Abstract
Background
Lateral abdominal wall hernias (LAWH) constitute about 1–4% of hernia surgical procedures. They represent a unique surgical challenge on account of their potential for anatomical complexity and consequent operative technical demand. Furthermore, LAWH repairs are currently not standardized, and remain contentious, despite a variety of approaches. These repairs are attendant with not insignificant morbidity and recurrence rates. We profile here our endoscopic and hybrid surgical approach to the management of LAWH and early therapeutic outcomes.
Methods
A retrospective review of our hernia clinical database between March 2018 and December 2020 was performed to extract all LAWH (with and without an associated midline component) patients, who underwent an enhanced-view totally extra peritoneal (eTEP) hernia repair with a transversus abdominis release (TAR), or a hybrid repair. Initial outcome data (6-month follow-up) is profiled here. The primary outcome measures were hernia recurrence and hernia-site bulging. The secondary measures were surgical site occurrence (SSO) and hernia-related quality of life (QoL).
Results
A total of 33 LAWH patients underwent an eTEP TAR or hybrid hernia repair. 11 patients had an associated midline defect and 12 were recurrent hernias. The mean hernia defect area was 84.2 ± 49 cm2 and mean mesh size was 859.6 ± 263 cm2. There was no hernia recurrence at initial follow-up of 24 months. The SSO rate was 12%. The CCS QoL scores were 34.6 ± 2 pre-operatively, and improved to 27.2 ± 4 at 6 months.
Conclusions
Our endoscopic and hybrid technique is a safe, reproducible, and technically promising approach for the repair of LAWH. Thorough knowledge of the surgical anatomy of the lateral abdominal wall and advanced endosurgical skills are imperative for good outcomes. We await the long-term results of our LAWH cohort to confirm the findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lateral abdominal wall defects are encountered much less frequently and their surgical management is difficult and not standardized [1]. Lateral defects vary significantly in their anatomy and behavior from midline hernias and can lead to significant morbidity for the patient. The main etiology is surgical or iatrogenic trauma involving tissue extirpative surgery or denervation of the musculature [2]. These lateral incisional hernias (LIHs) occur in a semi-rigid closed space limited by bony and musculo-aponeurotic margins; therefore, their repairs would impose greater limitations on the surgical technique [1]. In comparison to the central abdominal wall, the anatomy of the lateral wall has a smaller fascia to muscle ratio, and due to this large muscular surface area, these defects can progress rapidly and lead to cosmetic and physiological problems for the patient [2,3,4].
Unlike midline hernias, where vector forces are distributed centrally and evenly, a unilateral lateral wall defect causes asymmetrical shift of forces to the ipsilateral side [3]. This uneven pull can lead to lumbar spine ligamentous strain, back pain, changes in normal spinal curvature, poor bowel movements, and progressive herniation [3, 5]. Indications for operative repair are largely based on patient symptoms which are mainly pain and bulging. If the hernia interferes with the patient’s daily activities, then a definitive repair is recommended [6]. Due to their wide neck, lateral abdominal hernias are less likely to strangulate. Therefore, if asymptomatic, repair of these defects is generally not recommended [1, 6]. Treatment of lateral abdominal wall hernias is controversial, and multiple techniques have been described both via laparoscopic or open approaches. Surgical repair may help improving the bulging of the lateral abdominal wall [6]. Most series include a small number of patients and often combine different types of non-midline hernias [1].
The asymmetric forces caused by the independent contraction of anterior and posterior muscle units leads to strain and hernia progression. These features necessitate the use of wide load-bearing underlay mesh repairs supported by the static pillars of the lateral abdominal wall [2, 3].
The main objective of this study is to describe in details the technological deliberations of endoscopic approach and to evaluate the early results of minimal invasive surgical (MIS) technique of abdominal wall reconstruction (AWR) in the form of enhanced view totally extra peritoneal Rives–Stoppa repair with transversus abdominis release (eTEP TAR) for LIHs. Few hybrid cases (combination of laparoscopic and open) were also included in the study where major surgical component is laparoscopic. Recurrences and bulging were primary endpoints. The secondary objectives were to analyze the short- and long-term outcomes mainly in the form of complications, such as surgical site occurrences (SSOs) and surgical site infections (SSIs). An SSO was defined as any event that resulted in delayed healing of the wound, viz. cellulitis, seroma, hematoma [7]. An SSI was defined according to criteria established by the Center for Disease Control, and was classified as superficial, deep, or organ/space [8]. After discharge, the patients were routinely reviewed at 1 and 6 months, and then at the end of 1 year.
Materials and methods
A retrospective review of the institutional electronic medical records was done to identify all patients who underwent either eTEP repair with unilateral or bilateral TAR, or hybrid repair for large, complex lateral incisional hernia with or without midline component, between March 2018 and December 2020, at Sir Ganga Ram Hospital, New Delhi. Patient demographics included age, sex, body mass index (BMI), prior surgical history, social history of smoking and alcohol and associated comorbidity. Hernia characteristics captured included hernia site and size according to the EHS staging system, number of previous repairs if any, history of wound or mesh infection, as well as intraoperative and postoperative metrics. Assessment of the postoperative quality of life (QoL) in these patients was carried out using the Carolina Comfort Scale (CCS) as a validated questionnaire up till 6 months and expressed as mean ± SD.
Patient selection and workup
The pre-operative workup of all the patients included detailed clinical history, thorough abdominal and systemic examination, hematological and biochemical workup, besides ASA grading. Physical examination included confirming hernia location and size, contents and number of defects with their reducibility, quality of previous scars, prior or current wound complications. All patients underwent CT scan of the whole abdomen and pelvis prior to admission for assessing hernia characteristics and procedure planning. The diagnosis of LIHs was based on clinical examination and imaging from a computed tomography (CT). Hernias have been classified according to the criteria established by the European Hernia Society (EHS) [9]. We only included patients with lateral hernia (L1–L4 EHS classification) and those with associated midline defects (M1–M5 EHS classification). As a result, we excluded patients with primary, non-incisional lateral hernias, such as Spigelian and Lumbar. We also excluded those LIHs which were associated with parastomal defects in patients with permanent stoma.
All patients followed a similar, preoperative optimization program, which included endocrinologic and nutritional evaluations, abstinence from smoking, weight loss, and respiratory physiotherapy. Smoking cessation 1 month before operation was mandatory. Weight loss was encouraged but without any mandatory numerical cutoff.
Surgical technique
Minimally invasive retromuscular repair of lateral incisional hernias is a challenging endeavor and the technique warrants through knowledge of abdominal wall anatomy, with special reference to lateral abdominal wall. The technique is based on the eTEP approach for ventral hernias as described by Belyansky et al. with addition of endoscopic transversus abdominis release (TAR) added to it, thereby creating a large retromuscular pre-peritoneal space for prosthetic reinforcement of the lateral wall defects [10].
Positioning of patient
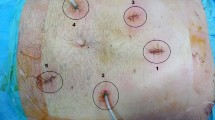
Patient positioning depends on various factors, such as hernial defect site, previous surgical scars (if any) and planned position of ports. For subcostal (L1) defects, the patient was laid supine with legs split apart as in modified lithotomy position to facilitate dissection in cranial direction with the surgeon standing in between the legs. When defects were located in the flank or iliac regions (L2 or L3), the patient was laid supine with arms tucked by the side of the body and the table extended at subcostal level (Fig. 1).
In cases where lateral defects (L1–L3) crossed the anterior axillary line, or were located in the lumbar region (L4), the patient was positioned in semilateral decubitus with the forearm supported.
In all cases, the urinary bladder was emptied using Foley balloon catheter, with the patient well-strapped to the operating table so as to allow changes in table position. The antero-lateral abdominal wall is marked to define the anatomical landmarks, defect site and size, previous surgical scars and probable port locations.
Port placements
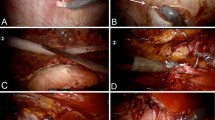
The initial point of entry was the contralateral retrorectus space high up in the left subcostal region for right sided L1–L3 defects that were encroaching the semilunar line or had a cross boundary component (Fig. 2a), i.e., extending medially into anterior compartment, laterally beyond posterolateral musculature or superiorly into the costal margin. An ipsilateral retrorectus access was employed in cases with L2–L4 defects without any cross-boundary component (Fig. 2b). In all cases, initial entry was accomplished using a 12 mm optical trocar and 10 mm 0-degree telescope.
The decision to perform only an ipsilateral Rives–Stoppa dissection, without violating the linea alba, was taken by preoperatively noting the absence of a midline defect, and the presence of a healthy at least 7 cm distance between the linea alba and the medial extent of the defect.
Once the entered retro rectus space was developed using telescopic dissection, secondary ports were placed. Location of placement of secondary ports depended on certain factors such as site of defect and whether a superior or inferior crossover is required. For superior crossover ports were placed in the same manner as in eTEP RS for midline hernias [11], while for inferior crossover after initial optical entry secondary ports were placed below the level of arcuate line for bottom-up dissection (Fig. 2c). In cases where ipsilateral retro rectus space was entered initially, secondary ports were placed in the midline along the linea alba, one above or below the umbilicus and another in the suprapubic region below the arcuate line (Fig. 2b). The exact distance between ports varied with the patient’s build and torso length.
Retro-rectus dissection and crossover
After placing the secondary ports, the retrorectus space was further developed to expose the complete length and width of the ipsilateral rectus muscle. For defects in subcostal (L1) region, to avoid scarred areas, inferior crossover was preferred, thereby facilitating retro rectus dissection in a cranial direction toward the defect and/or scarred tissue. In cases of L2–L4 defects where contralateral retro rectus dissection was required, crossover was done by superior approach through the subxiphoid pre-peritoneal fatty triangle of Schumpelick. In cases with cross boundary component, the retrorectus space was developed all around the edge of the defect before commencing TAR.
Transversus abdominis release (TAR)
When a lateral hernia is approached through the midline, a posterior component separation in the form of TAR is a usual, integral part of retromuscular repairs. In the direct, lateral approach, however, TAR is not required. TAR in cases of lateral wall hernia is typically challenging owing to multiple factors, such as tissue scarring in the region of previous scar and incision, denervation injury of muscle fibres secondary to neurovascular damage and lost planes from previous scars of drain sites, stomas or mesh repairs.
Depending on the location of the defect and its cross-boundary component, TAR was done by bottom-up or top-down, or a combination of both (Fig. 3a, b).
TA release was typically commenced in a relatively untouched area where tissues were not scarred, and gradually moving toward the defect, thereby preventing inadvertent tears in peritoneum and also preventing incorrect plane of entry. The TAR was usually commenced in the subcostal area. After the fleshy TA fibres were divided, the lateral extraperitoneal plane was entered by sweeping the fibres of the muscle off the fascia transversalis and moving further caudally and medially (Fig. 4) [12]. This maneuver from lateral to medial allowed a safer, controlled and easy separation of the transversus abdominis aponeurosis off the underlying thin peritoneum and thus made the caudal transection of the transversus aponeurosis easier.
Subcostal (L1) and flank (L2) defects were managed by a combination of bottom-up and top-down TAR approach, helping reach the lateral edge of the defect and further the lateral dissection (Fig. 5). On the other hand, in the iliac (L3) defects TAR was done in a top-down manner. Lumbar defects and those without cross boundary component could be managed by either of the approaches of doing TAR as per surgeon’s choice or by lateral approach to avoid TA release.
Lateral dissection
Once the hernial contents were reduced and dissection completed around the defect to reach up to the lateral edge (Fig. 5), extraperitoneal dissection was extended further laterally in pre-transversalis fascia plane. While doing this all precautions were taken to prevent button hole punctures in the peritoneum or entering into the wrong planes. Understanding of the CT images of the abdomen and their per-operative correlation helped in easier navigation through the planes.
In lateral wall hernias, due to the narrow musculo-aponeurotic space and the denervated musculature, the extent of lateral dissection was typically at least 5–10 cm beyond the lateral edge of the defect, in this process exposing the medial border of psoas major muscle. While doing this all precautions were taken to prevent inadvertent injury to the nerves running in this plane, such as ilio-inguinal, ilio-hypogastric and genito-femoral nerves.
Superior and inferior dissection
After the division of the TA, the dissection proceeded cephalad under the costal margin. The transition of retromuscular plane between the TA and the diaphragm was identified by their orientation, TA muscle fibres being oriented transversely and interdigitating with the longitudinally aligned diaphragmatic muscle fibres (Fig. 6). A fat deposit was often a landmark between this junction.
Complete release of TA muscle is essential to facilitate adequate dissection in supracostal region besides allowing better medialization of separated posterior component. The extent of this dissection was 6–10 cm beyond the costal margin laterally and medially up to the central tendon of diaphragm. Utmost care was taken to prevent injury to diaphragmatic fibres and an iatrogenic Morgagni hernia by directing the TAR more medially at the cephalad region of dissection. At the convergence of the costal margins, one could identify or visualize the connection of three different planes, i.e., pre-transversalis fascia plane, retro-rectus plane and pre-peritoneal plane, to each other (Fig. 7). Dissection in the space of Retzius and space of Bogros in preperitoneal space below the level of arcuate lines was done to expose Cooper’s ligament.
Posterior peritoneal and anterior defect closure
Closing the posterior peritoneal layer was not difficult in our series, thanks to abundant peritoneum that was available from the hernia sac and from the TAR effect on the posterior elements.
Defect closure was treated as a vital part of the operation to fulfil the principles of modern abdominal wall reconstruction.
The fascial defects were closed using non absorbable or delayed absorbable 1–0 PDS barbed suture in continuous fashion. Bites were taken in a staggered manner and tightened after a few throws so as to take away the tension from the suture line. Closure was done in two layers to provide better surface for mesh deployment and integration.
Mesh measurement and deployment
Measurement of the required size of the mesh was done at the widest points both in transverse and cranio-caudal directions. Because of the non-geometric shape of the space created, mesh measurement and tailoring were done in a way to not only provide wide coverage to the repair but also prevent folding of edges especially at corners.
For adequate overlap, the mesh was half rolled, and the posterolateral free edge is tucked on the exposed retroperitoneal bed (Fig. 8). The peritoneum with contents were allowed to fall over the flat mesh to keep it in place. Securing the mesh to the lateral abdominal wall muscles was sparingly done to prevent iatrogenic neural injury. At specific safe points, the mesh was fixed using glue, tacks or sutures, varying from case to case as per availability of the products. The mesh was then unrolled till the medial and superior edges were flatly placed to cover all the dissected areas well beyond the repair.
Results
A total of 33 patients with large, complex, symptomatic lateral abdominal hernia underwent e-TEP-RS with unilateral or bilateral Transversus Abdominis Release (TAR) or hybrid repair, between August 2018 and December 2020. Twenty-four (73%) of the patients were female, with a mean age of 57 ± 10 years and BMI 30 ± 5 kg/m2. Of these 12% were smokers, 27% diabetic and 42% hypertensive. Two patients had coronary artery disease (CAD) and one had chronic obstructive pulmonary disease (COPD). Patient demographics and co-morbidity details are profiled in Table 1.
All patients had large lateral incisional hernias, that included recurrent hernias with or without concomitant midline component. A total of 11 (33%) patients had associated midline defect and 12 (36%) had recurrent hernias of which previous mesh was seen in 10 patients, with 04 having onlay mesh and 06 with intraperitoneal mesh. Lateral incisional hernias were classified and categorized as per EHS classification (Tables 1 and 2). The mean defect area was 84 ± 49 cm2 and mean mesh area was 859 ± 263 cm2, with the largest defect dimension being 18 cm (Table 3). A heavy weight polypropylene (HWPP) mesh was used in 23 (70%) cases and in those with no associated denervated injury, medium weight polypropylene (MWPP) mesh (30%).
An ipsilateral RS TAR procedure was performed in 8 (24%) cases, eTEP unilateral TAR in 22 (67%) and eTEP bilateral TAR in 3 (9%) cases. Out of all these, hybrid mesh repair was done in 6 (18%) cases. Inferior crossover was done in 10 (30%) cases, while in 15 (45%) a superior crossover was done, no crossover was required in 8 cases where only ipsilateral Rives–Stoppa dissection was done (Table 4). We did inferior crossover in cases having subcostal defects with or without associated midline component, due to the scarring in subxiphoid area precluding the choice of an upper crossover.
None of the patients with previous indwelling mesh required explantation. No synchronous non-hernia surgery was performed in any of the patients. Patients requiring bilateral TAR, were further classified according to its indication, such as larger defect size, presence of defects on either side of midline or associated large midline component (Table 4). In our study eTEP TAR with hybrid repair was indicated in 6 patients for reasons, such as dense bowel adhesions, larger defect mandating open closure or ugly scars requiring revision. There were no events of intraoperative enterotomy or other bowel complications.
Mean duration of surgery was 250 ± 52 min and mean blood loss was 63 ± 28. Mesh was fixed to the Cooper’s ligament in 13 cases, all of which had iliac or flank defects or had associated midline infraumbilical component. Drainage of the space was done in only 8 (24%) out of 33 cases, all of them either had hybrid repair or bilateral TAR or had associated large midline defect (Table 4). The mean length of stay was 3.2 ± 1.9 days.
Repairs were classified according to procedure done into ipsilateral RS TAR, eTEP with unilateral or bilateral TAR and eTEP TAR hybrid, and compared on pre-operative parameters viz. mean defect size, mesh size, operative time, blood loss, drain placement and length of stay (Table 5).
The CCS QoL scores were 34.6 ± 2 pre-operatively when the patients struggled with symptoms pertaining to their hernias. At 1 month, the scores were 38.2 ± 7, probably because of the added factor of mesh sensation contributing to the scores. This improved to 27.2 ± 4 at the end of 6 months. On comparative analysis, the scores at 1 month and 6 months reflected a statistically significant improvement and hence the quality of life in these patients (Table 6). There was no recurrence during follow-up which ranged from 12 to 40 months, with a mean of 24 months. Major complications were seroma and prolonged ileus, while minor complications such as wound or mesh infection were not seen except chronic pain (Table 7). All patients with seroma formation had either hybrid repair or associated midline defect and were improved with masterly inactivity. Those with chronic pain required medical management using analgesics for a period of 2 weeks.
Discussion
Lateral incisional hernias are less common, occurring in 1–4% of surgical procedures as compared to 14–19% for midline incisional hernias [13,14,15]. Although rare, these hernias are distressing to patients not only because of the asymmetrical deformity of the lateral abdominal wall but also for the progressive growth of the hernia.
Flank, iliac and lumbar hernias are infrequently reported in the literature, usually with case series, retrospective reviews, and case reports to guide the general surgeon in decision making and patient counselling. Available relevant literature neither describes the best approach for these lateral incisional hernia repair nor has described a formal comparison between open and laparoscopic techniques. There are no randomized trials on the subject to provide strong recommendations regarding these complex hernias [16]. Given this paucity of evidence, optimal techniques and approaches remain elusive [6]. There are very few published laparoscopic series of lateral hernia repair. Heniford described the first laparoscopic approach for lumbar hernias in 1997 [17]. Moreno-Egea presented the largest series of laparoscopic treatment of lateral hernia, including inguinal and subcostal defects [18]. Clinical results for open and laparoscopic repairs differ in many ways, not only in technique, but also in recurrence rates, wound complications, length of stay, and other outcomes [6]. Primary fascial coaptation may not be as easily attained, because there is no analogous, tension-reducing fascial or component release that can be performed [2].
On examining preoperative CT scans, patients seemed to have certain common features, regardless of defect size and the location of the true parietal defect. The muscles were often reduced in thickness and retracted, thus making the entire lateral abdominal wall unstable. Moreover, the hernia defect often included more than one area, as described in the EHS classification of lateral hernia [16]. In our case series also, we have observed that lateral wall defects tend to include more than one zone and a few also had associated midline component or cross boundary component. Inclusion of concomitant midline defects is not by choice but are associated with lateral hernias as main primary complaint.
The classic repair of lateral hernias involves the fixation of the mesh to the bony structures: the costal margin superiorly and the iliac crest inferiorly. Katkhouda et al. believe that without this anchoring, the patient may develop a bulge or hernia recurrence and that this crucial anchoring step is best accomplished with an open approach. Katkhouda described placing the mesh between the external oblique muscle and the internal oblique muscle and fixing it to a bony structure [1]. Minimally invasive techniques are feasible but make fixation to the bony structures technically more challenging, with the potential for damage to the postero-lateral nerves [19].
Philips and Rosen believe that lateral hernias are best approached through an open repair, avoiding the extensive enterolysis and allowing for muscle approximation that addresses the skin deformity. They prefer the retro muscular preperitoneal repair of flank hernias to achieve a large mesh overlap, thus avoiding the creation of skin flaps. Phillips et al. also suggested that standard repair techniques often do not provide a long-term durable repair, as half of their patients had multiple recurrent hernias [20]. The same was observed in our series of patients, 36% of whom reported one or more previous attempts at hernia repair. In endoscopic retro muscular approach also minimal adhesiolysis is required in and around the area of defect, thereby reducing chances of inadvertent bowel injury. One of the major advantages of following the retro muscular preperitoneal dissection laterally is its ability to extend the space far beyond the bony limits. The bony landmarks (costal arch and iliac bone) are often too close to the hernia defect and are recognized as the most difficult obstacles for a surgeon attempting to create a large pocket for an adequate mesh overlap, thereby resulting in a smaller sized prosthesis [6, 20, 21].
Regardless of the mesh placement technique, it is generally recommended that the mesh area be much greater than the area of the hernia defect, i.e., the mesh should overlap the hernia defect by 5–10 cm in all directions [1, 9, 22,23,24,25,26]. This extensive mesh overlap made mesh fixation unnecessary [16]. Avoiding mesh fixation, especially on the iliacus or psoas muscle, reduces the risk of post-operative pain [20, 22, 25]. While this lack of chronic post-operative pain could be explained by the reduced use of fixation, we should also recognize that other factors, such as level of preoperative pain, patient expectations and psychological status, may also have an influence on pain and have not been analyzed. We also followed the same criteria for mesh overlap with minimum of 6 cm overlap and preferred to obtain extensive overlap over mesh fixation. Mesh size in our series seems smaller when compared to available literature, reason for this could be small defects or smaller build of the patients.
In the literature, different planes for mesh placement have been proposed, such as external mesh onlay [27], intraperitoneal mesh underlay [23, 28, 29], (open or laparoscopic), or mesh between external and internal oblique layers [1, 28,29,30].
Nielsen et al. proposed a peritoneal flap to bridge the fascial gap and placement of a mesh in the retrorectus space medially and the avascular plane between the internal and external oblique muscles laterally, with fixation of the mesh to the posterior musculo-fascial layer [24].
Robotic-assisted transabdominal preperitoneal hernia repair seems to be a promising technique, but to date, only a few studies have been published. It provides the benefits of both open and minimally invasive approaches, making it possible to place the prosthesis in an extraperitoneal space and suturing the defect by better approximation of the edges, and thus reducing the bulge effect [6].
No major complications were recorded. Our post-operative seroma rate (18%) was a bit higher than that reported in the literature (8–12.5%) [31, 32], most of which are small sized and all managed conservatively. To the best of our knowledge, there are no studies with large enough cohorts to effectively compare outcomes of lateral hernias depending on mesh type. A recent systematic review also concluded the same, secondary to the heterogeneity of operative technique and low patient numbers [21].
Conclusions
Though ours is a retrospective study with small heterogeneous patient population, our aim is to describe the feasibility and safety of the endoscopic retromuscular repair technique for the management of so called difficult to treat lateral abdominal wall hernias.
In our experience with this small cohort, we have tried to standardize the endoscopic retromuscular technique for management of these complex lateral hernias. In our opinion and experience, eTEP TAR approach with some contextual technical variations can be utilized to manage appropriately chosen patients with lateral abdominal wall defects so as to get desirable and comparable results, with wider coverage of both the repair and the area of denervation.
Our endoscopic technique is a safe, feasible, and reproducible treatment for these challenging surgical hernias and warrants thorough knowledge of lateral abdominal wall anatomy.
References
Katkhouda N, Alicuben ET, Pham V (2020) Management of lateral abdominal hernias. Hernia 24:353–358
Kapur SK, Butler CE (2018) Lateral abdominal wall reconstruction. Semin Plast Surg 32(3):141–146. https://doi.org/10.1055/s-0038-1666801
Baumann DP, Butler CE (2012) Lateral abdominal wall reconstruction. Semin Plast Surg 26(1):40–48. https://doi.org/10.1055/s-0032-1302465
Stamatiou D, Skandalakis JE, Skandalakis LJ, Mirilas P (2009) Lumbar hernia: surgical anatomy, embryology, and technique of repair. Am Surg 75(3):202–207
Moreno-Egea A, Baena EG, Calle MC, Martinez JA, Albasini JL (2007) Controversies in the current management of lumbar hernias. Arch Surg 142(1):82–88. https://doi.org/10.1001/archsurg.142.1.82
Befa LR, Margiotta AL, Carbonell AM (2018) Flank and lumbar hernia repair. Surg Clin North Am 98(3):593–605. https://doi.org/10.1016/j.suc.2018.01.009
Ventral Hernia Working Group, Breuing K, Butler CE et al (2010) Incisional ventral hernias review of the literature and recommendations regarding the grading and technique of repair. Surgery 148:544–558
Mangram AJ, Horan TC, Pearson ML et al (1999) (1999) Guideline for prevention of surgical site infection, Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol 20:250–278
Muysoms FE, Miserez M, Berrevoet F et al (2009) Classifcation of primary and incisional abdominal wall hernias. Hernia 13(4):407–414
Belyansky I, Ramana B et al (2017) A novel approach using the enhanced-view totally extraperitoneal (eTEP) technique for laparoscopic retromuscular hernia repair. Surg Endosc 32:1531–1532
Khetan M, Ramana B et al (2021) eTEP repair for midline primary and incisional hernia: technical considerations and initial experience. Hernia. https://doi.org/10.1007/s10029-021-02397-6
Gibreel W, Sarr MG, Rosen M, Novitsky Y (2016) Technical considerations in performing posterior component separation with transverse abdominis muscle release. Hernia. https://doi.org/10.1007/s10029-016-1473-Y
Halm JA, Lip H, Schmitz PI, Jeekel J (2009) Incisional hernia after upper abdominal surgery: a randomized controlled trial of midline versus transverse incision. Hernia 13(3):275–280. https://doi.org/10.1007/s10029-008-0469-7
Greenall MJ, Evans M, Pollock AV (1980) Midline or transverse laparotomy? A random controlled clinical trial. Part II: influence on postoperative pulmonary complications. Br J Surg 67(3):191–194
Kendall SW, Brennan TG, Guillou PJ (1991) Suture length to wound length ratio and the integrity of midline and lateral paramedian incisions. Br J Surg 78(6):705–707
Cavalli M, Aiolfi A, Morlacchi A, Bruni PG, Del Ferraro S, Manfredini L, Campanelli G (2021) An extraperitoneal approach for complex flank, iliac, and lumbar hernia. Hernia 25(2):535–544. https://doi.org/10.1007/s10029-020-02214-6
Heniford BT, Iannitti DA, Gagner M (1997) Laparoscopic inferior and superior lumbar hernia repair. Arch Surg 132(10):1141–1144
Moreno-Egea A, Carrillo A, Aguayo JL (2008) Midline versus nonmidline laparoscopic incisional hernioplasty: a comparative study. Surg Endosc 22(3):744–749. https://doi.org/10.1007/s00464-007-9480-9
Dakin GK, Kendrick ML (2013) Challenging hernia locations: flank hernias. The SAGES manual of hernia repair. Springer, Berlin, pp 531–540
Phillips MS, Krpata DM, Blatnik JA, Rosen MJ (2012) Retromuscular preperitoneal repair of flank hernias. J Gastrointest Surg 16(8):1548–1553. https://doi.org/10.1007/s11605-012-1890-x
Zhou DJ, Carlson MA (2018) Incidence, etiology, management, and outcomes of flank hernia: review of published data. Hernia 22(2):353–361
Edwards C, Geiger T, Bartow K et al (2009) Laparoscopic transperitoneal repair of flank hernias: a retrospective review of 27 patients. Surg Endosc 23(12):2692–2696
Zieren J, Menenakos C, Taymoorian K, Muller JM (2007) Flank hernia and bulging after open nephrectomy: mesh repair by flank or median approach? Report of a novel technique. Int Urol Nephrol 39(4):989–993
Nielsen MF, de Beaux A, Damaskos D, Tulloh B (2019) Peritoneal flap hernioplasty for reconstruction of transverse incisional hernia. Hernia. https://doi.org/10.1007/s10029-019-02099-0
Patel PP, Warren JA, Mansour R, Cobb WS, Carbonel AM (2016) A large single-center experience of open lateral abdominal wall hernia repairs. Am Surg 82(7):608–615
Welty G, Klinge U, Klosterhalfen B, Kasperk R, Schumpelick V (2001) Functional impairment and complaints following incisional hernia repair with different polypropylene meshes. Hernia 5(3):142–147
Hofman RS, Smink DS, Noone RB, Noone RB Jr, Smink RD Jr (2004) Surgical repair of the abdominal bulge: correction of a complication of the flank incision for retroperitoneal surgery. J Am Coll Surg 199(5):830–835
Luc G, David A, Couzi L, Midy D, Collet D, Dubuisson V (2014) Lateral incisional hernia after renal transplantation: a comparative study. World J Surg 38(11):2791–2796
Purnell CA, Park E, Turin SY, Dumanian GA (2016) Postoperative flank defects, hernias and bulges: a reliable method for repair. Plas Reconstr Surg 137(3):994–1001
Veyrie N, Poghosyan T, Corigliano N, Canard G, Servajean S, Bouillot JL (2013) Lateral incisional hernia repair by retromuscular approach with polyester standard mesh; topographic considerations and long-term follow-up of 61 consecutive patients. World J Surg 37(3):538–544
Kaafarani HM, Hur K, Hirter A, Kim LT, Thomas A, Berger DH, Reda D, Itani KM (2009) Seroma in ventral incisional herniorrhaphy: incidence, predictors and outcome. Am J Surg 198(5):639–644
Pring CM, Tran V, O’Rourke N, Martin IJ (2008) Laparoscopic versus open ventral hernia repair: a randomized controlled trial. ANZ J Surg 78(10):903–906
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors Mukund Khetan, Sudhir Kalhan, Suviraj John, Daksh Sethi, Pankaj Kannaujiya and Ramana Balasubramaniam have no relevant conflicts of interest or financial ties to disclose.
Ethical approval
All authors certify that they accept responsibility as an author and have contributed to the concept, data gathering, analysis, manuscript drafting, and give their final approval. This retrospective study was approved by local ethical committee as per the specific requirements of the country.
Informed consent
Informed consent of the patients is not possible as being a retrospective analysis of already available data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khetan, M., Kalhan, S., John, S. et al. MIS retromuscular repair of lateral incisional hernia: technological deliberations and short-term outcome. Hernia 26, 1325–1336 (2022). https://doi.org/10.1007/s10029-022-02671-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-022-02671-1