2.13, 95% CI 1.11, 4.07, p = 0.02, Nagelkerke R 2 0.07).
Replacing CAN in the model with HRV and spectral analysis variables demonstrated the following to be independent predictors of eGFR change: E/I ratio (β = 54.5, p = 0.015, R 2 = 0.11), deep breathing LFa (β = 2.8, p = 0.03, R 2 = 0.1), standing LFa (β = 2.9, p = 0.03, R 2 = 0.1), deep breathing SDNN (β = 7.25, p = 0.01, R 2 = 0.11), deep breathing RMSSD (β = 5.2, p = 0.04, R 2 = 0.1), deep breathing pNN50 (β = 2.78, p = 0.03, R 2 = 0.08), Valsalva SDNN (β = 5.8, p = 0.04, R 2 = 0.1). The direction of the relationship (the β values) suggests that the better the autonomic function, the smaller the decline in eGFR.
CAN and progression to CKD and albuminuria
Out of the 162 patients with baseline and study-end ACR measurements, 55 had evidence of albuminuria at baseline, which left 107 patients (40 and 67 with and without CAN, respectively) for the longitudinal analysis. Out of 167 patients with CKD status determined at baseline and at the end of the study, 72 had CKD at baseline, leaving 95 patients (34 with and 61 without CAN, respectively) in whom CKD progression was assessed.
Progression to CKD and albuminuria was more common in patients with CAN than in those without but the difference was not statistically significant (CKD: 26.5% [n = 9] vs 13.1% [n = 8], p = 0.1; albuminuria: 25% [n = 10] vs 16.4% [n = 11], p = 0.3).
Discussion
We have demonstrated that CAN is associated with CKD, DN and albuminuria in patients with type 2 diabetes. More importantly, CAN was associated with a greater decline in eGFR over the follow-up period, independent of potential confounders. To the best of our knowledge this is the first report to assess the impact of CAN on CKD prospectively in patients with type 2 diabetes.
The prevalence of CAN and CKD in our cohort is similar to the prevalence reported in the literature [5, 6, 8, 34]. In addition, the characteristics of our study population are similar to those reported in other studies conducted in the UK in which South Asians and white Europeans were included [38], suggesting that the current study sample was representative of the wider type 2 diabetes population in secondary care. However, whether our findings are applicable to patients typically managed in primary care and to those with a shorter duration of diabetes remains to be examined.
The baseline cross-sectional association between CAN, CKD and DN is not surprising as these complications are thought to share common mechanisms of pathogenesis, which are primarily driven by hyperglycaemia, hypertension and obesity [39, 40]. This is further supported by the higher prevalence of sight-threatening retinopathy and DN in patients with CAN in our study.
Although patients with and without CAN in our study were matched for some major CKD-determining risk factors, including systolic BP, HbA1c and medication use (including RAAS inhibitors), there were differences between the groups—patients with CAN were older and had longer diabetes duration. Nonetheless, CAN remained an independent predictor of study-end eGFR and decline in eGFR over the follow-up period despite adjustment for baseline differences. Unlike spectral analysis variables, CAN was not independently associated with eGFR, CKD, DN or albuminuria after adjustment in the cross-sectional analysis at baseline. There could be several explanations for this. The diagnosis of CAN in our study was based on the widely accepted criteria of the presence of two abnormal tests out of four. By the time the two tests are abnormal, CAN is usually clinically apparent. In contrast, spectral analysis has greater sensitivity and variables can be abnormal (or approach abnormality) before CAN is evident clinically (i.e. subclinical CAN). Hence, while CAN as a dichotomous variable was not independently associated with eGFR, CKD, DN or albuminuria cross-sectionally, some of the spectral analysis variables demonstrated an association, suggesting that the relationship between CAN and CKD starts when autonomic dysfunction is still subclinical. Another contributing factor is that the traditional tests for CAN largely depend on parasympathetic function while spectral analysis is also sensitive to additional variables including sympathetic (e.g. LFa) dysfunction.
The direction of the relationship between HRV and spectral analysis variables on one hand and eGFR, albuminuria and CKD on the other suggests that better autonomic function was associated with higher eGFR and less CKD, and albuminuria at baseline and with a lesser decline in eGFR over the follow-up period, independent of baseline differences between patients with and without CAN. HRV and spectral analysis largely reflects parasympathetic activity although sympathetic activity contributes to some variables such as LFa. Hence our findings indicate that in diabetes both parasympathetic and sympathetic deficits are associated with CKD and greater decline in eGFR.
It is biologically plausible that the relationship between CAN and eGFR could be bi-directional. As we mentioned above, CAN might contribute to the development and progression of CKD by its impact on BP (lack of nocturnal dipping, postural hypotension) and by causing haemodynamic changes, which can affect the intraglomerular pressure, the glomeruli and the microvasculature surrounding the renal tubules [10–16]. On the other hand CKD may contribute to the development of CAN [41] through the lack of clearance of leptin, which can stimulate the sympathetic nervous system [42].
Our data demonstrate that CAN at baseline predicts decline in eGFR over a period of 2.5 years. Thus, the detection of CAN may be of value for the identification of patients who require more intensive treatment early in the course of CKD with the aim of delaying or preventing the development of ESRD. Changes in lifestyle and improvements in metabolic control, including glycaemia, BP and lipid profiles, are well known to slow the progression and/or prevent the development of CAN, but such treatments are already well established in the management of CKD and DN.
Although our data demonstrate that CAN is an independent predictor of worsening eGFR, CAN was not a predictor of the development of CKD or albuminuria alone. This could be explained by the relatively short duration of follow-up, the small sample size of the subgroup that did not have CKD at baseline or the definition of CKD (which included an eGFR < 60 ml min−1 1.73 m−2 [36]). Furthermore, decline in eGFR can be the sole manifestation of CKD progression in patients with diabetes, without accompanying evidence of albuminuria in up to 30% of cases [36, 43].
Our study has several strengths and limitations. We used a single random measurement of urine albumin to assess albuminuria rather than three measurements; again this approach has been used previously by many other investigators [34, 35] and recent data suggest that single urinary albumin measurements are accurate (with a high sensitivity, specificity and positive and negative predicting values) in predicting nephropathy and are appropriate for epidemiological studies [35]. Furthermore, the performance of single vs multiple ACR measurement in our sample was satisfactory (see ESM). The cross-sectional analysis of the relationship between CAN and CKD makes it difficult to ascertain causality, but the longitudinal analysis of the impact of CAN on decline in eGFR suggests a cause–effect relationship. The missing follow-up data, particularly for albuminuria, is a weakness and a potential source for bias, although no differences in characteristics of participants vs patients lost to follow-up were observed. The use of a wide spectrum of assessments of CAN lends strength to our study as it allowed a more in-depth assessment of CAN. Another strength of our study was the well-characterised population; the measurement of a wide range of demographic and clinical variables allowed adjustment for a wide range of potential confounders. Furthermore, the study included patients of both sexes and of South Asian and white European ethnicity. In addition, we focused on patients relatively early in the course of CKD; this population would be a prime target for treatment that might slow the progression towards ESRD.
In conclusion, CAN is associated with CKD in patients with type 2 diabetes and predicts decline in eGFR early in the course of CKD independently of possible confounders. CAN might be used as potential risk factor to identify patients who are at increased risk of developing ESRD and therefore require more intensive preventative strategies. Further studies examining the mechanisms underpinning the complex relationship between CAN and DN are needed.
Abbreviations
- ACR:
-
Albumin/creatinine ratio
- CAN:
-
Cardiac autonomic neuropathy
- CKD:
-
Chronic kidney disease
- DN:
-
Diabetic nephropathy
- E/I:
-
Expiratory/inspiratory
- ESRD:
-
End-stage renal disease
- HRV:
-
Heart rate variability
- LFa:
-
Low-frequency area
- pNN50:
-
Percentage of adjacent R–R intervals that varied by more than 50 ms
- RAAS:
-
Renin–angiotensin–aldosterone system
- RFa:
-
Respiratory-frequency area
- RMSSD:
-
Square root of the mean squared differences of successive RR intervals
- RRT:
-
Renal replacement therapy
- SDNN:
-
Standard deviation of normal RR intervals
References
Leiter LA (2005) The prevention of diabetic microvascular complications of diabetes: is there a role for lipid lowering? Diabetes Res Clin Pract 68:S3–S14
Bakris GL (2011) Recognition, pathogenesis, and treatment of different stages of nephropathy in patients with type 2 diabetes mellitus. Mayo Clin Proc 86:444–456
Dronavalli S, Duka I, Bakris GL (2008) The pathogenesis of diabetic nephropathy. Nat Clin Pract End Metab 4:444–452
Afghahi H, Cederholm J, Eliasson B et al (2011) Risk factors for the development of albuminuria and renal impairment in type 2 diabetes—the Swedish National Diabetes Register (NDR). Nephrol Dial Transplant 26:1236–1243
Low PA, Benrud-Larson LM, Sletten DM et al (2004) Autonomic symptoms and diabetic neuropathy: a population-based study. Diabetes Care 27:2942–2947
Gæde P, Vedel P, Larsen N, Jensen GVH, Parving HH, Pedersen O (2003) Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med 348:383–393
Ziegler D, Mayer P, Mühlen H, Gries FA (1991) The natural history of somatosensory and autonomic nerve dysfunction in relation to glycaemic control during the first 5 years after diagnosis of type 1 (insulin-dependent) diabetes mellitus. Diabetologia 34:822–829
Valensi P, Pariès J, Attali JR (2003) Cardiac autonomic neuropathy in diabetic patients: influence of diabetes duration, obesity, and microangiopathic complications the French multicenter study. Metabolism 52:815–820
Joles JA, Koomans HA (2004) Causes and consequences of increased sympathetic activity in renal disease. Hypertension 43:699–706
Kuehl M, Stevens MJ (2012) Cardiovascular autonomic neuropathies as complications of diabetes mellitus. Nat Rev Endocrinol 8:405–416
Maser RE, Lenhard MJ (2005) Cardiovascular autonomic neuropathy due to diabetes mellitus: clinical manifestations, consequences, and treatment. J Clin Endocrinol Metab 90:5896–5903
Spallone V, Gambardella S, Maiello MR, Barini A, Frontoni S, Menzinger G (1994) Relationship between autonomic neuropathy, 24-h blood pressure profile, and nephropathy in normotensive IDDM patients. Diabetes Care 17:578–584
Sundkvist G, Lilja B (1993) Autonomic neuropathy predicts deterioration in glomerular filtration rate in patients with IDDM. Diabetes Care 16:773–779
Kempler P, Amarenco G, Freeman R et al (2011) Management strategies for gastrointestinal, erectile, bladder, and sudomotor dysfunction in patients with diabetes. Diabetes Metab Res Rev 27:665–677
Salman IM, Ameer OZ, Sattar MA et al (2011) Renal sympathetic nervous system hyperactivity in early streptozotocin-induced diabetic kidney disease. Neurourol Urodyn 30:438–446
Luippold G, Beilharz M, Mühlbauer B (2004) Chronic renal denervation prevents glomerular hyperfiltration in diabetic rats. Nephrol Dial Transplant 19:342–347
Pop-Busui R, Kirkwood I, Schmid H et al (2004) Sympathetic dysfunction in type 1 diabetes: association with impaired myocardial blood flow reserve and diastolic dysfunction. J Am Coll Cardiol 44:2368–2374
Moran A, Palmas W, Field L et al (2004) Cardiovascular autonomic neuropathy is associated with microalbuminuria in older patients with type 2 diabetes. Diabetes Care 27:972–977
Sterner NG, Nilsson H, Rošen U, Lilja B, Sundkvist G (1997) Relationships among glomerular filtration rate, albuminuria, and autonomic nerve function in insulin-dependent and non-insulin-dependent diabetes mellitus. J Diabetes Complicat 11:188–193
Smulders YM, Jager A, Gerritsen J et al (2000) Cardiovascular autonomic function is associated with (micro-)albuminuria in elderly Caucasian subjects with impaired glucose tolerance or type 2 diabetes: the Hoorn Study. Diabetes Care 23:1369–1374
Duvnjak L, Vuckoviç S, Car N, Metelko Ž (2001) Relationship between autonomic function, 24-h blood pressure, and albuminuria in normotensive, normoalbuminuric patients with type 1 diabetes. J Diabetes Complicat 15:314–319
Lafferty AR, Werther GA, Clarke CF (2000) Ambulatory blood pressure, microalbuminuria, and autonomic neuropathy in adolescents with type 1 diabetes. Diabetes Care 23:533–538
Poulsen PL, Ebbehøj E, Hansen KW, Mogensen CE (1997) 24-h blood pressure and autonomic function is related to albumin excretion within the normoalbuminuric range in IDDM patients. Diabetologia 40:718–725
Forsén A, Kangro M, Sterner G et al (2004) A 14-year prospective study of autonomic nerve function in type-1 diabetic patients: association with nephropathy. Diabet Med 21:852–858
Piya MK, Shivu GN, Tahrani A et al (2011) Abnormal left ventricular torsion and cardiac autonomic dysfunction in subjects with type 1 diabetes mellitus. Metabolism 60:1115–1121
Colombo JP, Shoemaker WCM, Belzberg HM, Hatzakis GM, Fathizadeh PM, Demetriades DM (2008) Noninvasive monitoring of the autonomic nervous system and hemodynamics of patients with blunt and penetrating trauma. J Trauma Inj Infect Crit Care 65:1364–1373
Vinik AI, Ziegler D (2007) Diabetic cardiovascular autonomic neuropathy. Circulation 115:387–397
Spallone V, Ziegler D, Freeman R et al (2011) Cardiovascular autonomic neuropathy in diabetes: clinical impact, assessment, diagnosis, and management. Diabetes Metab Res Rev 27:639–653
Bernardi L, Spallone V, Stevens M et al (2011) Methods of investigation for cardiac autonomic dysfunction in human research studies. Diabetes Metab Res Rev 27:654–664
Ziegler D, Laux G, Dannehl K et al (1992) Assessment of cardiovascular autonomic function: age-related normal ranges and reproducibility of spectral analysis, vector analysis, and standard tests of heart rate variation and blood pressure responses. Diabet Med 9:166–175
Levey AS, Coresh J, Greene T et al (2006) Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145:247–254
Tahrani AA, Ali A, Raymond NT et al (2013) Obstructive sleep apnea and diabetic nephropathy: a cohort study. Diabetes Care 36:3718–3725
Chronic Kidney Disease Prognosis Consortium (2010) Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375:2073–2081
Parving HH, Lewis JB, Ravid M, Remuzzi G, Hunsicker LG (2006) Prevalence and risk factors for microalbuminuria in a referred cohort of type II diabetic patients: a global perspective. Kidney Int 69:2057–2063
Pugliese G, Solini A, Fondelli C et al (2011) Reproducibility of albuminuria in type 2 diabetic subjects. Findings from the Renal Insufficiency and Cardiovascular Events (RIACE) study. Nephrol Dial Transplant 26:3950–3954
Molitch ME, Steffes M, Sun W et al (2010) Development and progression of renal insufficiency with and without albuminuria in adults with type 1 diabetes in the diabetes control and complications trial and the epidemiology of diabetes interventions and complications study. Diabetes Care 33:1536–1543
Zoppini G, Targher G, Chonchol M et al (2012) Predictors of estimated GFR decline in patients with type 2 diabetes and preserved kidney function. Clin J Am Soc Nephrol 7:401–408
Abbott CA, Chaturvedi N, Malik RA et al (2010) Explanations for the lower rates of diabetic neuropathy in Indian Asians versus Europeans. Diabetes Care 33:1325–1330
Tahrani AA, Askwith T, Stevens MJ (2010) Emerging drugs for diabetic neuropathy. Expert Opin Emerg Drugs 15:661–683
Brownlee M (2005) The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625
Krishnan AV, Kiernan MC (2007) Uremic neuropathy: clinical features and new pathophysiological insights. Muscle Nerve 35:273–290
Nasrallah MP, Ziyadeh FN (2013) Overview of the physiology and pathophysiology of leptin with special emphasis on its role in the kidney. Semin Nephrol 33:54–65 (Abstract)
Kramer HJNQ (2003) Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA 289:3273–3277
Tahrani AA, Ali A, Raymond NT et al (2012) Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. Am J Respir Crit Care Med 186:434–441
Acknowledgements
A. Tahrani is a Clinician Scientist fellow supported by the National Institute for Health Research (UK). The views expressed in this publication are those of the author(s) and not necessarily those of the National Health Service, the National Institute for Health Research or the Department of Health.
We acknowledge F. Hanna, H. Hodgson and R. Barakam (University Hospital of North Staffordshire, Stoke-on-Trent, UK) for their help in recruitment.
Funding
This project was funded by the National Institute for Health Research (UK), the UK Novo Nordisk Research Foundation and Sanofi Aventis.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
AAT was responsible for the conception and design of the study and obtaining funding, acquired, analysed and interpreted data and wrote the first draft of the manuscript. NTR was responsible for statistical analysis and interpretation and reviewed the manuscript. SB acquired data, designed the study and reviewed the manuscript. KD and QAA designed the study and reviewed the manuscript. HS acquired data and reviewed the manuscript. MKP acquired data and reviewed the manuscript. MJS was responsible for the study conception and design, analysed and interpreted data and reviewed the manuscript. All authors gave final approval for the publication of this manuscript.
AAT is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM
(PDF 853 kb)
Rights and permissions
About this article
Cite this article
Tahrani, A.A., Dubb, K., Raymond, N.T. et al. Cardiac autonomic neuropathy predicts renal function decline in patients with type 2 diabetes: a cohort study. Diabetologia 57, 1249–1256 (2014). https://doi.org/10.1007/s00125-014-3211-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-014-3211-2