Abstract
Thermotolerant yeast strains were isolated from nuruk, a traditional Korean fermentation starter in which variety of microorganisms are present. Among the isolates, the MBY1358 identified as yeast Pichia kudriavzevii showed significantly higher growth rate (0.59 ± 0.00 1/h) at 44 °C than other strains. Maximum ethanol concentration of 8.35 ± 0.03 g/L was obtained from 20 g/L glucose with yield of 0.44 ± 0.01 g/g at 44 °C, which is 1.14 times ethanol production of the control strain of P. kudriavzevii. The MBY1358, which was significantly more thermotolerant than the control strain and fermented 200 g/L glucose to 107.33 ± 5.03 g/L ethanol at 44 °C, was deposited to Korean Collection for Type Cultures (KCTC) under the accession number 27654.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, there has seen the introduction of large-scale processing for the bioconversion of renewable biomass including feed stock, agricultural crops and animal wastes [1, 2]. The most important economic obstacles for the production of ethanol from biomass are the cost of the enzyme and loss of productivity due to inactivation of yeasts by various inhibitory conditions such as heat, acid, and fermentation inhibitors [3, 4]. In most cases, large-scale ethanol fermentation is performed at high concentrations of sugar and ethanol, and high temperatures, and these conditions cause stress to the yeast cells [5, 6]. Thus, tolerance to high temperature is one of the most desired properties of microorganisms of interest to fermentation industry. In particular, this property is advantageous for the reduction of cooling costs and contamination by other strains [7]. Simultaneous saccharification and fermentation (SSF) of biomass requires the use of microorganisms capable of working at high temperature [8]. The optimum temperature ranges is 40–60 °C for saccharification and 25–35 °C for fermentation [8].
Nuruk is a traditional Korean fermentation starter that contains a variety of microorganisms [9,10,11] including yeasts and other fungi, and bacteria [12]. Yeasts isolated from nuruk have been used to produce an alcoholic beverage and traditional fermented foods [13]. Non-conventional yeast species such as Pichia stipitis [14], Kluyveromyces lactis [15], Candida thermophila [16] and Kluyveromyces marxianus [17] have advantageous characteristics such as tolerance to acid or heat, or the ability to metabolize carbohydrates that Saccharomyces cerevisiae does not naturally metabolize [18]. Isono et al. [19] reported the ability of the yeast Pichia kudriavzevii to grow and produce ethanol at temperatures up to 43 °C.
In this study, thermotolerant P. kudriavzevii strains were isolated from nuruks and their growth and ethanol production properties were examined and compared with those of the control strain.
Materials and methods
Collection of nuruk
A variety of nuruks manufactured in different areas in Korea were purchased from local markets. A total of 12 nuruks were collected from Busan (1), Jinju (2), Gwangju (2), Jeju (1), Sangju (1), Yesan (2), Chuncheon (1), and Yongin (2).
Strain isolation and growth conditions
One gram of nuruk was suspended in peptone water [20] and plated onto YEPD agar (1% bacto-yeast extract, 2% bacto-peptone, 2% glucose, and 2% bacto-agar, w/v) supplemented with chloramphenicol (0.01%, w/v) [20]. The plates were incubated at 30 °C for 48 h. As a result, 200 strains were isolated from nuruk and each colony was picked up, purified by streaking on YEPD agar, suspended in YEPD broth, supplemented with 15% glycerol, and stored at −80 °C. To evaluate cell growth rate and ethanol productivity, a colony was transferred from a YEPD plate into 10 mL of YEPD broth, incubated overnight at 30 °C, diluted to an OD600 of 0.2 with the same sterile medium, and cultivated to an exponential growth phase. Cells were then harvested, washed, inoculated into 100 mL of fresh YEPD in 500-mL shake flasks, and incubated at 30, 37, and 44 °C for 48 h. An API 20C kit (BioMérieux, France) was used examine the utilization of various substrates as carbon source. Pichia kudriavzevii KCTC17763 isolated from cabbage was used as control strain.
Strain identification
Genomic DNA was extracted from yeast cells grown in YEPD broth. Cells were collected by brief centrifugation, and genomic DNA was purified using a GenEx™ genomic Sx kit (GeneAll, Korea) according to the manufacturer’s instructions. To identify yeast strains, primers ITS1, ITS4 [21] were used to amplify the internal transcribed spacer (ITS) [22]. Polymerase chain reaction (PCR) was performed at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 58 °C for 30 s, and extension at 72 °C for 1 min, with a final extension at 72 °C for 5 min [20]. PCR products were T-A cloned and sequenced; the sequences were analyzed using the BLAST program (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Analytical methods
Cell growth was monitored by measuring the optical density at 600 nm (OD600) using a spectrophotometer (Amersham Biosciences, USA). A high-performance liquid chromatograph LC-20 (Shimadzu, Japan) equipped with a Rezex ROA-Organic Acid H+ column (Phenomenex, USA) and a refractive index detector (Shimadzu) was used to determine the concentrations of ethanol and glucose. The column was maintained at 65 °C. H2SO4 (5 mM) solution at a flow rate of 0.6 mL/min was used as a mobile phase.
Statistical analysis
Data were expressed as mean ± standard error of the mean using SigmaPlot 12 software (SPSS Inc., USA). A Duncan’s multiple range test [23] was performed using PASW 18.0 (SPSS Inc.) to verify the group results.
Results and discussion
Isolation and identification of thermotolerant yeast strains
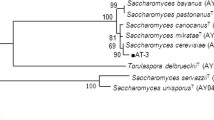
Several hundred colonies were isolated from nuruks purchased from local markets. To select thermotolerant yeast strains, single colonies were inoculated and cultivated in YEPD broth at 44 °C for 48 h. Five strains (MBY1325, 1330, 1337, 1357, and 1358) which showed rapid growth and high ethanol productivity were identified based on the ITS region of the rDNA gene. Interestingly, all strains were identified as P. kudriavzevii; their ITS regions shared over 99% similarity with that of the type strain (GenBank No. KM234478 or KF959839). MBY1325, 1330, 1337, 1357, and 1358 strains were isolated from the Yesan nuruk. MBY1358 showed approximately 99% homology with P. kudriavzevii (LC014798). Phylogenetic tree of ITS region of the rDNA gene with other various yeasts is shown in Fig. 1.
The phylogenetic tree of the P. kudriavzevii ITS region. The tree was constructed with the neighbor-joining method. The numbers on the nodes correspond to the percentages, with which a cluster appears in a bootstrap test based on 1000 pseudoreplicates. The bars denote the relative branch length. The ITS region was identified by their GenBank accession number in parentheses
Carbon assimilation patterns of the isolated P. kudriavzevii strains were examined. All strains except MBY1325 assimilated glucose and glycerol. None of the tested strains utilized d-xylose, adonitol, xylitol, galactose, inositol, sorbitol, α-methyl-d-glucoside, cellobiose, lactose, maltose, sucrose, trehalose, or melezitose as sole carbon source. In addition, contrast to the control strain, P. kudriavzevii KCTC17763, all P. kudriavzevii isolates were unable to utilize raffinose as carbon source.
Growth characteristics of P. kudriavzevii isolates
Thermotolerance of P. kudriavzevii MBY1325, 1330, 1337, 1357, and 1358 were compared with that of KCTC17763. All strains showed the maximum growth rate at 37 °C (Table 1) and cell growth was retarded with the increase in cultivation temperature. MBY1325, 1337 and KCTC17763 were more sensitive to temperature than other strains, whereas MBY1358 showed thermotolerance at 44 °C.
Cell growth, glucose consumption, and ethanol production rates of the P. kudriavzevii isolates were examined in shake-flask cultures grown for 48 h at various temperatures (Table 1; Fig. 2). KCTC17763 produced 9.80 ± 0.32 g ethanol from 20 g glucose at 30 °C, corresponding to an ethanol yield of 0.49 ± 0.00 g/g based on the amount of glucose consumed; this strain showed a specific growth rate of 0.41 ± 0.00 1/h. At 30 °C, MBY1357 (0.60 ± 0.01 1/h) and 1358 (0.60 ± 0.02 1/h) showed higher specific growth rates than KCTC17763, MBY1325 (0.41 ± 0.00 1/h), MBY1330 (0.38 ± 0.01 1/h), and MBY1337 (0.38 ± 0.01 1/h). In case the temperature was increased to 37 °C, cell growth of KCTC17763 was significantly decreased, as was the amount of ethanol formed from 20 g glucose (8.08 ± 0.29 g/L). At 37 °C, MBY1358 produced 8.86 ± 0.28 g ethanol from 20 g glucose with a productivity of 0.18 ± 0.01 g/L h. As the temperature was increased further, MBY1358 showed specific growth rate superior to those of the other strains and produced a maximum ethanol concentration of 8.35 ± 0.03 g/L; its ethanol production was 13.6% higher than that of KCTC17763. At 44 °C (Fig. 2), the specific growth rate of MBY1358 (0.59 ± 0.00 1/h) was 15.6% higher than that of KCTC17763 (0.51 ± 0.01 1/h).
Profiles of cell growth (filled triangle), glucose consumption (filled square), and ethanol production (filled circle) by P. kudriavzevii KCTC17763 and MBY1358 grown in YEPD at various temperatures (30, 37, 44 °C) for 48 h. Averages and standard errors determined from three independent cultivations are shown
Cell growth and ethanol production by MBY1358 and control strain were further examined in shake-flasks cultures using an elevated glucose concentration. The MBY1358 consumed 200 g/L glucose for 40 h to produce 107.33 ± 5.03 g/L ethanol (Fig. 3). However, KCTC17763 did not utilize glucose completely, i.e., 31.10 ± 0.34 g/L glucose was present after 48 h. Ethanol concentration of 54.00 ± 3.80 g/L was obtained by control strain KCTC17763. Dhaliwal et al. [24] reported P. kudriavzevii, isolated from sugarcane, produced 71.9 g/L ethanol from sugarcane juice at 40 °C. Pichia kudriavzevii PBB511-1 produced 42.4 g/L ethanol for 48 h at 45 °C from 180 g/L reducing sugar obtained from cassava [25]. These results indicate that MBY1358 might be a promising thermotolerant P. kudriavzevii strain capable of fermenting glucose to produce ethanol.
Profiles of cell mass (filled triangle), glucose consumption (filled square), and ethanol production (filled circle) by P. kudriavzevii KCTC17763 (A) and MBY1358 (B). Shake-flask cultures were grown in YEPD medium containing 200 g/L glucose at 44 °C. Averages and standard errors determined from three independent cultivations are shown
Strains able grow at high temperatures are industrially useful, especially in bioethanol production, because the risk of contamination by other microorganisms and the cost of reactor cooling are reduced [26, 27]. For bioethanol production from lignocellulosic biomass, SSF is performed by enzymatic hydrolysis, usually followed by yeast fermentation. Optimal temperature for substrate degradation by cellulolytic enzymes is around 55 °C [26, 27], which means that the availability of a thermotolerant yeast strain is crucial for SSF-based bioethanol production. A number of studies reported thermotolerant yeasts such as Kluyveromyces marxianus [17], Hansenula polymorpha [28], Debaryomyces hansenii [29], and P. kudriavzevii [30]. Koutinas et al. [31] reported that P. kudriavzevii KVMP10 produces ethanol from glucose at 42 °C. Pichia kudriavzevii DMKU 3-ET15 [32] was reported to produce 7.35% (w/v) ethanol with a volumetric productivity of 2.23 g/L h. Pichia kudriavzevii was also engineered to produce value-added organic acids such as lactic acid [33], succinic acid [34], and xylonic acid [30].
In conclusion, P. kudriavzevii strain MBY1358 showing significantly higher thermotolerance and ethanol productivity than the control strain was isolated from nuruk in this study. Further studies including improvement of stress tolerance and construction of gene expression vectors, are underway to enable utilization of this promising yeast strain.
References
Caspeta L, Nielsenb J. Thermotolerant yeast strains adapted by laboratory evolution show trade-off at ancestral emperatures and preadaptation to other stresses. MBio 6: 4 (2015)
Shinechi H, Koh J, Fujita Y, Matsumoto T, Bito Y, Ueda M. Direct production of ethanol from raw corn starch via fermentation by use of a novel surface-engineered yeast strain codisplaying glucoamylase and α-amylase. Appl. Environ. Microbiol. 70: 5037-5040 (2004)
Caspeta L, Buijs NAA, Nielsen J. The role of biofuels in the future energy supply. Energy Environ. Sci. 6: 1077-1082 (2013)
Lynd LR, Cushman JH, Nichols RJ, Wyman CE. Fuel ethanol from cellulosic biomass. Science 251: 1318-1323 (1991)
Abdel-Banat BM, Hoshida H, Ano A, Nonklang S, Akada R. High-temperature fermentation: how can processes for ethanol production at high temperatures become superior to the traditional process using mesophilic yeast? Appl. Microbiol. Biotechnol. 85: 861-867 (2010)
Abreu-Cavalheiro AMG, Monteiro G. Solving ethanol production problems with genetically modified yeast strains. Braz. J. Microbiol. 44: 665-671 (2013)
Matsumoto N, Fukushi O, Miyanaga M, Kakihara K, Nakajima E, Yoshizumi H. Industrialization of a noncooking system for alcoholic fermentation from grains. Agric. Biol. Chem. 46: 1549-1558 (1982)
Edgardo A, Carolina P, Manuel R, Juanita F, Jaime B. Selection of thermotolerant yeast strains Saccharomyces cerevisiae for bioethanol production. Enzyme. Microb. Tech. (2008)
Song SH, Lee C, Lee S, Park JM, Lee HJ, Bai DH. Analysis of microflora profile in Korean traditional Nuruk. J. Microbiol. Biotechnol. 23: 40-46 (2013)
Kim HS, Hyun JS, Kim J, Ha HP, Yu TS. Characteristics of useful fungi isolated from traditional korean Nuruk. J. Korean Soc. Food Sci. Nutr. 26: 767-774 (1997)
Park JH, Chung CH. Characteristics of Takju (a cloudy korean rice wine) prepared with Nuruk (a traditional korean rice wine fermentation starter), and identification of lactic acid bacteria in Nuruk. Korean J. Food Sci. Technol. 46: 153-164 (2014)
Rhee SJ, Lee JT, Kim KK, Lee CH. Comparison of the traditional (samhaeju) and industrial (cheongju) rice wine brewing in Korea. Korean J. Food Sci. Technol. 12: 242-247 (2003)
Kang SH, Kim HR, Kim JH, Ahn BH, Kim TW, Lee JE. Identification of wild yeast strains and analysis of their b-glucan and glutathione levels for use in Makgeolli brewing. Mycobiology 42: 361-367 (2014)
Ilmén M, Koivuranta K, Ruohonen L, Suominen P, Penttilä M. Efficient production of L-lactic acid from xylose by Pichia stipitis. Appl. Environ. Microbiol. 73: 117-123 (2006)
Jiménez JJ, Borrero J, Diep DB, Gútiez L, Nes IF, Herranz C. Cloning, production, and functional expression of the bacteriocin sakacin A (SakA) and two SakA-derived chimeras in lactic acid bacteria (LAB) and the yeasts Pichia pastoris and Kluyveromyces lactis. J. Ind. Microbiol. Biotechnol. 40: 977-993 (2013)
Thongekaew J, Boonchird C. Molecular cloning and functional expression of a novel extracellular lipase from the thermotolerant yeast Candida thermophila. FEMS Yeast Res 7: 232-243 (2007)
Yamamoto H, Shima T, Yamaguchi M, Mochizuki Y, Hoshida H, Kakuta S, Kondo-Kakuta C, Noda NN, Inaqaki F, Itoh T, Akada R, Ohsumi Y. The thermotolerant yeast Kluyveromyces marxianus is a useful organism for structural and biochemical studies of autophagy. J. Biol. Chem. 115: 6833-6842 (2015)
Spencer JFT, Ragout de Spencer AL, Laluce C. Non-conventional yeasts. Appl. Micobiol. Biotechnol. 58: 147-156 (2002)
Isono N, Hayakawa H, Usami A, Mishima T, Hisamatsu M. A comparative study of ethanol production by Issatchenkia orientalis under stress conditions. J. Biosci. Bioeng. 113: 76-78 (2012)
Choi DH, Park EH, Kim MD. Characterization of starch-utilizing yeast Saccharomycopsis fibuligera isolated from Nuruk. Korean J. Microbiol. Biotechnol. 42: 407-412 (2014)
Yun HJ, Lee YJ, Yeo SH, Choi HS, Park HY, Park HD, Baek SY. The isolation and culture characterization of a lipolytic enzyme producing strain from meju. Korean J. Microbiol. Biotechnol. 2: 98-103 (2012)
Hillis DM, Dixon MT. Ribosomal DNA: molecular evoluiton and phylogenetic inference. Quart. Rev. Biol. 66: 411-453 (1991)
Ducan DB. Multiple range and multiple F tests. Biometrics 11: 1-42 (1955)
Dhaliwal SS, Oberoi HS, Sandhu SK, Nanda D, Kumar D, Uppal SK. Enhanced ethanol production from sugarcane juice by galactose adaptation of a newly isolated thermotolerant strain of Pichia kudriavzevii. Bioresour. Technol. 102: 5968-5975 (2011)
Kaewkrajay C, Dethoup T, Limtong S. Ethanol production from cassava using a newly isolated thermotolerant yeast strain. Sci. Asia 40: 268-277 (2014)
Arora R, Behera S, Sharma NK, Kumar S. A new search for thermotolerant yeasts, its characterization and optimization using response surface methodology for ethanol production. Front Microbiol. 6: 889 (2015)
Choudhary J, Singh S, Nain L. Thermotolerant fermenting yeasts for simultaneous saccharification fermentation of lignocellulosic biomass. Electro. J. Biotecnol. 21: 82-92 (2016)
Kurylenko OO, Ruchala J, Hryniv OB, Abbas CA, Dmytruk KV, Sibirny AA. Metabolic engineering and classical selection of the methylotrophic thermotolerant yeast Hansenula polymorpha for improvement of high-temperature xylose alcoholic fermentation. Microb. Cell Fact. 13: 122 (2014)
Prakash G, Varma AJ, Prabhune A, Shouche Y, Rao M. Microbial production of xylitol from D-xylose and sugarcane bagasse hemicellulose using newly isolated thermotolerant yeast Debaryomyces hansenii. Bioresour. Technol. 102: 3304-3308 (2011)
Toivari M, Vehkomaki ML, Nygard Y, Penttila M, Ruohonen L, Wiebe MG. Low pH D-xylonate production with Pichia kudriavzevii. Bioresour. Technol. 133: 555-562 (2013)
Koutinas M, Patsalou S, Stavrinou S, Vyrides I. High termperature alcoholic fermentation of orange peel by the newly isolated thermotolerant Pichia kudriavzevii KVMP10. Lett. Appl. Microbiol. 62: 75-83 (2015)
Yuangsaard N, Yongmanitchai W, Yamada M, Limtong S. Selection and characterization of a newly isolated thermotolerant Pichia kudriavzevii strain for ethanol production at high temperature from cassava starch hydrolysate. Anton. Van Leeuw. 103: 577-588 (2013)
Sirisan V, Pattarajinda V, Vichitphan K, Leesing R. Isolation, identification and growth determination of lactic acid-utilizing yeasts from the ruminal fluid of dairy cattle. Lett. Appl. Microbiol. 57: 102-107 (2013)
Ahn JH, Jang YS, Lee SY. Production of succinic acid by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 42: 54-66 (2016)
Acknowledgements
This work was supported by the Human Resource Training Program for Regional Innovation and Creativity through the Ministry of Education and National Research Foundation of Korea (NRF-2014H1C1A1073145).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Choi, DH., Park, EH. & Kim, MD. Isolation of thermotolerant yeast Pichia kudriavzevii from nuruk . Food Sci Biotechnol 26, 1357–1362 (2017). https://doi.org/10.1007/s10068-017-0155-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-017-0155-6