Abstract
Purpose
Lateral abdominal wall hernias are rare defects but, due to their location, repair is difficult, and recurrence is common. Few studies exist to support a standard protocol for repair of these lateral hernias. We hypothesized that anchoring our repair to fixed bony structures would reduce recurrence rates.
Methods
A retrospective review of all patients who underwent lateral hernia repair at our institution was performed.
Results
Eight cases (seven flank and one thoracoabdominal) were reviewed. The median defect size was 105 cm2 (range 36–625 cm2). The median operative time was 185 min (range 133–282 min). There were no major complications. One patient who was repaired without mesh attachment to bony landmarks developed a recurrence at ten months and subsequently underwent reoperation. Patients with mesh secured to bony landmarks were recurrence free at a median follow-up of 171 days.
Conclusions
Lateral hernias present a greater challenge due to their anatomic location. An open technique with mesh fixation to bony structures is a promising solution to this complex problem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
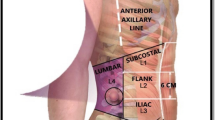
Lateral abdominal hernias are rare and their surgical management is difficult and not standardized. Most series include a small number of patients and often combine different types of non-midline hernias including inguinal and subcostal sub-groups. These lateral hernias occur in a semi-rigid space limited by the costal margin superiorly, the iliac crest inferiorly, the linea semilunaris medially, and the paraspinal muscles posteriorly. Therefore, they differ greatly from the classic midline ventral hernias from the standpoint of both their pathophysiology and repair technique.
Classic midline ventral hernias are commonly centered on defects in the tendinous sheath of the rectus muscle. A recent meta-analysis failed to find a significant difference between open and laparoscopic techniques on the basis of complications and hernia recurrences [1]. Our preferred method is the retrorectus repair described by Stoppa and Rives, which involves mesh placement between vascularized tissue planes and allows for rapid tissue ingrowth of the prosthesis. The presence of the posterior sheath in the midline provides a strong foundation to support the mesh while providing surface area for mesh overlap. In comparison to the central abdominal wall, the anatomy of the lateral wall has a smaller fascia to muscle ratio, which does not allow for the dual-layer closure as in ventral midline hernia repair [2]. The limited availability of landmarks with tensile strength to securely attach the mesh can result in a “parachuting” mesh that merely bridges the defect without the strength provided by the tendinous anchoring structures used in the repair of midline hernias.
Due to their wide neck, lateral abdominal hernias are less prone to strangulation. However, given the large muscular surface area of the lateral wall, these defects can progress rapidly and lead to cosmetic and physiological problems for the patient [3]. Unlike ventral midline hernias, whose vector forces are distributed centrally and evenly, a unilateral lateral wall defect can cause an asymmetrical shift of forces to the ipsilateral side [2]. This uneven pull from the hernia can lead to lumbar spine ligamentous strain, back pain, progressive flank herniation, changes in spinal curvature, poor bowel function, loss of core muscles, and paradoxical breathing [2, 4].
We present our experience of management of these complex lateral hernias with a special focus on surgical anatomy.
Relevant anatomy and operative technique
Surgical anatomy and classification
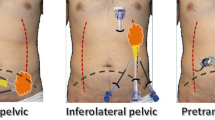
Lateral abdominal wall defects include subcostal, paramedian, flank, and lumbar triangle hernias. The most common lateral hernias are flank hernias that involve the mid-portion of the external oblique, internal oblique, and transversus abdominis as well as their attachment to the linea semilunaris and paraspinal muscles (Fig. 1). Paramedian hernias involve defects along the linea semilunaris with an intact linea alba and include Spigelian hernias. Lumbar hernias can occur in the superior or inferior lumbar triangles. The superior triangle of Grynfellt is an inverted triangle bounded by the posterior border of the internal oblique anteriorly, the anterior border of the paraspinal muscles posteriorly, and the 12th rib superiorly [3, 5]. The inferior triangle of Petit, is bounded by the posterior border of the external oblique anteriorly, the anterior border of the latissimus dorsi posteriorly, and the iliac crest inferiorly [5]. Thoracoabdominal lateral hernias are extremely rare and occur in the intercostal space secondary to rupture of the intercostal muscles (Fig. 2). They may allow the protrusion of the lung, liver, bowel, and spleen [6]. We have chosen to include them here as their management within the confines of a rigid bony space draws similarities to the lateral abdominal hernias.
Innervation of the lateral abdominal muscles (external oblique, internal oblique and transversus abdominis) arises from branches of the intercostal nerves (T7–T11), subcostal nerve (T12), and the iliohypogastric and ilioinguinal nerves (L1) [7]. The lower branches, starting at the rami of the 11th intercostal nerve, can be injured by incisions and lead to muscle denervation and loss of function [8].
True hernias with a musculotendinous defect are usually caused by denervation of the large muscles following transverse or oblique incisions of the flank for retroperitoneal surgery of the kidney, aorta, or spine [4]. These differ from bulges, as the latter are a myofascial laxity without defects. Non-operative management of bulges is a classic recommendation, but we believe that the asymmetrical functional impairment, the rapid growth of the bulge, and the severe strain on the spinal muscles warrant repair. In summary, the pathophysiology of these acquired lateral hernias is based on a traumatic denervation of lateral wall muscles with resulting amyotrophy, a disruption of the origin/insertion points, or a combination of these two factors.
Surgical technique
In the classic repair of the median ventral hernia, the technique invariably utilizes the tendinous component of the rectus sheath to centralize the rectus muscle. The addition of a component separation establishes a symmetrical and physiologically balanced and dynamic repair. Repair of lateral hernias is limited by the lack of midline fascia to anchor the mesh to. Thus, the repair of lateral hernias involves the fixation of the mesh to fixed bony structures: the costal margin superiorly and the iliac crest inferiorly. Without this anchoring, the patient may develop a bulge or hernia recurrence. We believe this crucial anchoring step is best accomplished with an open approach. Minimally invasive techniques are feasible but make fixation to the bony structures described above more technically challenging, with the potential for damage to the lateral femoral cutaneous and ilioinguinal nerves [9]. Results in the literature of the robotic approach are limited to case series [10]. As such, more patients and outcomes should be compiled to provide a higher level of evidence than expert opinion.
An oblique incision of the flank is made, the hernia defect is circumscribed, and all of the hernia contents are carefully reduced. In the case of a bulge, the external oblique is divided in the direction of its muscle fibers. Care is taken to avoid the branches of the iliohypogastric and ilioinguinal nerves. The external oblique is then separated from the underlying internal oblique. The plane between the internal oblique and the transversus abdominis must be avoided to prevent injury to the neurovascular bundle [11, 12]. The periosteum of the iliac crest and the costal cartilage are carefully exposed using electrocautery over a distance of approximately 4 to 5 cm in width and 0.5 cm deep, creating a thin strip just wide enough to hold the anchoring sutures. The internal oblique and transversus muscles are then reapproximated in an overlap fashion with non-absorbable sutures.
A lightweight, large pore, polypropylene mesh (Ultrapro®, Ethicon, Cincinnati, Ohio) is then inserted between the external oblique and internal oblique and fixed superiorly to the costal cartilage with four stitches of 2–0 polypropylene. Alternatively, a drill can be used through the ribs, or the sutures can be wrapped around the ribs if the periosteum itself is of poor quality. Care must be taken to avoid the neurovascular bundle localized at the inferior border of the rib.
Inferiorly, a Mitek system (Mitek Surgical Products, Westwood, MA) is used to drill (Stryker, Kalamazoo, MI) four holes into the iliac crest, and subsequently, four non-absorbable anchor stitches are placed to allow for the inferior suture fixation of the mesh. Posteriorly, the mesh is fixed transfascially to the paraspinal muscles and anteriorly to the edge of the external oblique or the linea semilunaris (Fig. 3). A drain is placed in this space and the external oblique is approximated over the mesh.
For thoracoabdominal hernias, the technique is more complex and involves a thoraco-abdominal incision and the opening of the chest and abdomen. The diaphragm is usually stretched by the organ protrusion and requires plication over pledgets to minimize the redundancy. The mesh is then placed as an overlay on the defect and bridged from one costal margin to the lower one, as the approximation of the ribs in large hernias is usually impossible. The mesh is fixed after drilling several holes in the costal cartilage, and subsequently, attached medially and laterally transfascially. A chest tube and a drain are placed in the pleural cavity and above the internal oblique, respectively, prior to closing.
Results
Between June 2011 and December 2017, seven patients (two male, five female) underwent elective open mesh repair by one surgeon (NK) at a tertiary medical center. Seven flank hernias (six right-sided and one left-sided) and one right thoracoabdominal hernia were repaired. One patient underwent two repairs for a total of eight procedures. The median age was 58 years (range 44–69) and the median body mass index (BMI) was 29.8 kg/m2 (range 25.8–36.9), (see Table 1 for demographics). Three hernias were recurrent and five were primary hernias. Four of the patients had incisions from previous lumbar spine surgery, one had a prior bilateral adrenalectomy, one had a prior nephrectomy, and one patient had whooping cough leading to the development of a thoracoabdominal hernia. One patient was our own recurrence, as the mesh had not been affixed to bone superiorly and inferiorly. The patient subsequently developed a recurrence at ten months that was repaired with the attachment of mesh with sutures to the costal margin and to the iliac crest using bone anchors. The remaining seven surgical cases were repaired using the same technique of securing the mesh to bony landmarks. Due to his morbid obesity, the patient with the thoracoabdominal hernia underwent a sleeve gastrectomy prior to his hernia reconstruction. His hernia was repaired after a 50-lb weight loss four months after the gastrectomy.
The median defect size was 105 cm2 with a range of 36–625 cm2. The median operating time was 185 min (range 133–282 min). There were no intraoperative or immediate post-operative complications. Two patients developed a small seroma that required an ultrasound-guided drainage. Within the seven patients that had mesh fixation to bony landmarks, there have been no recurrences at a median follow up of 171 days (range of 74–1200 days). One patient presented with a complaint of an abdominal wall bulge which on exam was found to be laxity likely related to having had multiple surgical procedures as there was no fascial defect.
Discussion
Lateral abdominal hernias are rare occurring in 1–4% versus 14–19% for midline incisional hernias [13,14,15]. Although rare, these hernias are distressing to patients because of the asymmetrical deformity of the flank and the progressive growth of the hernia. The resulting back pain due to the strain on the spinal muscles is another reason for prompt consultation.
Most published series include a small number of patients, but more importantly, combine several types of hernias under the description of non-midline hernias [16]. Previous authors have reported on the difficulty of defining a standardized technique. We have chosen to focus on a specific group of lateral hernias that share common characteristics. These lateral hernias occur in a confined space bordered by rigid bony structures and thus a repair that does not involve fixation to cartilage superiorly and inferiorly will likely recur as in our first patient.
Philips and Rosen prefer the retromuscular preperitoneal repair of flank hernias to achieve a large mesh overlap, thus avoiding the creation of skin flaps [17]. They caution against the possibility of injury to retroperitoneal vascular and nerve structures. We believe that this wide retroperitoneal dissection carries an inherently significant potential for morbidity, as was the case with one patient in their series who sustained a ureteral injury. One other patient required a blood transfusion of two units, most likely in relation to a hematoma due to the wide preperitoneal dissection. This risk is also increased in patients with coagulopathy.
There are very few published laparoscopic series of lateral hernia repair. Heniford described the first laparoscopic approach for lumbar hernias in 1997 [18]. Moreno-Egea presented the largest series of laparoscopic treatment of lateral hernia, including inguinal and subcostal defects [16]. His intraoperative morbidity was 7.5%, bleeding complications occurred in 25%, and the average length of stay was 3 days. In addition, he reported a 40% “post-operative weakness” which is most likely a parachuting of the bridging mesh leading to mesh eventration. The size of the defects treated was also much smaller than in the series of the open approach. We agree, therefore, with Philips and Rosen that lateral hernias are best approached through an open repair, avoiding the extensive enterolysis and allowing for muscle approximation that addresses the skin deformity.
Veyrie and Stumpf insisted that the space between the transversus abdominis and the internal oblique should not be opened to avoid injuring the major thoracoabdominal neurovascular bundle that runs in this plane [11, 12]. The ideal space of dissection and positioning of the mesh is between the external oblique and the internal oblique. Some authors have raised concerns about the potential lack of tissue ingrowth between the mesh and the bone [17], but we have not observed this problem with longer follow-up. Veyrie uses a technique similar to ours and places the mesh in the same space anchored to the iliac bone. His superior fixation, however, is muscular only and does not use the costal cartilage. This could explain a recurrence rate of 5% in a comparable follow-up time to ours. Baumann in a recent review article recommended the same bony fixation superiorly and inferiorly to the costal margin and the iliac crest as in our patients [2]. Blair recently reported on the use of bone anchors for inferior iliac bone mesh fixation in patients with lumbar and suprapubic hernias [19]. No osteomyelitis was noted and in broader terms, this complication while described rarely in Urology in the treatment of incontinence was not observed in the treatment of ventral hernias.
We routinely use a lightweight, large pore polypropylene mesh (Ultrapro®, Ethicon, Cincinnati, Ohio), and have not encountered any mesh infection or superficial surgical site infection. The incidence of infection in other series using other types of mesh, especially polyester (69% of all meshes used), was 6% for superficial wound infection and 6% for mesh infection requiring debridement [17]. Another study demonstrated a mesh infection rate of 6.9% using acellular dermal matrix [20]. A recent systematic review concluded that due to the heterogeneity of operative technique and low patient numbers, it was difficult to make any meaningful comparison of mesh-related outcomes [21]. To our knowledge, there are no studies with large enough cohorts to effectively compare outcomes of lateral hernias depending on mesh type.
Our study has some limitations with the small number of patients included and a relatively short follow-up time. However, given the successful results obtained and the high patient satisfaction, we believe that this technique is durable and has become our routine standard for the repair of lateral hernia defects and bulges of the abdominal wall.
Conclusions
Compared to midline hernias, lateral hernias present a greater challenge for surgeons due to their anatomic location. An open technique with mesh fixation to the bony structures may represent a durable solution to this complex problem and deserves further evaluation.
References
Dietz UA, Menzel S, Lock J, Wiegering A (2018) The treatment of incisional hernia. Deutsches Aerzteblatt. https://doi.org/10.3238/arztebl.2018.0031
Baumann DP, Butler CE (2012) Lateral abdominal wall reconstruction. Semin Plast Surg 26(1):40–48. https://doi.org/10.1055/s-0032-1302465
Stamatiou D, Skandalakis JE, Skandalakis LJ, Mirilas P (2009) Lumbar hernia: surgical anatomy, embryology, and technique of repair. Am Surg 75(3):202–207
Moreno-Egea A, Baena EG, Calle MC, Martinez JA, Albasini JL (2007) Controversies in the current management of lumbar hernias. Arch Surg 142(1):82–88. https://doi.org/10.1001/archsurg.142.1.82
Macchi V, Porzionato A, Morra A, Picardi EEE, Stecco C, Loukas M, Tubbs RS, De Caro R (2016) The triangles of Grynfeltt and Petit and the lumbar tunnel: an anatomo-radiologic study. Hernia. https://doi.org/10.1007/s10029-016-1509-3
Wigley J, Noble F, King A (2014) Thoracoabdominal herniation but not as you know it. Ann R Coll Surg Engl 96(5):e1–e2. https://doi.org/10.1308/003588414x13814021679032
Standring S (2015) Gray's anatomy E-book: the anatomical basis of clinical practice. Elsevier Health Sciences, Amsterdam
Gardner GP, Josephs LG, Rosca M, Rich J, Woodson J, Menzoian JO (1994) The retroperitoneal incision. An evaluation of postoperative flank 'bulge'. Arch Surg 129(7):753–756
Dakin GK, Kendrick ML (2013) Challenging hernia locations: flank hernias. The SAGES manual of hernia repair. Springer, Berlin, pp 531–540
Beffa LR, Margiotta AL, Carbonell AM (2018) Flank and lumbar hernia repair. Surg Clin North Am 98(3):593–605. https://doi.org/10.1016/j.suc.2018.01.009
Veyrie N, Poghosyan T, Corigliano N, Canard G, Servajean S, Bouillot JL (2013) Lateral incisional hernia repair by the retromuscular approach with polyester standard mesh: topographic considerations and long-term follow-up of 61 consecutive patients. World J Surg 37(3):538–544. https://doi.org/10.1007/s00268-012-1857-9
Stumpf M, Conze J, Prescher A, Junge K, Krones CJ, Klinge U, Schumpelick V (2009) The lateral incisional hernia: anatomical considerations for a standardized retromuscular sublay repair. Hernia 13(3):293–297. https://doi.org/10.1007/s10029-009-0479-0
Halm JA, Lip H, Schmitz PI, Jeekel J (2009) Incisional hernia after upper abdominal surgery: a randomised controlled trial of midline versus transverse incision. Hernia 13(3):275–280. https://doi.org/10.1007/s10029-008-0469-7
Greenall MJ, Evans M, Pollock AV (1980) Midline or transverse laparotomy? A random controlled clinical trial. Part II: Influence on postoperative pulmonary complications. Br J Surg 67(3):191–194
Kendall SW, Brennan TG, Guillou PJ (1991) Suture length to wound length ratio and the integrity of midline and lateral paramedian incisions. Br J Surg 78(6):705–707
Moreno-Egea A, Carrillo A, Aguayo JL (2008) Midline versus nonmidline laparoscopic incisional hernioplasty: a comparative study. Surg Endosc 22(3):744–749. https://doi.org/10.1007/s00464-007-9480-9
Phillips MS, Krpata DM, Blatnik JA, Rosen MJ (2012) Retromuscular preperitoneal repair of flank hernias. J Gastrointest Surg 16(8):1548–1553. https://doi.org/10.1007/s11605-012-1890-x
Heniford BT, Iannitti DA, Gagner M (1997) Laparoscopic inferior and superior lumbar hernia repair. Arch Surg 132(10):1141–1144
Blair LJ, Cox TC, Huntington CR, Ross SW, Kneisl JS, Augenstein VA, Heniford BT (2015) Bone anchor fixation in abdominal wall reconstruction: a useful adjunct in suprapubic and para-iliac hernia repair. Am Surg 81(7):693–697
Pezeshk RA, Pulikkottil BJ, Bailey SH, Schaffer NE, Reece EM, Thornton NJ, Gupta AR, Hoxworth RE (2015) An evidence-based model for the successful treatment of flank and lateral abdominal wall hernias. Plast Reconstr Surg 136(2):377–385. https://doi.org/10.1097/PRS.0000000000001432
Zhou DJ, Carlson MA (2018) Incidence, etiology, management, and outcomes of flank hernia: review of published data. Hernia 22(2):353–361. https://doi.org/10.1007/s10029-018-1740-1
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
All procedures performed in studies involving human participants were in accordance with The ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Human and animal rights
This study does not include any animal trial.
Informed consent
For this type of article, informed consent is not required.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Katkhouda, N., Alicuben, E.T., Pham, V. et al. Management of lateral abdominal hernias. Hernia 24, 353–358 (2020). https://doi.org/10.1007/s10029-020-02126-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-020-02126-5