Abstract
The principal desirable characteristics of the yeasts used in order to maintain the yield and the efficiency high during the process of ethanol production are mainly thermotolerance, Killer activity and high resistance to inhibitor compounds like acetic acid. Yeast represents the most important factor to achieve an efficient conversion process of the different raw materials used to produce ethanol like sorghum and sugarcane juice or their lignocellulosic materials. The aim of this work was to isolate and select native yeasts from sweet sorghum juice with thermotolerance and resistance to toxic compounds like acetic acid, furfural and 5-hydroxymethylfurfural, capable of producing high ethanol concentrations. The results obtained showed that native yeast Pichia kudriavzevii ITV-S42 exhibit thermotolerance at 40 °C, positive Killer activity, tolerant to acetic acid up to 24 g/L initial concentration, and tolerant to furfural and 5-hydroxymethylfurfural up to 1 g/L for both compounds. An ethanol productivity and yield of 0.54 g/Lh and 0.376 g ethanol/g glucose, respectively, were obtained. These results show that the desirable characteristics of Pichia kudriavzevii ITV-S42 could be interesting for their implementation in first and second generation ethanol process production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Large-scale ethanol production is carried out by the fermentation of different types of raw materials rich in fermentable sugars such as sugarcane, molasses, beet and corn. Currently, sweet sorghum (Sorghum bicolor (L.) Moench) has begun to be considered for ethanol production in some countries as an alternative to sugarcane since it has similar characteristics (Almodares and Hadi 2009), specifically its stem is rich in fermentable sugars, harvest period is short, it grows in different types of climates and soils, it is resistant to drought, salinity and floods and the whole plant can be used (Ratnavathi et al. 2011).
Yeasts have certain advantages in alcoholic fermentation because they are capable of using a wide variety of substrates, are more resistant to ethanol and have higher yields, their membranes are rich in sterols, so they maintain their fermentative activity for longer, and the residual biomass they generate can be used as livestock feed.
Desirable characteristics have been reported in yeasts such as easy propagation, being genetically stable, resistant to toxic compounds, capable of using various substrates, resistant to low pH, acid-tolerant, osmotolerant, thermotolerant and possessing Killer activity (Panchal 1990).
Therefore, it is necessary to search for yeasts that help to counteract the problems present in industrial alcoholic fermentations due to ethanol and substrate inhibition and microbial contamination produced by the presence of bacteria and yeasts that lead to a decrease in ethanol production, economic losses (Ortiz-Zamora et al. 2007). This study deals with the isolation and screening of highly efficient ethanol fermenting yeasts which are also thermotolerant and resistant to inhibitor compounds. The yeasts selected were identified based on phenotypic characteristics, biochemical tests and amplification of the ITS1-5.8S rADN-ITS2 region by means of oligonucleotide primer (ITS1 and ITS4) gene sequence analysis.
Materials and Methods
Isolation and Selection
For the isolation of the yeasts, sweet sorghum juice was fermented spontaneously at 30 °C, 250 rpm for 36 h. Dilutions were prepared from the fermented juice (1 × 10–4 and 1 × 10−5cel/mL), which were seeded by means of the plate extension technique in a culture medium containing 20 g/L glucose, 10 g/L yeast extract, 20 g/L agar–agar and 0.1 g/L antibiotic (chloramphenicol).
The isolated strains were selected through fermentations in semi-synthetic medium proposed by Strehaiano (1984) (20 g/L glucose, 5 g/L KH2PO4, 2 g/L (NH4) 2SO4, 0.4 g/L MgSO4·7H2O, 1 g/L yeast extract) based on its capacity to produce ethanol with ethanol yields above 0.35 g/g.
Subsequently, a secondary selection was carried out in a semi-synthetic medium to determine if the isolated strains showed to be resistant to high temperatures and preserve their fermentative capacity. The temperatures evaluated were: 30, 34, 36, 38, 40 °C.
Killer activity was evaluated using the method described by Rosini (1983): the yeast strain sensitive to Killer toxin was activated in semi-synthetic medium at 30 °C for 24 h at 250 rpm. Subsequently, 25 × 106 viable cells/mL were inoculated in a medium containing YPD-Agar-BM (20 g/L glucose, 20 g/L Peptone, 10 g/L Yeast Extract, 30 g/L agar–agar, 0.003 g/L methylene blue) at 45 °C, which was homogenized and poured into Petri dishes. Once solidified, wells were made on the agar, where the selected yeast biomass with possible Killer Activity was inoculated for 48–72 h at 22 °C. The formation of an inhibition halo surrounded by blue around the well where the biomass was deposited, denotes Killer activity.
In order to evaluate the resistance of yeasts in the presence of toxic compounds present in lignocellulosic hydrolysates, different concentrations of acetic acid (0, 3, 7, 9, 18, 24 g/L), 5-hydroxymethylfurfural (5-HMF) and furfural (0.5, 1.0, 1.5 g/L) were evaluated.
Statistical Analysis
Data means were compared using an analysis of variance (ANOVA) to test the significant differences (P = 0.05) between treatments, with a post hoc Tukey multiple range test between all group mean values to pinpoint the location of any significant differences. All data analyses were conducted using GraphPad Prism version 5.
Analytic Methods
Biomass Analysis
Cellular growth was measured using a correlation between optic density (620 nm) and dry weight. The correlation was also checked using direct count of the cells with a Thoma chamber (Blau Brand, Germany).
Substrate and Product Analysis
Samples from the fermentation kinetics were centrifuged (10 min; 4 °C; 10,000 rpm). Glucose, fructose, ethanol, acetic acid, glycerol, furfural and 5-hydroxymethyl furfural concentrations were measured using High-Performance Liquid Chromatography (HPLC) (Waters 600, TSP Spectra System, Waters, Milford, MA, USA), with a Waters 2414 index detector (TPS Refracto Monitor V Waters, Milford, MA, USA) at 50 °C, using a Shodex SH 1011 column (8 × 300 mm); the mobile phase was a 5 mM H2SO4 solution with a 0.6 mL/min flow rate (Díaz-Nava et al. 2017).
Identification of Yeasts
Identification was carried out by means of the amplification of the ITS1-5.8S rADN-ITS2 region by means of oligonucleotide primers (ITS1 and ITS4). Sequencing was carried out in the DNA Synthesis and Sequencing Unit (USSDNA) of the Biotechnology Institute (Autonomous National University of Mexico, UNAM, Mexico City) the sequences obtained being that were compared with those of reference sequences reported in the Gene bank using the NCBI database.
Stoichiometric and Kinetic Parameters
The kinetic and stoichiometric parameters were carried out from the Monod Model (1949), this model is often used to predict fermentations process and describes the interaction between the growth of microorganisms in a batch culture. Where ethanol yields (YEt/s) were defined as the ratio between final ethanol concentration (P) and glucose consumed during fermentation (S0–S); biomass yield (Yx/s) was defined as the ratio between final biomass concentration and the concentration of glucose consumed. Ethanol productivity (Qp) was considered as the ratio between final ethanol or biomass concentration (X) and end fermentation time (t).
Results and Discussion
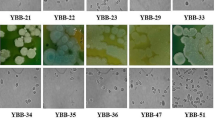
Sixty strains of ethanol-producing yeasts were isolated from the spontaneous fermentation of the juice of different varieties of sweet sorghum. The colonial morphology of these strains had the following characteristics: white, cream and yellowish, with viscous appearance, creamy, convex, rough on the periphery, with brightness. With respect to microscopic morphology, most of the isolated strains had an ellipsoid or ovoid shape, with mono-polar budding, and some of them tended to be grouped into clusters of 5 to 6 cells whose dimensions ranged from 2 to 5 μm in width and 3–7 μm in length (Fig. 1).
Fermentations were carried out in a synthetic medium in which the ethanol production capacity of the 60 isolates was evaluated. A primary selection was made taking as a criterion those yeast strains that presented ethanol yields above 0.35 g/g, since the theoretical yield for glucose conversion to ethanol is 0.511 g/g, however, the yields achieved in the industry vary between 87 and 93% of the theoretical yield (Vázquez and Dacosta 2007); only 22 strains met this criterion (Fig. 2). Most of the yields were between 0.35 and 0.38 g/g, however six strains stood out for having yields above 0.4 g/g, similar to those reported for Saccharomyces cerevisiae, Pichia stipitis, and Kluveromyces marxianus starting from the same initial sugar concentration (20 g/L) (Rouhollah et al. 2007).
Seven strains were selected, which were identified as Pichia kudriavzevii (Issatchenkia orientalis). This identification was carried out by amplification of the ITS1-5.8S rDNA-ITS2 region by means of primer oligonucleotides (ITS1 and ITS4). The sequence analysis of the ITS1-5.8S rDNA-ITS2 region was carried out using the BLAST tool from the NCBI website to identify the yeast strains isolated from sorghum juice. Analysis of the seven strains revealed about 99% similarity to the Pichia kudriavzevii species. With this information, a phylogenetic tree (Fig. 3) was elaborated using the Neighbor Joining method and 1000 replicas to obtain the Bootstrapping value.
The effect of temperature on glucose consumption and ethanol production in the selected strains was evaluated. The phenomena related to thermotolerance and tolerance to ethanol in yeasts have been investigated by several authors, who have suggested that tolerance to ethanol and high temperatures interact with each other, that is, high concentrations of ethanol decrease the optimal temperature of growth and an increase in temperature increases the inhibitory effect of ethanol (Ingram and Buttke 1985; D’Amore et al. 1990). This may be due to the fact that the mechanism of cellular response to stress by temperature and by ethanol is essentially similar (Piper 1995) and consists of changes in the lipid composition of the plasma membranes.
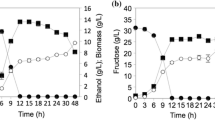
The results showed that both glucose consumption and ethanol production remained constant up to 38 °C, with 100% glucose consumption and maximum ethanol concentrations of 20 g/L. However, there is a notable decrease in the production of ethanol when exposed to 40 °C (Fig. 4) which may be related to the report by Sohn et al. (1994) who mention that certain yeasts are able to grow up to temperatures between 40 and 45 °C but their fermentative capacity decreases or disappears above 40 °C.
Product yield values between 0.31 to 0.38 g/g were observed for all evaluated strains; however, ethanol productivity showed a notable decrease. Up to 38 °C, the strains presented productivities around 0.8 g/Lh and that when exposed to 40 °C they decreased by up to 50% (Table 1). This has been previously observed by Hernández et al. (1986), who reports that due to the heating of the culture media by the exothermic reactions and by the environmental temperature, the temperatures in the bioreactors reach 40 °C causing thermal stress and decreasing the productivity.
According to some researchers (Lee and Kodama 1993) thermotolerant yeasts are those capable of growing and fermenting at temperatures above 38 °C. Although Kluyveromyces marxianus is considered a thermoethalogenic yeast commonly used in industrial processes (Ballesteros et al. 2004; Costa et al. 2014; Arora et al. 2015), species such as Pichia kudriavzevii (Koutinas et al. 2016; Chamnipa et al. 2018; Rahman et al. 2021) have received great interest in the ability to produce ethanol at elevated temperatures.
The selected strains that were isolated from sweet sorghum game are considered to be thermotolerant at 40 °C. According to the studies of Chamnipa et al. (2018), this characteristic suggests that they could manifest an adaptive response at elevated temperatures and thus modify the composition of the cell membrane or synthesize HSP proteins, which have been identified in microorganisms such as S. cerevisiae and K. Marxianus by conferring thermotolerance (Charoensopharat et al. 2015).
Each of seven strains with desirable characteristics was evaluated in order to determine if any of them had Killer activity. Only two strains, ITV-S30 and ITV-S42, exhibited Killer activity. Killer yeasts or their toxins can be used as biological control agents against plant pathogens, food spoilage, fungi, yeasts, and bacteria (Hernandez et al. 2008). The fact that a yeast strain has Killer activity is very important in fermentative processes because, for the most part, they do not use sterile media, and can be used to control undesirable microorganisms in the process, since it reduces the risk of contamination from other strains of yeast or bacteria.
The presence of Killer activity has been demonstrated in strains of Pichia farinosa and Pichia anomala (Suzuki et al. 2001; Polonelli et al. 2011).
Given the problems with the hydrolysis process of lignocellulosic materials in ethanol production from agro-industrial waste, the objective was to evaluate the effect of inhibitory compounds, because this process generates toxic compounds that have a negative influence on the respiration-fermentative metabolism of the yeast (Martin et al. 2007): acetic acid, furfural (product of pentose degradation) and 5-hydroxymethylfurfural (5-HMF) (product of hexose oxidation) mainly.
Concentrations of acetic acid, furfural and 5-HMF were selected based on critical concentrations of yeast toxic compounds present in traditional cane bagasse hydrolysates reported in the literature (Ortiz-Muñiz et al. 2010a, 2010b). Because the selected yeast strains were seen to be of the same genus and species, similar behaviors were expected among them, so it was decided to test 4 yeast strains. Two of them with Killer activity (ITV-S30 and ITV-S42) and two that did not present (ITV-S6 and ITV-S16) and that were also found in nearby neighboring nodes of the phylogenetic tree obtained.
Figure 5 shows the results obtained from the effect of acetic acid concentration on the ethanol production and acetic acid consumption. Maximum ethanol production was observed to be around 20 g/L in the four strains, and no significant differences were found between the evaluated concentrations (0, 3, 7, 9 and 18 g/L acetic acid). In all the selected strains, an acetic acid consumption between 10 and 90% was observed when an initial concentration of 3–24 g/L was evaluated. This consumption decreased as concentrations increased. Otherwise Table 2 shows the productivity obtained under the influence of the previous concentrations ranged around 1.2 g/Lh in all strains; however, when subjected to a concentration of 24 g/L acetic acid, productivity was reduced by approximately 50% (0.56 g/Lh).
Felipe et al. (1997) and Morita et al. (2000) observed that acetic acid could be used by yeast as an additional nutrient for growth, which may explain why it was consumed partially during the fermentation process. Reports by Ortiz-Muñiz (2010a, 2010b) show that native strains of P. stipitis and S. cerevisiae have inhibitory effects on cell growth (acetic acid concentrations of 4.8 and 8.46 g/L, respectively), while P. kudriavzevii ITV-S42 was able to use this acid as an alternative carbon source and produce ethanol with acetic acid concentrations of 18 g/L, outlining the guidelines for the use of these strains in later work.
With furfural and 5-HMF inhibitors, 0.5, 1.0 and 1.5 g/L concentrations were evaluated and it was observed that maximum ethanol production in a medium supplemented with furfural, no changes were observed up to a 1.0 g/L concentration; however, ethanol production was affected when furfural concentration in the medium increased to 1.5 g/L, unlike 5-HMF where strains presented similarity in terms of ethanol production, (approximately 20 g/L) and did not present variation when initial 5-HMF concentration increased.
Ethanol productivity in media supplemented with furfural reached values of around 0.43 g/Lh in the four strains, while in media supplemented with 5-HMF productivity was approximately 0.52 g/Lh, compared to the yield obtained by the strains without the addition of inhibitory compounds ranging from 1 to 1.2 g/Lh (Figs. 6 and 7).
Cell growth inhibition was affected in the presence of both compounds. It was observed that at a 1.5 g/L concentrations cell growth inhibition of the four selected strains was above 90% in the case of furfural and around 70% for 5-HMF. This inhibitory effect is due to the inhibition mechanism of furfural and 5-HMF in the central enzymes of glycolysis and the repression of the enzymes of the tricarboxylic acid cycle by the consumption of NADH leading to cell death (Rangel 2006). In a similar way with that reported by Delgenes et al. (1998) who found that the inhibitory concentrations for furfural and 5-HMF were 1.0 and 0.5 g/L, respectively, the yeasts isolated from sweet sorghum juice showed inhibitory effects for these two compounds up to a 1.0 g/L concentration.
The inhibitory compounds acetic acid, furfural and 5HMF not only decreased cell performance as concentrations in the medium increased, but also the cell growth phase was prolonged. The ethanol productivity was reduced by approximately 50% from that reported for values in the absence of toxic compounds, which was around 1.2 g/L. However, with the exception of furfural, the maximum ethanol production was not affected in the presence of inhibitors.
Conclusions
A native strain of Pichia kudriavzevii ITV-S42 was isolated and identified from the spontaneous fermentation of sweet sorghum juice. This strain has presented desirable characteristics in the ethanol production process, mainly thermotolerance and high resistance to acetic acid, resistance to furfural and 5-HMF and presenting positive Killer activity. It should be noted that there is little information about this species of yeast, and the results obtained are of great interest, since they provide the basis for its application in the production of first and second generation ethanol.
References
Almodares, A., and M.R. Hadi. 2009. Production of bioethanol from sweet sorghum: A review. African Journal of Biotechnology 9: 77–780. https://doi.org/10.5897/AJAR.9000567.
Arora, R., S. Behera, N.K. Sharma, and S. Kumar. 2015. A new search for thermotolerant yeasts, its characterization and optimization using response surface methodology for ethanol production. Frontiers in Microbiology 6: 889. https://doi.org/10.3389/fmicb.2015.00889.
Ballesteros, M., J.M. Oliva, M.J. Negro, P. Manzanares, and I. Ballesteros. 2004. Ethanol from lignocellulosic materials by a simultaneous saccharification and fermentation process (SFS) with Kluyveromyces marxianus CECT 10875. Process Biochemistry 39: 1843–1848. https://doi.org/10.1016/j.procbio.2003.09.011.
Chamnipa, N., S. Thanonkeo, P. Klanrit, and P. Thanonkeo. 2018. The potential of the newly isolated thermotolerant yeast Pichia kudriavzevii RZ8-1 for high-temperature ethanol production. Brazilian Journal of Microbiology 49: 378–391. https://doi.org/10.1016/j.bjm.2017.09.002.
Charoensopharat, K., P. Thanonkeo, S. Thanonkeo, and M. Yamada. 2015. Ethanol production from Jerusalem artichoke tubers at high temperature by newly isolated thermotolerant inulin-utilizing yeast Kluyveromyces marxianus using consolidated bioprocessing. Anton Van Leeuwenhoek 108: 173–190. https://doi.org/10.1007/s10482-015-0476-5.
Costa, D.A., C.J. de Souza, P.S. Costa, M.Q. Rodrigues, A.F. dos Santos, M.R. Lopes, H.L. Genier, W.B. Silveira, and L.G. Fietto. 2014. Physiological characterization of thermotolerant yeast for cellulosic ethanol production. Applied Microbiology and Biotechnology 98: 3829–3840. https://doi.org/10.1007/s00253-014-5580-3.
D’Amore, T., C.J. Panchal, I. Russell, and G.G. Stewart. 1990. A study of ethanol tolerance in yeast. Critical Reviews in Microbiology 9: 287–304. https://doi.org/10.3109/07388558909036740.
Díaz-Nava, L.E., N. Montes-Garcia, J.M. Domínguez, and M.G. Aguilar-Uscanga. 2017. Effect of carbon sources on the growth and ethanol production of native yeast Pichia kudriavzevii ITV-S42 isolated from sweet sorghum juice. Bioprocess and Biosystems Engineering 40: 1069–1077. https://doi.org/10.1007/s00449-017-1769-z.
Delgenes, J.P., M.C. Escare, J.M. Laplace, R. Moletta, and J.M. Navarro. 1998. Biological production of industrial chemicals, i.e. xylitol and ethanol, from lignocelluloses by controlled mixed culture systems. Industrial Crops and Products 7: 101–111. https://doi.org/10.1016/S0926-6690(97)00038-1.
Felipe, M.G.A., I.M. Mancilha, S.S. Silva, and M. Vitolo. 1997. Environmental parameters affecting xylitol production from sugarcane bagasse hemicellulosic hydrolyzate by Candida guilliermondii. Journal of Industrial Microbiology and Biotechnology 18: 251–254. https://doi.org/10.1038/sj.jim.2900374.
Hernández, M.T., T. Sais and O. Sánchez. 1986. Microbiología de la Producción Azucarera. Producciones Microbianas Derivadas. Edición UCLV, Santa Clara, Cuba.
Hernandez, A., A. Martin, M.G. Cordoba, and M.J. Benito. 2008. Determination of Killer activity in yeasts isolated from the elaboration of seasoned green table olives. International Journal of Food Microbiology 121: 178–188. https://doi.org/10.1016/j.ijfoodmicro.2007.11.044.
Ingram, L.O., and T.M. Buttke. 1985. Effects of alcohols on microorganisms. Advances in Microbial Physiology 25: 253–300. https://doi.org/10.1016/S0065-2911(08)60294-5.
Koutinas, M., M. Patsalou, S. Stavrinou, and I. Vyrides. 2016. High temperature alcoholic fermentation of orange peel by the newly isolated thermotolerant Pichia kudriavzevii KVMP10. Letters in Applied Microbiology 62: 75–83. https://doi.org/10.1111/lam.12514.
Lee, C., T. Kodama, and T. Yamakawa. 1993. Rapid growth of thermotolerant yeast on palm oil. World Journal of Microbiology and Biotechnology 9: 187–190. https://doi.org/10.1007/BF00327834.
Martin, C., M. Galbe, L.J. Jönsson, and N.O. Nilvebrant. 2007. A study of three strategies for improving the fermentability of sugarcane bagasse hydrolysates for fuel ethanol production. International Sugar Journal 109: 33–39.
Monod, J. 1949. The growth of bacterial cultures. Annual Review of Microbiology 3: 371. https://doi.org/10.1146/annurev.mi.03.100149.002103.
Morita, T.A., S.S. Silva, and M.G.A. Felipe. 2000. Effects of initial pH on biological synthesis of xylitol using xylose-rich hydrolysate. Applied Microbiology and Biotechnology 84–86: 751–759. https://doi.org/10.1385/ABAB:84-86:1-9:751.
Ortiz-Muñiz, B., M.G. Aguilar-Uscanga, O. Carvajal-Zarrabal, and B. Torrestiana-Sánchez. 2010. Kinetic study on ethanol production using Saccharomyces cerevisiae ITV-01 yeast isolated from sugarcane molasses. Journal of Chemical Technology and Biotechnology 85: 1361–1367. https://doi.org/10.1002/jctb.2441.
Ortiz-Muñiz, B. 2010b. Estudio de la fisiología de Saccharomyces cerevisiae ITV-01 y su deficiente respiratoria para la producción de etanol. Doctoral thesis. Instituto Tecnológico de Veracruz .Veracruz, México.
Ortiz-Zamora, O., M.G. Aguilar-Uscanga, R. Cortés-García, J. Gómez-Rodríguez, and M. Ramírez-Lepe. 2007. Isolation and Selection of ethanol-resistant and osmotolerant yeasts from regional agricultural sources in Mexico. Journal of Food Process Engineering 32: 775–786. https://doi.org/10.1111/j.1745-4530.2008.00244.x.
Panchal, C.J. 1990. Yeast strain selection. Biotechnology and bioprocessing series. New York: Marcel Dekker Inc.
Piper, P.W. 1995. The heat-shock and ethanol stress responses of yeast exhibit extensive similarity and functional overlap. FEMS Yeast Research 134: 121–127. https://doi.org/10.1111/j.1574-6968.1995.tb07925.x.
Polonelli, L., W. Magliani, T. Ciociola, L. Giovato, and S. Conti. 2011. From Pichia anomala Killer toxin through Killer antibodies to Killer peptides for a comprehensive anti-infective strategy. Antonie Van Leeuwenhoek 99: 35–41. https://doi.org/10.1007/s10482-010-9496-3.
Rahman, K., K. Ismail, and N. Najimudin. 2021. Growth of thermotolerant Pichia kudriavzevii UniMAP 3–1 strain for ethanol production using xylose and glucose at different fermentation temperatures. IOP Conference Series: Earth and Environmental Science. 765: 012107. https://doi.org/10.1088/1755-1315/765/1/012107.
Rangel, L.E. 2006. Efectos de los requerimientos nutricionales y de productos tóxicos presentes en hidrolizados de lignocelulósicos sobre la producción de xylitol por la capa IEC5-ITV. Masters thesis. Instituto Tecnológico de Veracruz. Veracruz. México.
Ratnavathi, C.V., S.K. Chakravarthy, U.D. Chavan, V.V. Komala, and J.V. Patil. 2011. Sweet sorghum as feedstock for bio-fuel production: A review. Sugar Tech 13: 399–440. https://doi.org/10.1007/s12355-011-0112-2.
Rosini, G. 1983. The occurrence of killer characters in yeasts. Canadian Journal of Microbiology 29: 1462–1464. https://doi.org/10.1139/m83-224.
Rouhollah, H., E. Giti, N. Iraj, and A. Sorah. 2007. Mixed sugar fermentation by Pichia stipitis, Saccharomyces cerevisiae, and an isolated xylose fermenting Kluyveromyces marxianus and their co-cultures. African Journal of Biotechnology 6: 1110–1114. https://doi.org/10.4314/ajb.v6i9.57123.
Sohn, H.Y., I. Jin, W. Park, and J.H. Seu. 1994. The fermentation characteristics of newly selected thermotolerant yeasts at high temperature. Journal of Microbiology and Biotechnology 4: 222–229.
Strehaiano, P. 1984. Phénomenes d’ inhibition et fermentation alcoolique, Tesis Doctoral. I. P. N. Toulouse, Francia.
Suzuki, C., Y. Ando, and S. Machida. 2001. Interaction of SMKT, a Killer toxin produced by Pichia farinosa, with the yeast cell membranes. Yeast 18: 1471–1478. https://doi.org/10.1002/yea.791.
Vázquez, H.J., and O. Dacosta. 2007. Fermentación alcohólica: Una opción para la producción de energía renovable a partir de desechos agrícolas. Ingeniería Investigación y Tecnología 8: 249–259.
Acknowledgements
Authors acknowledge the economic support from the National Council of Science and Technology (CONACyT) through the doctoral grant of Libia Diaz-Nava and also the critical reading of Dulce Maria Barradas-Dermitz MSc. and Patricia Margaret Hayward-Jones MSc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Díaz-Nava, L.E., Aguilar-Uscanga, M.G., Ortiz-Muñiz, B. et al. Acetic Acid-Tolerant Native Yeast Pichia kudriavzevii ITV-S42 Isolated from Sweet Sorghum Juice for Ethanol Production. Sugar Tech 24, 576–584 (2022). https://doi.org/10.1007/s12355-021-01040-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-021-01040-z