Abstract
The aim of this study is to investigate the association between serum albumin concentrations and nerve conduction (NC) parameters in Chinese patients with type 2 diabetes (T2DM). A total of 409 T2DM patients were enrolled between October 2010 and April 2014. All participants underwent nerve conduction studies. The composite Z scores for NC parameters including conduction velocity (CV), amplitude, and latency were calculated as well. Serum albumin was measured by Bromcresol Green dye-binding method. The composite Z scores of CV and amplitude increased with the increasing albumin tertiles (test for trend, both P < 0.001), while the composite Z score of latency decreased with increasing albumin tertiles (test for trend, P < 0.001). After adjusting for age, sex, duration, and HbA1c, higher serum albumin concentrations were associated with higher composite Z scores of CV (β = 0.314, P < 0.001), amplitude (β = 0.279, P < 0.001), and lower composite Z score of latency (β = −0.279, P < 0.001). When participants were stratified into albuminuria and normoalbuminuria group, we found the associations of serum albumin with composite Z scores of NC parameters remained significant only in the albuminuria group (CV Z score: β = 0.253, P = 0.002; amplitude Z score: β = 0.233, P = 0.006; latency Z score: β = −0.217 P = 0.013) after further adjustment for urinary albumin to creatinine ratio. The optimal cutoff point of serum albumin to indicate abnormal peripheral nerve function was 36.75 g/L in T2DM patients with albuminuria, with a sensitivity of 65.6 % and a specificity of 78.0 %. Serum albumin was independently associated with peripheral nerve function in T2DM patients, especially in those with albuminuria. Serum albumin could be a potential biomarker for diabetic peripheral neuropathy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prevalence of diabetes mellitus has reached epidemic proportions worldwide [1]. Diabetic peripheral neuropathy (DPN)—the most common chronic complication of diabetes, occurs in up to 50 % of all patients with diabetes [2] and is widely considered as a main risk factor for foot ulceration and lower limb amputation [3, 4]. Early detection of DPN is of great importance because it can increase opportunities for both physicians and patients to benefit from better glycemic control and to initiate strategies to prevent its complications such us foot ulcers [5, 6]. Yet detection and diagnosis of DPN can be complex. Consensus definitions for distal symmetric polyneuropathy consistently recommend a combination of neuropathic symptoms and signs in addition to specific abnormalities in nerve conduction studies (NCSs) as criteria for diagnosis [7, 8]. NCSs are considered to be the most sensitive, accurate, and reliable method for detecting DPN [9–11]. Despite the diagnostic benefits of NCSs in evaluation of DPN, they are not widely utilized due to availability and complexity. Simple and effective biomarkers are needed to detect the population with diabetes who are at high risk of DPN.
Albumin, the most abundant circulating protein in plasma, possesses many important physiological and pharmacological functions [12]. Among the various functions, the anti-oxidative and anti-inflammatory properties are of paramount importance [13–15]. As oxidative stress and inflammation are considered to be important contributors to the development of diabetic microvascular complication including neuropathy [16–26], serum albumin concentrations may be associated with peripheral nerve function. Therefore, by performing NCSs, the current study sought to investigate the cross-sectional association between serum albumin concentrations and nerve conduction (NC) parameters in patients with type 2 diabetes (T2DM).
Materials and methods
Study subjects
The study enrolled a total of 424 T2DM inpatients from Shanghai Jiao Tong University Affiliated Sixth People’s Hospital Jinshan Branch between October 2010 and April 2014. All participants underwent NCSs. Diabetes mellitus was diagnosed in accordance with the 1999 World Health Organization criteria (fasting plasma glucose ≥7.0 mmol/L, 2 h plasma glucose ≥11.1 mmol/L, or both). Subjects were excluded if they had other severe illness, such as acute infectious disease, progressive malignancy, or severe renal impairment. In addition, 15 patients were excluded because of lack of data on serum albumin. Thus, final analyses were performed on 409 subjects (214 men and 195 women), who were divided into tertiles according to albumin levels.
This study was approved by the institutional review board of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and in accordance with the principle of the Helsinki Declaration II. Written informed consent was obtained from each participant.
Clinical and laboratory measurements
Body height and body weight were measured with light clothes and bare feet, and body mass index (BMI) was calculated by dividing weight in kilograms by the square of height in meters. Patients’ information was obtained by interviews during medical examinations.
Blood samples were obtained after an overnight fast for at least 8 h. Serum albumin was measured by Bromcresol Green (BCG) dye-binding method, using a Hitachi 7600 analyzer. Lipid profiles [including total cholesterol, triglycerides, high-density lipoprotein (HDL), and low-density lipoprotein (LDL)], were measured on a Hitachi 7600 analyzer using an enzymatic assay. Glycated hemoglobin (HbA1c) was estimated by high-performance liquid chromatography using an analyzer (HLC-723 G7, Tosoh, Japan).
A first morning spot urine sample was collected at the survey. The concentrations of urinary albumin and urinary creatinine were measured by Hitachi 7600 biochemistry autoanalyzer (albumin: immune turbidimetric assay; creatinine: enzymatic assay). Urinary albumin to creatinine ratio (ACR) was calculated by dividing the urinary albumin concentration in micrograms by the urinary creatinine concentration in milligrams. Individuals were classified into two groups as “Normoalbuminuria” or “Albuminuria” based on whether they were above or below the ACR cutoff point of 30 μg/mg. Normoalbuminuria was defined as ACR < 30 μg/mg, and albuminuria was defined as ACR ≥ 30 μg/mg [27].
Fundus photography was performed following a standardized protocol at the Department of Ophthalmology, Shanghai Jiao Tong University Affiliated Sixth People’s Hospital Jinshan branch. Retinopathy was defined according to the International Classification of Diabetic Retinopathy [28].
Cardiovascular disease (CVD) was considered to be present if the patients had ever experienced one or more cardiovascular events including ischemic heart disease, heart failure, cerebrovascular accident, or peripheral arterial disease [29].
Electrophysiological assessments were performed prior to knowledge of the laboratory results using EMG Myto, EBNeuro (Esaote, Florence, Italy). Examinations were performed by trained electrophysiologists with extensive experience in recording and interpreting clinical data. Sensory and motor nerves of the nondominant side were tested. Motor nerve studies were performed using stimulation of the median, ulnar, and tibial nerves. For each motor nerve, distal latency, compound muscle action potential (CMAP) amplitude, and conduction velocity (CV) were obtained or calculated. Sensory nerve studies were performed using the median, ulnar, and sural nerves. The following parameters were measured: onset latency, sensory nerve action potential (SNAP) amplitude, and CV. Temperatures were maintained at ≥35 °C for the upper and ≥32 °C for the lower extremities during testing. All data obtained were compared with reference values from our laboratory. Abnormal peripheral nerve function is defined as abnormality of one or more parameters in two or more anatomical nerves tested [30]. In addition, we constructed composite Z scores for CV, amplitude and latency, respectively. For each individual, every CV value was transformed into a Z score using the formula: Z score = (individual value of patient—mean value of control group)/SD of control group. A composite CV Z score was then calculated as [(Z score motor median CV) + (Z score sensory median CV) + (Z score motor ulnar CV) + (Z score sensory ulnar CV) + (Z score tibial CV) + (Z score sural CV)]/6. The composite Z scores for amplitude and latency were calculated similarly.
Statistical analysis
For continuous variables, results were presented as mean ± standard deviation or median (25th–75th percentiles), and differences among groups were evaluated by one-way ANOVA (normally distributed data) or the Kruskal–Wallis test (Skewly distributed data). Categorical variables were presented as n (%), and intergroup comparisons were analyzed using a χ 2 test. Skewly distributed quantitative traits (such as albumin and ACR) were logarithmically transformed to approximate normality. The differences of composite Z scores of CV, amplitude, and latency across the tertiles of albumin were analyzed with Bonferroni method and test for trend. Multiple linear regression analysis was performed with the composite Z scores of CV, amplitude, and latency designated as the dependent variables, and serum albumin concentration, age, sex, duration of diabetes, HbA1c, and ACR designated as independent variables. Furthermore, receiver-operating characteristic (ROC) analysis was conducted to identify the optimal cutoff point of albumin for indicating abnormal peripheral nerve function. All statistical analyses were performed with the SPSS version 19. All tests were two-sided and performed at a 5 % significance level.
Results
The characteristics of the study subjects by tertiles of serum albumin are given in Table 1. The serum albumin concentration cutoff value between the low and the medium albumin groups was 34.7 g/L. The cutoff value between the medium and the high albumin groups was 37.6 g/L. The age of the enrolled subjects ranged from 21 to 88 years, with a mean age of 62.07 ± 11.82 years. The proportions of men and women were comparable among the three groups (53.3 % men vs. 51.8 % men vs. 51.9 % men, P = 0.963). Age, BMI, duration of diabetes, HbA1c, LDL-C, triglyceride, total cholesterol, ACR, and almost all NC parameters tested were significantly different among the three tertiles (P < 0.05).
Figure 1 illustrates that the composite Z scores of CV and amplitude increased (the higher the better) with increasing albumin tertiles (test for trend, both P < 0.001), while the composite Z score of latency decreased (the lower the better) with increasing albumin tertiles (test for trend, P < 0.001). Bonferroni corrections revealed significant differences in all composite Z scores of three parameters between the low versus the high albumin groups (all P < 0.01) and the medium versus high albumin groups (all P < 0.01).
To assess the independent correlation between serum albumin levels and peripheral nerve function, we carried out multiple linear regression analysis adjusted for potential confounders. As shown in Table 2, after adjusting for age, sex, duration, and HbA1c, higher serum albumin concentrations were associated with higher composite Z scores of CV (β = 0.314, P < 0.001), amplitude (β = 0.279, P < 0.001), and lower composite Z score of latency (β = −0.279, P < 0.001).
Since it was reported by previous studies that serum albumin was inversely associated with urinary albumin level [31–33], ACR was additionally included in the multivariate linear regression model for adjustment. The results revealed that the associations of serum albumin with the composite Z scores of CV (β = 0.197, P < 0.001), amplitude (β = 0.161, P = 0.004), and latency (β = −0.177, P = 0.003) remained significant (Table 2). All individuals were further classified into two groups as “Normoalbuminuria” or “Albuminuria” based on ACR levels. As depicted in Table 3, in the normoalbuminuria group, there was no significantly association between serum albumin and the composite Z scores of NC parameters (all P > 0.05) after adjusting for age, sex, duration, and HbA1c. However, in the albuminuria group, serum albumin was positively associated with the composite Z scores of CV (β = 0.253, P = 0.002), amplitude (β = 0.233, P = 0.006), and reversely associated with that of latency (β = −0.217, P = 0.013) even after adjustment for age, sex, duration, HbA1c, and ACR.
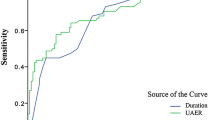
Finally, in T2DM patients with albuminuria, ROC analysis revealed that the optimal cutoff point of serum albumin to indicate abnormal peripheral nerve function was 36.75 g/L (AUC = 0.735; 95 % CI, 0.643–0.828; Youden index = 0.436; sensitivity, 65.6 %; specificity, 78.0 %) (Fig. 2).
Discussion
In this study, we analyzed the NCSs data of 409 T2DM participants to investigate the association between serum albumin and peripheral nerve function. Our study had two major findings. First, a lower serum albumin level was significantly related to worse nerve conduction outcomes even after controlling for age, sex, duration, HbA1c, and ACR. Second, those associations of serum albumin with NC parameters were only significant among T2DM patients with albuminuria.
Albumin, the most abundant protein in human plasma, has important antioxidant properties resulting from its transition metal ion-binding activity and its reactive oxygen intermediate scavenging activity [34–38]. For example, Lang et al. reported that treatment of bovine aortic endothelial cells with human albumin in vitro attenuated HOCl-induced oxidative damage in a dose-dependent manner [35]. On the other hand, there is increasing evidence supporting the anti-inflammatory property of albumin [15, 39, 40]. In a rat endotoxemia model [40], for instance, albumin decreased myocardial nitric oxide synthase II messenger RNA and protein expression, thereby ameliorating intramyocardial inflammatory response. Given that oxidative stress and inflammation play important roles in diabetic microvascular complications [16–26], it is possible that serum albumin levels may be associated with the development of these complications. Indeed, a cohort study of 343 T2DM patients demonstrated that hypoalbuminemia was independently associated with progression of nephropathy as evaluated by rate of decline of estimated glomerular filtration rate [41]. Additionally, in a Japanese study comprising 130 T2DM patients [42], Iwasaki et al. reported that serum albumin was significantly associated with the severity of neuropathy, retinopathy, and nephropathy. With respect to neuropathy, it is notable that serum albumin was independently related to median motor nerve CV after adjusting for three variables (age, sex, serum C-reactive protein). Consistent with their study, the association of serum albumin with median motor nerve CV was also observed in our study (data not shown). Besides, for the first time to our best knowledge, we constructed composite Z scores of CV, amplitude, and latency respectively for six nerves including both sensory (median, ulnar, and sural) and motor nerves (median, ulnar, and tibial nerves), which can provide more global representation of peripheral neuropathy function [43]. Results obtained in the current study demonstrated that serum albumin concentrations were independently associated with composite Z scores of all NC parameters, implying that serum albumin might affect peripheral nerve function in an extensive way.
It is noteworthy that the association of serum albumin with peripheral nerve function was significant only in T2DM patients with albuminuria but not in those with normoalbuminuria. Interestingly, there are ample evidences that compared to those without albuminuria, patients with albuminuria could have more severe oxidative stress and inflammation [44–46], which are all important contributors in the development of DPN [23, 24, 26]. For instance, a previous study by Aslan et al. showed that oxidative stress, as evaluated by total oxidant status (TOS) and oxidative stress index (OSI), increases in patients with diabetic nephropathy compared to diabetic patients without nephropathy, they also found that this increase in oxidative stress seems to be related to the severity of microalbuminuria levels [45]. Furthermore, another study observed that patients with microalbuminuria or proteinuria had greater concentrations of high-sensitivity C-reactive protein (hs-CRP) and tumor necrosis factor-α (TNF-α) (both parameters of inflammation) than normoalbuminuric patients with diabetes [46]. Therefore, it is reasonable to postulate that albumin, as an antioxidant and anti-inflammatory agent, might play a greater role in this subset of patients. In accord with this hypothesis, we found that serum albumin was not associated with the composite Z scores of NC parameters among healthy controls (data not shown).
Our observations implied that serum albumin could be a potential biomarker for DPN especially in T2DM patients who had albuminuria. We found that the optimal cutoff point of serum albumin to indicate abnormal peripheral nerve function was 36.75 g/L in T2DM patients with albuminuria. It is well established that NCSs are the most sensitive, accurate, and reliable method for detecting DPN [9–11]. Despite that NCSs have considerable diagnostic benefits in evaluation of DPN, the routine referral of patients with diabetes for NCSs is not practical and would impose a high cost on patients. While serum albumin is easily measurable, making it a potentially simple and efficient method in screening for specific individuals who are at high risks of DPN.
In conclusion, our data suggests that serum albumin is independently associated with peripheral nerve function in T2DM patients, especially in those with albuminuria. Serum albumin levels could be a potential biomarker for DPN. Further larger and prospective studies are needed to confirm our findings.
Abbreviations
- T2DM:
-
Type 2 diabetes
- DPN:
-
Diabetic peripheral neuropathy
- NCSs:
-
Nerve conduction studies
- NC:
-
Nerve conduction
- CV:
-
Conduction velocity
- ACR:
-
Urinary albumin to creatinine ratio
- HbA1c:
-
Glycated hemoglobin
References
S. Wild, G. Roglic, A. Green, R. Sicree, H. King, Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27(5), 1047–1053 (2004)
S. Tesfaye, A.J. Boulton, P.J. Dyck, R. Freeman, M. Horowitz, P. Kempler, G. Lauria, R.A. Malik, V. Spallone, A. Vinik, L. Bernardi, P. Valensi, Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 33(10), 2285–2293 (2010). doi:10.2337/dc10-1303
A.J. Boulton, L. Vileikyte, G. Ragnarson-Tennvall, J. Apelqvist, The global burden of diabetic foot disease. Lancet 366(9498), 1719–1724 (2005). doi:10.1016/s0140-6736(05)67698-2
R.A. Malik, Which test for diagnosing early human diabetic neuropathy? Diabetes 63(7), 2206–2208 (2014). doi:10.2337/db14-0492
Writing Team for the Diabetes, C., Complications Trial/Epidemiology of Diabetes, I., and Complications Research, G, Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 287(19), 2563–2569 (2002)
N. Singh, D.G. Armstrong, B.A. Lipsky, Preventing foot ulcers in patients with diabetes. JAMA 293(2), 217–228 (2005). doi:10.1001/jama.293.2.217
N. Papanas, D. Ziegler, New vistas in the diagnosis of diabetic polyneuropathy. Endocrine 47(3), 690–698 (2014). doi:10.1007/s12020-014-0285-z
P.J. Dyck, J.W. Albers, H. Andersen, J.C. Arezzo, G.J. Biessels, V. Bril, E.L. Feldman, W.J. Litchy, P.C. O’Brien, J.W. Russell, Diabetic polyneuropathies: update on research definition, diagnostic criteria and estimation of severity. Diabetes Metab. Res. Rev. 27(7), 620–628 (2011). doi:10.1002/dmrr.1226
B.A. Perkins, V. Bril, Diabetic neuropathy: a review emphasizing diagnostic methods. Clin. Neurophysiol. 114(7), 1167–1175 (2003)
J.D. England, G.S. Gronseth, G. Franklin, R.G. Miller, A.K. Asbury, G.T. Carter, J.A. Cohen, M.A. Fisher, J.F. Howard, L.J. Kinsella, N. Latov, R.A. Lewis, P.A. Low, A.J. Sumner, Distal symmetrical polyneuropathy: definition for clinical research. Muscle Nerve 31(1), 113–123 (2005). doi:10.1002/mus.20233
A. Weisman, V. Bril, M. Ngo, L.E. Lovblom, E.M. Halpern, A. Orszag, B.A. Perkins, Identification and prediction of diabetic sensorimotor polyneuropathy using individual and simple combinations of nerve conduction study parameters. PLoS ONE 8(3), e58783 (2013). doi:10.1371/journal.pone.0058783
P. Caraceni, M. Domenicali, A. Tovoli, L. Napoli, C.S. Ricci, M. Tufoni, M. Bernardi, Clinical indications for the albumin use: still a controversial issue. Eur. J. Intern. Med. 24(8), 721–728 (2013). doi:10.1016/j.ejim.2013.05.015
W.J. Zhang, B. Frei, Albumin selectively inhibits TNF alpha-induced expression of vascular cell adhesion molecule-1 in human aortic endothelial cells. Cardiovasc. Res. 55(4), 820–829 (2002)
K. Oettl, R.E. Stauber, Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br. J. Pharmacol. 151(5), 580–590 (2007). doi:10.1038/sj.bjp.0707251
N. Ishizaka, Y. Ishizaka, R. Nagai, E. Toda, H. Hashimoto, M. Yamakado, Association between serum albumin, carotid atherosclerosis, and metabolic syndrome in Japanese individuals. Atherosclerosis 193(2), 373–379 (2007). doi:10.1016/j.atherosclerosis.2006.06.031
F. Barutta, G. Bruno, S. Grimaldi, G. Gruden, Inflammation in diabetic nephropathy: moving toward clinical biomarkers and targets for treatment. Endocrine (2014). doi:10.1007/s12020-014-0437-1
A.M. Vincent, J.W. Russell, P. Low, E.L. Feldman, Oxidative stress in the pathogenesis of diabetic neuropathy. Endocr. Rev. 25(4), 612–628 (2004). doi:10.1210/er.2003-0019
H.Y. Jin, K.A. Lee, J.Z. Wu, H.S. Baek, T.S. Park, The neuroprotective benefit from pioglitazone (PIO) addition on the alpha lipoic acid (ALA)-based treatment in experimental diabetic rats. Endocrine 47(3), 772–782 (2014). doi:10.1007/s12020-014-0198-x
A.M. Joussen, V. Poulaki, M.L. Le, K. Koizumi, C. Esser, H. Janicki, U. Schraermeyer, N. Kociok, S. Fauser, B. Kirchhof, T.S. Kern, A.P. Adamis, A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J. 18(12), 1450–1452 (2004). doi:10.1096/fj.03-1476fje
Dalla Vestra, M. Mussap, M. Gallina, P. Bruseghin, A.M. Cernigoi, A. Saller, M. Plebani, P. Fioretto, Acute-phase markers of inflammation and glomerular structure in patients with type 2 diabetes. J. Am. Soc. Nephrol. 16(Suppl 1), S78–S82 (2005)
J.F. Navarro, C. Mora, Role of inflammation in diabetic complications. Nephrol. Dial. Transplant. 20(12), 2601–2604 (2005). doi:10.1093/ndt/gfi155
R. Pazdro, J.R. Burgess, The role of vitamin E and oxidative stress in diabetes complications. Mech. Ageing Dev. 131(4), 276–286 (2010). doi:10.1016/j.mad.2010.03.005
R. Sayin, M. Aslan, M.E. Kucukoglu, A. Luleci, M. Atmaca, R. Esen, H. Demir, Serum prolidase enzyme activity and oxidative stress levels in patients with diabetic neuropathy. Endocrine 47(1), 146–151 (2014). doi:10.1007/s12020-013-0136-3
R. Sandireddy, V.G. Yerra, A. Areti, P. Komirishetty, A. Kumar, Neuroinflammation and oxidative stress in diabetic neuropathy: futuristic strategies based on these targets. Int. J. Endocrinol. 2014, 674987 (2014). doi:10.1155/2014/674987
R. Stavniichuk, H. Shevalye, S. Lupachyk, A. Obrosov, J.T. Groves, I.G. Obrosova, M.A. Yorek, Peroxynitrite and protein nitration in the pathogenesis of diabetic peripheral neuropathy. Diabetes Metab. Res. Rev. 30(8), 669–678 (2014). doi:10.1002/dmrr.2549
R.D. Hoeldtke, K.D. Bryner, G.R. Hobbs, G.G. Horvath, J.E. Riggs, I. Christie, G. Ganser, S.M. Marcovina, A. Lernmark, Antibodies to glutamic acid decarboxylase and peripheral nerve function in type 1 diabetes. J. Clin. Endocrinol. Metab. 85(9), 3297–3308 (2000). doi:10.1210/jcem.85.9.6830
M.E. Molitch, R.A. DeFronzo, M.J. Franz, W.F. Keane, C.E. Mogensen, H.H. Parving, M.W. Steffes, Nephropathy in diabetes. Diabetes care 27(Suppl 1), S79–S83 (2004)
C.P. Wilkinson, F.L. Ferris 3rd, R.E. Klein, P.P. Lee, C.D. Agardh, M. Davis, D. Dills, A. Kampik, R. Pararajasegaram, J.T. Verdaguer, Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 110(9), 1677–1682 (2003). doi:10.1016/s0161-6420(03)00475-5
L. Rodriguez-Rodriguez, C. Gonzalez-Juanatey, R. Palomino-Morales, T.R. Vazquez-Rodriguez, J.A. Miranda-Filloy, B. Fernandez-Gutierrez, J. Llorca, J. Martin, M.A. Gonzalez-Gay, TNFA -308 (rs1800629) polymorphism is associated with a higher risk of cardiovascular disease in patients with rheumatoid arthritis. Atherosclerosis 216(1), 125–130 (2011). doi:10.1016/j.atherosclerosis.2010.10.052
P.J. Dyck, R.E. Carter, W.J. Litchy, Modeling nerve conduction criteria for diagnosis of diabetic polyneuropathy. Muscle Nerve 44(3), 340–345 (2011). doi:10.1002/mus.22074
N.H. Kim, K.B. Kim, D.L. Kim, S.G. Kim, K.M. Choi, S.H. Baik, D.S. Choi, Y.S. Kang, S.Y. Han, K.H. Han, Y.H. Ji, D.R. Cha, Plasma and urinary vascular endothelial growth factor and diabetic nephropathy in type 2 diabetes mellitus. Diabetic Med. 21(6), 545–551 (2004). doi:10.1111/j.1464-5491.2004.01200.x
Z. Wang, W.E. Hoy, Z. Wang, The correlates of urinary albumin to creatinine ratio (ACR) in a high risk Australian aboriginal community. BMC Nephrol. 14, 176 (2013). doi:10.1186/1471-2369-14-176
H. Taskapan, M.C. Taskapan, I. Orman, O. Ulutas, A. Yigit, F. Ozyalin, S. Yologlu, NGAL and NT-proBNP levels in diabetic patients with macroproteinuria. Ren. Fail. 35(9), 1273–1277 (2013). doi:10.3109/0886022x.2013.824336
L. Djousse, K.J. Rothman, L.A. Cupples, D. Levy, R.C. Ellison, Serum albumin and risk of myocardial infarction and all-cause mortality in the Framingham Offspring Study. Circulation 106(23), 2919–2924 (2002)
J.D. Lang Jr, M. Figueroa, P. Chumley, M. Aslan, J. Hurt, M.M. Tarpey, B. Alvarez, R. Radi, B.A. Freeman, Albumin and hydroxyethyl starch modulate oxidative inflammatory injury to vascular endothelium. Anesthesiology 100(1), 51–58 (2004)
G.J. Quinlan, S. Mumby, G.S. Martin, G.R. Bernard, J.M. Gutteridge, T.W. Evans, Albumin influences total plasma antioxidant capacity favorably in patients with acute lung injury. Crit. Care Med. 32(3), 755–759 (2004)
M. Roche, P. Rondeau, N.R. Singh, E. Tarnus, E. Bourdon, The antioxidant properties of serum albumin. FEBS Lett. 582(13), 1783–1787 (2008). doi:10.1016/j.febslet.2008.04.057
M. Taverna, A.L. Marie, J.P. Mira, B. Guidet, Specific antioxidant properties of human serum albumin. Ann. Intensive Care 3(1), 4 (2013). doi:10.1186/2110-5820-3-4
S.S. Awad, S. Sawada, O.S. Soldes, P.B. Rich, R. Klein, W.H. Alarcon, S.C. Wang, R.H. Bartlett, Can the clearance of tumor necrosis factor alpha and interleukin 6 be enhanced using an albumin dialysate hemodiafiltration system? ASAIO J. 45(1), 47–49 (1999)
K.R. Walley, T.E. McDonald, Y. Wang, S. Dai, J.A. Russell, Albumin resuscitation increases cardiomyocyte contractility and decreases nitric oxide synthase II expression in rat endotoxemia. Crit. Care Med. 31(1), 187–194 (2003). doi:10.1097/01.ccm.0000037157.55600.7f
D.J. Leehey, H.J. Kramer, T.M. Daoud, M.P. Chatha, M.A. Isreb, Progression of kidney disease in type 2 diabetes—beyond blood pressure control: an observational study. BMC Nephrol. 6, 8 (2005). doi:10.1186/1471-2369-6-8
T. Iwasaki, Y. Togashi, Y. Terauchi, Significant association of serum albumin with severity of retinopathy and neuropathy, in addition to that of nephropathy, in Japanese type 2 diabetic patients. Endocr. J. 55(2), 311–316 (2008)
P.J. Dyck, W.J. Litchy, J.R. Daube, C.M. Harper, P.J. Dyck, J. Davies, P.C. O’Brien, Individual attributes versus composite scores of nerve conduction abnormality: sensitivity, reproducibility, and concordance with impairment. Muscle Nerve 27(2), 202–210 (2003). doi:10.1002/mus.10320
A. Festa, R. D’Agostino, G. Howard, L. Mykkanen, R.P. Tracy, S.M. Haffner, Inflammation and microalbuminuria in nondiabetic and type 2 diabetic subjects: the Insulin Resistance Atherosclerosis Study. Kidney Int. 58(4), 1703–1710 (2000). doi:10.1046/j.1523-1755.2000.00331.x
M. Aslan, T. Sabuncu, A. Kocyigit, H. Celik, S. Selek, Relationship between total oxidant status and severity of diabetic nephropathy in type 2 diabetic patients. Nutr. Metab. Cardiovasc. Dis. 17(10), 734–740 (2007). doi:10.1016/j.numecd.2006.08.005
J.F. Navarro, C. Mora, M. Maca, J. Garca, Inflammatory parameters are independently associated with urinary albumin in type 2 diabetes mellitus. Am. J. Kidney Dis. 42(1), 53–61 (2003)
Acknowledgments
We would like to thank all of the involved clinicians, nurses, and technicians for dedicating their time and skill to the completion of this study. This work was supported by the Science and Technology Innovation Fund of Shanghai Jinshan district, China (2011-3-16) and the Programs of the National Natural Science Foundation of China (81170760; 81300666).
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Lu Li and Bo Liu have contributed equally to this study.
Rights and permissions
About this article
Cite this article
Li, L., Liu, B., Lu, J. et al. Serum albumin is associated with peripheral nerve function in patients with type 2 diabetes. Endocrine 50, 397–404 (2015). https://doi.org/10.1007/s12020-015-0588-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-015-0588-8