Abstract
At the local (within site) scale, soil heterogeneity can influence ecosystem function. However, in former agricultural systems, soil heterogeneity can be low as a legacy of tillage (that is, via the process of soil homogenization). We investigated the relationship between soil homogenization and three ecosystem functional response variables—aboveground productivity, nitrogen retention (assessed via 15N tracer addition) and plant litter decomposition (assessed using litter bags)—during the first 2 years following a tallgrass prairie restoration in a former agricultural field. We compared plots with substrate heterogeneity (topsoil patches enriched with sand or woodchips) with homogeneous plots that contained the same overall ratios of the component materials. We also compared plots with topographic heterogeneity (pits and mounds) with flat plots. The ecosystem response variables varied significantly with soil homogenization, but the effect depended on the type of heterogeneity (sand vs. woodchips vs. microtopography), which was consistent with these sources of heterogeneity having variable effects on soil water and nutrient availability. Soil homogenization reduced aboveground productivity by 50% for the woodchip treatment, but it had the opposite effect for the topography treatment. Homogenization also reduced plant 15N retention for the woodchip treatment by 50%, but the rate of litter decomposition increased by 8%. Overall, variation in nitrogen retention and decomposition were associated with the treatment effects on aboveground productivity and the relative abundances of forbs and grasses. The latter results suggest that the influence of soil homogenization on ecosystem function may occur indirectly as a result of its effects on plant community assembly. Moreover, forb productivity and N retention along woodchip and mound edges differed from the means of the adjacent patches, which revealed that complementarity along microsite edges can contribute to soil heterogeneity effects on ecosystem function.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
-
Soil homogenization alters ecosystem-level processes via changes to plant species composition.
-
Microsites and microedges in heterogeneous soils facilitate functional complementarity.
-
Adding soil microsites to former cropland can promote recovery of ecosystem function.
Introduction
In North America, less than 1% of native tallgrass prairie remains, largely due to conversion to agriculture (Sampson and Knopf 1994). Ecological restoration of tallgrass prairie and other native grassland ecosystems is occurring worldwide, with the goal of restoring ecosystem functions and biodiversity (Neill and others 2015; Horrocks and others 2016). However, restoration of former cropland must address a legacy of soil disturbance (Krause and others 2016). In particular, soil homogenization from decades of tillage (mixing of the upper topsoil) increases the uniformity of habitat and soil properties in agricultural fields (Anderson and Coleman 1985; Elliott 1986). Productivity, nitrogen retention and decomposition are important components of ecosystem function, because they can regulate species composition and carbon storage (de Vries and Bardgett 2016; Zirbel and others 2017). Greenhouse experiments conducted using temperate grassland mesocosms have demonstrated that heterogeneous soil nutrient supply can increase above and belowground productivity, nitrogen use efficiency and nitrogen uptake via root proliferation into nutrient-rich patches (Maestre and others 2005, 2006, 2007; Maestre and Reynolds 2006a, b, 2007a, b; Liu and others 2017). Although field experiments in restored grasslands have been used to examine the relationship between soil heterogeneity and plant species diversity (Richardson and others 2012; Williams and Houseman 2014; Baer and others 2016), they have not examined the influence of soil heterogeneity (or homogenization) on ecosystem responses (García-Palacios and others 2012). The question therefore arises as to how soil homogenization may alter the ecosystem functioning of restored grassland ecosystems on former agricultural land.
Loss of distinct soil patches via soil homogenization could decrease productivity, ecosystem nitrogen retention and decomposition indirectly as a result of decreased plant species diversity. Niche theory suggests that a large number of species can coexist in an ecosystem where there is an abundance of distinct niches available for species to colonize and differentially dominate (Tilman and Pacala 1993). Environmental heterogeneity therefore can increase species diversity (Stein and others 2014) and increased species diversity can in turn benefit ecosystem function (Hooper and others 2005). Heterogeneity can improve ecosystem function by providing diverse niches for complementarity in resource use to occur (Tylianakis and others 2008). Conversely, reduced plant diversity could decrease the variability of rooting depths, rooting phenology and forms of nitrogen uptake in the community, decreasing overall nitrogen retention (McKane and others 2002). Similarly, the rate of decomposition can decrease with reduced species diversity, because the litter pool lacks structural and chemical diversity, as observed in litter mixture experiments, where the litter of individual species decomposes slower than that of multiple species mixes (Gartner and Cardon 2004).
Loss of plant functional group diversity, rather than species diversity, may impair ecosystem function most in the context of soil homogenization. For example, in pot experiments, the presence of specific plant functional groups facilitated an increase in productivity in response to increased soil nutrient heterogeneity, whereas manipulation of plant species diversity did not (Maestre and others 2006). Likewise, while carbon, phosphorus and nitrogen cycling (measured via beta-glucosidase and acid phosphatase enzyme activity, and in situ N availability) were not influenced directly by soil nutrient heterogeneity (García-Palacios and others 2011), specific plant functional groups and traits had large effects on the cycling of these nutrients in response to nutrient heterogeneity (García-Palacios and others 2013).
Nevertheless, soil homogenization could decrease ecosystem functioning directly by decreasing substrate diversity and heterogeneity (that is, by decreasing the frequency and spatial variability of distinct soil patches, otherwise known as microsites). For example, decomposition rates can vary with changes in soil moisture (Zirbel and others 2017), and variability in soil moisture can result from microtopographic heterogeneity (hummocks and hollows) and variability in soil depth to bedrock (Fridley and others 2011; Naeth and others 2018). Moreover, the interface between two microsites (microsite edges or “microedges”) may exhibit ecosystem functional properties that are not a simple additive effect of the adjacent microsites by possessing a blend of limiting resources from each neighboring microsite (Stover and Henry 2018). For example, soil microbial activity limited by a nutrient in one patch may be increased by the higher availability of the nutrient in a neighboring patch. Such complementarity along microedges would be analogous to the landscape-level phenomenon of increased elemental cycling in wetland–upland transition zones (McClain and others 2003).
We investigated the influence of soil homogenization on productivity, nitrogen retention and decomposition in a field experiment conducted in a former agricultural field restored to a grassland. We also examined how levels of productivity, decomposition and nitrogen retention along microedges (that is, the edges between patches in heterogeneous treatments) compared to the levels of the adjacent patches. We added patches of sand or woodchips to the soil to construct heterogeneous plots, and added the same materials, but mixed them, to construct corresponding homogenized plots. We also created patches with microtopographic relief (that is, pits and mounds) to construct heterogeneous plots, and we compared these with flat plots. To prevent the effect of homogenization from being confounded with differences in overall substrate composition, a key feature of our design was that overall substrate composition remained consistent at the plot level among the corresponding homogeneous and heterogeneous plots.
Although our experiment was designed to examine the mechanism of homogenization, and not designed to simulate the loss of naturally occurring soil patches per se, the sources of heterogeneity we examined (that is, soil particle size, organic matter and topography) nevertheless represent sources of variation commonly encountered in soil (for example, patches of organic matter are deposited via decaying plants); woody materials and microtopographic variation also are often used in soil and plant community restoration (Brown and Naeth 2014). Although the addition of sand would be expected to reduce both soil water holding capacity and nutrient availability, the addition of woodchips would be expected to increase soil water holding capacity and the soil nutrient immobilization potential. Microtopographic variation would be expected to increase variation in soil water content. Based on the direct and indirect mechanisms described above, we predicted that soil homogenization would decrease productivity, nitrogen retention and plant litter decomposition. Moreover, due to potential complementarity between adjacent microsites, we predicted that the productivity, nitrogen retention and plant litter decomposition of microedges would be greater than the mean values of the adjacent microsites.
Materials and Methods
Study Site
We conducted this study at the Environmental Sciences Western field station, located near Ilderton, Ontario, Canada (43o04′29″N, 81o20′18″W). The site had a mean air temperature of 7.9 °C and annual precipitation of 1012 mm (1981–2010 Canadian Climate Normals), and the soil was characterized as Bryanston silt loam, which is a Brunisolic Gray Brown Luvisol, and it had a mean pH of 7.5 (Hagerty and Kingston 1992; Environment and Climate Change Canada 2018). The region was rural, and the 4 ha research site was situated in a field formerly used for cash cropping under rotations of corn, soybean and winter wheat for decades until 2014.
Experimental Design
In May 2015, we established 18 experimental blocks at the field site. Each block contained homogeneous and heterogeneous treatment plots that were 50 × 100 cm and spaced 2 m apart (Figure 1). Heterogeneous plots were divided into two distinct halves: a 50 × 50 cm and 15 cm deep patch of tilled topsoil (mixed to a depth of 15 cm with shovels) and an adjacent 50 × 50 cm patch designed to provide either substrate heterogeneity (that is, a topsoil patch enriched with sand or a topsoil patch enriched with woodchips) or topographic heterogeneity (that is, a pit or a mound). The edge between the two patches in the center of each plot was defined as the microedge (Figure 1). Therefore, the heterogeneous plots had three distinct sampling areas (microsites): a topsoil patch, a microedge and a distinct microsite (sand–topsoil patch, woodchip–topsoil patch, pit or mound). The sand–topsoil patches were a 4:1 mixture of sand and topsoil, and the woodchip–topsoil patches were a 2:1 mixture of woodchips and topsoil. The pits were 15 cm in depth, and the mounds were 20 cm in height. The pits were underlain with 15 cm of topsoil and mounds with subsoil to make their substrate depth profiles equivalent to the other side of the plot (Figure 1). For each heterogeneous plot in a block, there was a corresponding homogeneous plot: The sand and woodchip plots were compared to plots with the corresponding ratios of topsoil, sand or woodchips tilled and mixed thoroughly across the entire 50 × 100 cm plot area, and the topographically heterogeneous plots were compared to a flat, tilled topsoil plot (Figure 1). Sand was obtained from a local quarry (calcium carbonate sand derived from limestone) and woodchips from sugar maple (Acer saccharum) located on site. The patch size (spatial scale of heterogeneity) was chosen to approximately match the rooting footprint of the herbaceous grassland plants used in the experiment. This approach was anticipated to maximize diversity based on the expectation it would promote species coexistence via the preferential occupation of separate patches by distinct suites of species (Tilman and Pacala 1993; Chesson 2000; Day and others 2003; Stover and Henry 2018).
In early June 2015, an equal amount of tallgrass prairie grass and forb seeds were sown on each plot. The areas outside the plots were sown separately with the same tallgrass prairie species. To increase the species pool, seeding of the plots was conducted a second and final time in late May 2016. Seeds were obtained by wild collection from local sites in southern Ontario or by purchase of regionally similar ecotypes from Ernst Conservation Seeds (Meadville, Pennsylvania, USA). Percent viable seed was determined either by using certificates obtained at the time of purchase (which listed percent germination) or, for wild collected seed, by sowing a predetermined amount of seed on potting soil-filled trays in a greenhouse, counting emerging seedlings for 14 days, and dividing the number of emerging seedlings by the number sown and multiplying by 100 to calculate percent emergence (Supplementary information Table S1).
Soil Properties
We assessed the properties of the sand, woodchips and topsoil used to create the homogeneous and heterogeneous treatment plots (Table S2). Topsoil was sampled by taking a 2-cm diameter by 15 cm deep soil core from a random location within each block just outside the study plots. Sand and woodchips samples were collected randomly from the stockpiles on site. Sand–topsoil and woodchip–topsoil mixtures were prepared for analysis using the same ratios as present in the experimental plots. Soil pH was measured by mixing 10 g of soil from each sample with deionized water in a 1:2 ratio to create a slurry and analyzing the slurry with a pH meter. Extractable NH4+ and NO-3 were measured by extracting 7 g of each soil sample in 35 ml of KCl for 1 h on a shaker, filtering through pre-leached cellulose filter paper, then analyzing the filtrate colorimetrically (NH4+-N: EPA method 353.2; NO3−-N: EPA method 350.1) using a SmartChem 140 discrete auto-analyzer (Westco Scientific Instruments, Brookfield, CT). Percent sand, silt and clay were determined for a 20-g subsample using a graduated cylinder, and soil organic matter as loss on ignition following drying of 1 g of the sample in a muffle furnace at 500 °C for 24 h. Total carbon and nitrogen analyses were conducted by the University of Western Ontario Biotron facility using a vario isotope cube (Elementar Analysensysteme GmbH, Langenselbold Germany).
Productivity and Diversity
In November 2016, aboveground shoots rooted in the heterogeneous plots were harvested by overlaying three sampling quadrats (25 × 25 cm), with one in the center of each distinct patch and one on the center of the edge between the patches. Aboveground shoots were harvested from the homogeneous plots using the same quadrats and positioning. Shoots were sampled during the second growing season following succession from bare cropland. Litter from the first growing season was relatively sparse (that is, it largely represented the first-year growth of the perennial species that established from seed) and it was easy to visually identify and exclude the previous year’s litter from the samples. Shoots were sorted into three functional group categories: grass, non-leguminous forb (forb) and leguminous forb (legume). Samples were dried at 60 °C until their mass became constant (approximately 4 days) and weighed. The total mass of aboveground shoots (grasses, forbs and legumes) was used to estimate aboveground productivity. The relative abundances of grasses, forbs and legumes were used to estimate functional group diversity. Species diversity and composition data are reported in Stover (2018).
Nitrogen Retention
Growing season (over summer) nitrogen retention was assessed by applying a 15N tracer solution (15NH154NO3 at a rate of 0.054 g 15N m−2) evenly over each plot on June 24, 2016. In November 2016, one soil core (2 cm diameter and 20 cm depth) was collected from the center of each homogeneous plot, and three were collected from each heterogeneous plot: one in the center of each distinct patch and one in the center of the edge between the two patches. We sampled to 20 cm depth to match the depth at which the roots of the grassland species were most heavily concentrated in the soil profile, and the depth at which the soils were manipulated in our experiment (15–20 cm depth; the mounds were 20 cm in height underlain by subsoil). Aboveground shoots and soil cores were sampled from outside the research blocks to provide a set of non-enriched control samples to establish the natural background level of 15N at the site.
Soil samples were dried at 60 °C until their masses became constant (approximately 4 days) and weighed. The grass, forb and legume aboveground biomass samples were bulked into a single sample for 15N analyses. Soil and biomass samples were ground and weighed into tin capsules (4 ± 0.5 mg subsamples for plant material and 40 ± 3 mg for soil). Soils were ground using a mortar and pestle, and plant samples were ground using a ball mill (SPEX Sample Prep Model 2000 Geno/Grinder, Metuchen, New Jersey, USA). The capsules were sent to the University of California Davis Stable Isotope Facility, where 15N, total N (atom%15N and atom%N) and total C (atom%C) were measured with a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20-20 isotope ratio mass spectrometer (Sercon Ltd., Cheshire, UK). The atom%15N natural abundance was estimated from control samples (0.367012 for plant and 0.36858% for soil) and subtracted from enriched samples to determine atom% excess 15N (de Vries and others 2012):
- (1)
atom% excess 15N = atom%15N enriched − atom%15N natural abundance
Percent sample excess 15N was then calculated as follows:
- (2)
% sample excess 15N = (atom% excess 15N/100) × % sample total N
The % sample excess 15N was then expressed as an amount (mass) of excess 15N per unit area:
- (3)
15N aboveground pool (g/m2) = (% sample excess 15N/100) × total aboveground biomass (g/m2)
- (4)
15N belowground pool (g/m2) = (% sample excess 15N/100) × mass of soil (g/m2)
Percent 15N retained was then calculated using the 15N tracer application rate (0.054 g 15N/m2) as follows:
- (5)
% 15N retention = (0.054 g 15N/m2 − 15N pool g/m2)/0.054 g 15N/m2 × 100
Over summer nitrogen retention was estimated by calculating the percent of 15N tracer added that was retained over the 2016 growing season (% 15N retention) in aboveground and belowground pools.
Decomposition
We initiated a decomposition experiment in the plots in fall 2015. Donor litter was collected from a nearby tallgrass prairie restoration site on November 3, 2015, because sufficient litter was not yet available at the recently planted research site. Andropogon gerardii Vitman (Big Bluestem) tussock leaf litter was collected from several plants over a 100 m2 area to a total of approximately 500 g (dry weight). The litter was collected on a hot, dry, sunny day and spread thinly to air dry for 1 week. Andropogon gerardii litter was chosen because it is a dominant species in tallgrass prairie, and it provided a uniform source of litter. Decomposition was assessed using 10 × 5 cm litterbags constructed using fiberglass window screen mesh sealed with hot glue (mesh hole size: 1 mm to balance exclusion of soil fauna against sample loss). Three hundred milligrams of litter were weighed and placed in each litter bag. Litter bags were positioned on the soil surface of the plots the week of November 23, 2015. Homogeneous plots had one litter bag in the center and heterogeneous plots had three litter bags (one in the center of each distinct patch and one on the center of the edge between the patches). In fall 2016 (1 year after placement), the litter bags were collected, oven-dried and reweighed. The final mass of the dry litter (mass remaining) was used to calculate percent mass loss: initial mass—final mass/initial mass × 100. Prior to drying and reweighing, the litter bags were gently rinsed with distilled water to clean off soil and debris.
Statistical Analyses
The experimental design (Figure 1) was a randomized complete block design with a hierarchical nested structure: quadrats (subplots) nested within plots nested within blocks. For the statistical analyses, homogenization was a fixed effect with two levels (homogeneous and heterogeneous). Subplot was a fixed effect with three levels unique to each heterogeneous plot to examine the effect of soil microsite (topsoil patch, microedge and sand/woodchips/pit/mound). The plots and blocks were defined as nested random effects. Our initial statistical model containing the above parameters was too complex for the data and did not converge, primarily due to heteroscedasticity of variance among the three heterogeneity sources (woodchips, sand and topography). Thus, the data for each source of heterogeneity were analyzed separately using linear mixed models with block as a random effect and homogenization as a fixed effect. Three separate models were used, one for each source of heterogeneity: (1) sand homogeneity versus sand heterogeneity, (2) woodchip heterogeneity versus woodchip homogeneity and (3) topographic heterogeneity, with three levels (pit plots, mound plots and flat topsoil plots). For the response variables, the three measurements collected for each heterogeneous plot were averaged to compare with the measurements from the corresponding homogeneous plots.
Within each of the four heterogeneous plots, mean levels of each response variable were compared among the topsoil patch, microedge and microsite (sand–topsoil patch, woodchip–topsoil patch, pit or mound). Four separate models were used, with one for each heterogeneous plot: (1) sand heterogeneous (topsoil patch, microedge and sand–topsoil patch), (2) woodchip heterogeneous (topsoil patch, microedge and woodchip–topsoil patch), (3) topographic heterogeneity: pit plots (topsoil patch, microedge and pit) and (4) topographic heterogeneity: mound plots (topsoil patch, microedge and mound). For significant treatment effects, an a priori contrast (assessed with a t test) was used to compare the heterogeneous mean to the homogeneous mean. To determine if the ecosystem properties along microedges were non-additive, an a priori contrast also was used to compare among the microedge and the mean of the two adjacent patches. A log10(y + 1) transformation was used for cases when residuals did not meet assumptions of normality or homogeneity of variance. Statistical analyses were conducted with R v. 3.3.3 (R Core Team 2017). Alpha was 0.05, but p values < 0.1 were reported as marginally significant, because of the inherently high variability in plant biomass and species composition among plots.
Results
Productivity
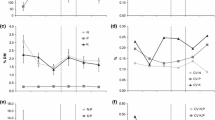
Total aboveground biomass and the biomass of individual plant functional groups varied significantly between homogeneous and heterogeneous plots, but the effect depended upon the type of heterogeneity (topography versus sand versus woodchips). Specifically, in the woodchip treatment, soil homogenization significantly decreased total aboveground biomass by approximately 50% (p = 0.028, t34 = 2.3) (Figure 2). The topsoil and microedge in woodchip heterogeneous plots had significantly greater biomass than the woodchip microsite (Table 1). Forb productivity along the woodchip microedges was marginally significantly greater than the average of the adjacent woodchip and topsoil patches (p = 0.097, t34 = − 1.71).
Mean total (A), forb (B) and grass (C) aboveground biomass (grams per plot: sum of the three 25 × 25 cm sampling quadrats). Means were back-transformed from the log scale log10(y + 1) and standard error in the positive direction is shown. Within each source of heterogeneity, heterogeneous treatments followed by * (p < 0.05), ** (p < 0.01) and *** (p < 0.001) are significantly different from the homogeneous treatment and + are marginally significantly different (p < 0.1).
In the topography treatment, flat topsoil had 50 and 30% more total aboveground biomass and 75 and 50% more forb biomass compared to the pit and mound plots, respectively (total p = 0.0006, t50 = 3.65 and p = 0.05, t50 = 1.98, respectively, and forb p = 0.001, t50 = 3.49 and p = 0.06, t50 = 1.92, respectively) (Figure 2A, B). Pit and mound microsites had less than half the biomass of the topsoil patches present in the topographically heterogeneous plots (Table 1). Within the mound heterogeneous plots, forb productivity along the microedges was marginally significantly greater than the average of the adjacent mound and topsoil patches (p = 0.067, t34 = − 1.9).
Soil homogenization marginally significantly decreased grass biomass in the sand treatment by 50% (p = 0.057, t17 = 2.04) (Figure 2C). Topsoil microsites in the sand heterogeneous plots had the greatest grass biomass, but they were not significantly different than the microedge and sand patches (Table 1). There were no treatment effects on legume biomass; the latter was extremely low (mostly zero) in all samples.
Nitrogen Retention
Soil homogenization altered nitrogen retention, but the effect varied based on the source of homogenization, and it varied aboveground versus belowground. Aboveground nitrogen retention significantly decreased by approximately 50% with homogenization for the woodchip treatment (p = 0.047, t22 = 2.1) (Figure 3A). As with productivity, topsoil and microedges in the woodchip heterogeneous plots had significantly greater retention compared to the woodchip patches (Table 2). Woodchip microedges had similar retention to topsoil patches alone, but marginally significantly greater retention than the average of the woodchip and topsoil patches (p = 0.072, t33 = − 1.86) (Table 2). Aboveground nitrogen retention increased by approximately 50% with homogenization for the topography treatment (pit p = 0.01, t50 = − 2.5 and mound p = 0.07, t50 = − 1.9, respectively) (Figure 3A). Belowground nitrogen retention was approximately double the aboveground retention overall, but there were few significant treatment effects (Figure 3B). Belowground nitrogen retention decreased in flat homogeneous plots by 40% compared to the mound topography treatment (p = 0.036, t30 = 2.2) (Figure 3B). Neither aboveground nor belowground retention was significantly different among microsites present within the pit and mound heterogeneous plots (Table 2).
Percent 15N retained (over summer/growing season nitrogen retention) in A aboveground and B belowground pools. Means were back-transformed from the log scale log10(y + 1) and standard error in the positive direction is shown. Within each source of heterogeneity, heterogeneous treatments followed by * (p < 0.05) are significantly different from the homogeneous treatment and + are marginally significantly different (p < 0.1).
Belowground total nitrogen decreased by approximately 20% with homogenization for the sand treatment (p = 0.01, t17 = 3) (Table 3). Belowground total nitrogen was greatest in the topsoil patch in the sand heterogeneous plots, and the microedge was intermediate between the sand and topsoil patches (Table 2). Soil homogenization increased belowground total nitrogen by approximately 8% for the topography treatment (pit compared to flat topsoil, p = 0.002, t33 = − 3.5) (Table 3). Belowground total nitrogen was lowest in the pits within the heterogeneous plots, and the microedge was intermediate between the pit and topsoil patches (Table 2). Belowground total nitrogen was lowest along the mound microedges (Table 2).
Decomposition
Homogenization increased mass loss by 8% in the woodchip treatment (p = 0.026, t24 = 2.37) (Figure 4). Mass loss was similar (averaging around 35%) among topsoil, microedge and woodchip patches in the woodchip heterogeneous plots (Table 4). Within the sand heterogeneous plots, mass loss was significantly lower in sand patches compared to topsoil patches (Table 4). No other significant treatment effects on mass loss were observed.
Mean percent mass loss in litter bags for decomposition experiment. Initial mass was 0.3 g and percentage mass loss after 12 months is presented. Higher mass loss infers greater decomposition rate. Error bars are standard error. Within each source of heterogeneity, heterogeneous treatments followed by * are significantly different from the homogeneous treatment (p < 0.05).
Discussion
We hypothesized that soil homogenization would decrease productivity, nitrogen retention and decomposition. Such a result would be consistent with studies of variation in soil nutrient heterogeneity, where decreased nutrient heterogeneity resulted in decreased productivity and decreased plant nitrogen uptake (Maestre and others 2005, 2006, 2007; Maestre and Reynolds 2006a, 2007a). Although our results demonstrated that these ecosystem responses indeed varied with soil homogenization, the direction of the responses varied based on the source of heterogeneity. Specifically, homogenization decreased productivity when woodchips were used to create heterogeneity, it increased productivity for the microtopography treatment and it resulted in no overall change in productivity in the sand treatment. Such variation in responses might be explained by the differences among these sources of heterogeneity in their likely effects on soil nutrient and water availability; for example, as described above, sand and woodchips could have opposing effects on soil water holding capacity, woodchips could increase nutrient immobilization, and microtopographic variation increases variation in soil water content. We also hypothesized that the ecosystem responses along microedges would differ from the mean responses of the adjacent microsites. Microedges in the woodchip and mound heterogeneous treatments exhibited greater forb productivity and aboveground N retention than the average of their neighboring patches, which revealed that microedges can be an important mechanism whereby soil heterogeneity can alter ecosystem function.
Overall, a high abundance of forbs and high overall productivity were associated with increased aboveground N retention. Homogenization, via a reduction in productivity, decreased aboveground N retention in the woodchip treatment. Heterogeneous woodchip plots had a patch of topsoil that featured high aboveground productivity, whereas homogeneous woodchip plots had woodchips spread throughout, which suppressed plant growth, possibly as a result of increased nutrient immobilization (the latter was consistent with reduced aboveground 15N recovery in these plots). The exact opposite response occurred in the topography treatment, where flat topsoil plots (the homogenization treatment) had higher aboveground biomass than the pits and mounds, where growth was suppressed. Pits also had lower belowground nitrogen than the flat topsoil plots, which may have occurred due to the suppression of root growth in response to the wetter conditions.
Our findings for aboveground N retention revealed that the abundance of the most productive functional group (forbs) predicted the outcome, which in line with the dominant plant species controlling ecosystem processes (that is, the mass ratio hypothesis—Grime 1998). However, changes in functional group diversity (the relative abundances of grasses, forbs and legumes) caused by soil homogenization also were associated with increased aboveground productivity and nitrogen retention, which is consistent with previous studies that have shown that functional group diversity is a strong predictor of ecosystem function (Maestre and others 2006; de Vries and Bardgett 2016). For the heterogeneous plots in the sand treatment, belowground nitrogen increased along with increased grass abundance. Grasses develop dense root systems and therefore promote high N retention in mature ecosystems (Phoenix and others 2008; Suding and others 2008). The heterogeneous conditions in the sand treatment also promoted increased diversity of plant functional groups (that is, a more balanced community of grasses and forbs compared to the forb-dominated homogenized plots). The latter resulted in increased complementarity, with the increased belowground sequestration of N from the dense grassroots being combined with the large N pool present in the “top-heavy” forbs.
Topographic heterogeneity can promote the development of temporal and spatial variability in vegetation cover in grassland restoration (Biederman and Whisenant 2011). In the mound heterogeneous plots, belowground nitrogen retention was significantly greater than in the flat topsoil plots, with forbs being most abundant along the mound edges and grasses most abundant in the topsoil patches. Thus, increased complementarity afforded by increased functional group diversity appeared to drive increased belowground N sequestration. Mounds also can increase forb production during grassland restoration by increasing water infiltration rates (Grant and others 1980; Naeth and others 2018). Moreover, mounding can directly affect nutrient cycling (Hough-Snee and others 2011), with the elevated temperatures on mounds increasing decomposition and nutrient availability (Walker and del Moral 2003; Bruland and Richardson 2005), which may have contributed to the increased belowground N retention in our study.
Consistent with our prediction, homogenization decreased litter decomposition in the mound treatment (albeit not significantly), whereas contrary to our prediction, litter mass loss increased significantly with homogenization in the woodchip treatment. Similar to the productivity and N retention results, these contrasting outcomes highlight the importance of the type of heterogeneity in determining the responses of ecosystem processes. In the case of the woodchip treatment, increased soil nutrient immobilization in the woodchip-dominated patch could have decreased decomposition rates in the heterogeneous plots.
Overall, the lowest rates of decomposition were observed in the plots with the greatest aboveground productivity, which suggests that a portion of the soil heterogeneity effects on decomposition may have been indirect. The ecosystem functional responses we investigated also were likely interrelated, given that the treatments with high aboveground productivity tended to have a low decomposition rates and belowground nitrogen retention, coupled with high aboveground nitrogen retention. Heterogeneous habitat conditions also can increase invertebrate species diversity and thereby affect ecosystem processes (Griffin and others 2009). In our study, macrofauna appeared to be most abundant in plots with topographic heterogeneity, and in particular, numerous ant colonies were observed on the mounds, which could further explain the increased decomposition in topographically heterogeneous plots.
Conclusions
Our study, which addressed changes in nitrogen retention, aboveground productivity and decomposition associated with soil homogenization in a grassland restoration, was the first field experiment to our knowledge to assess the relationship between soil heterogeneity and ecosystem functioning. Plant community responses to soil homogenization are clearly influential in driving the responses of ecosystem processes. To build upon this work, future field experiments could further uncover the mechanisms involved in these responses by investigating the role of plant functional traits using the response effect trait framework (García-Palacios and others 2012, 2013), or root system responses (Liu and others 2017). We demonstrated that soil homogenization has the potential to alter and increase uniformity of ecosystem properties across sites. In contrast, microsites and microedges in heterogeneous soils facilitate complementarity in plant functional groups that can increase ecosystem function. Therefore, the addition of contrasting soil patches to restoration sites could aid in restoring multiple ecosystem functions, while establishing structural diversity and biodiversity.
References
Anderson BDW, Coleman DC. 1985. Dynamics of organic matter in grassland soils. J Soil Water Conserv 40:211–6. http://www.jswconline.org/content/40/2/211.short.
Baer SG, Blair JM, Collins SL. 2016. Environmental heterogeneity has a weak effect on diversity during community assembly in tallgrass prairie. Ecol Monogr 86:94–106.
Biederman LA, Whisenant SG. 2011. Using mounds to create microtopography alters plant community development early in restoration. Restor Ecol 19:53–61.
Brown RL, Naeth MA. 2014. Woody debris amendment enhances reclamation after oil sands mining in Alberta, Canada. Restor Ecol 22:40–8.
Bruland GL, Richardson CJ. 2005. Hydrologic, edaphic, and vegetative responses to microtopographic reestablishment in a restored wetland. Restor Ecol 13:515–23.
Chesson P. 2000. General theory of competitive coexistence in spatially-varying environments. Theor Popul Biol 58:211–37. http://www.sciencedirect.com/science/article/pii/S0040580900914862. Last accessed 07/08/2014.
de Vries FT, Bardgett RD. 2016. Plant community controls on short-term ecosystem nitrogen retention. New Phytol 210:861–74.
de Vries FT, Bloem J, Quirk H, Stevens CJ, Bol R, Bardgett RD. 2012. Extensive management promotes plant and microbial nitrogen retention in temperate grassland. PLoS One 7:1–12.
Day KJ, Hutchings MJ, John EA. 2003. The effects of spatial pattern of nutrient supply on yield, structure and mortality in plant populations. J Ecol 91:541–53. http://apps.webofknowledge.com/full_record.do?product=UA&search_mode=GeneralSearch&qid=34&SID=2ERQwC4Hhoft5xxs4Iu&page=1&doc=1. Last accessed 28/08/2014.
Elliott ET. 1986. Aggregate structure and carbon, nitrogen, and phosphorus in native and cultivated soils. Soil Sci Soc Am J 50:627–33.https://dl.sciencesocieties.org/publications/sssaj/abstracts/50/3/SS0500030627. Last accessed 28/01/2015.
Environment and Climate Change Canada. 2018. Environment and Climate Change Canada National Climate Data and Information Archive. http://climate.weather.gc.ca/. Last accessed 04/06/2018.
Fridley JD, Grime JP, Askew AP, Moser B, Stevens CJ. 2011. Soil heterogeneity buffers community response to climate change in species-rich grassland. Glob Chang Biol 17:2002–11. http://doi.wiley.com/10.1111/j.1365-2486.2010.02347.x. Last accessed 21/08/2014.
García-Palacios P, Maestre FT, Bardgett RD, de Kroon H. 2012. Plant responses to soil heterogeneity and global environmental change. J Ecol 100:1303–14.
García-Palacios P, Maestre FT, Gallardo A. 2011. Soil nutrient heterogeneity modulates ecosystem responses to changes in the identity and richness of plant functional groups. J Ecol 99:551–62.
García-Palacios P, Maestre FT, Milla R. 2013. Community-aggregated plant traits interact with soil nutrient heterogeneity to determine ecosystem functioning. Plant Soil 364:119–29.
Gartner TB, Cardon ZG. 2004. Decomposition dynamics in mixed-species leaf litter. Oikos 104:230–46. http://apps.webofknowledge.com.proxy1.lib.uwo.ca/full_record.do?product=WOS&search_mode=GeneralSearch&qid=1&SID=1E61eOUR8AnHETZ4euy&page=1&doc=1. Last accessed 25/01/2015.
Grant W, French N, Folse JJ. 1980. Effects of pocket gopher mounds on plant production in shortgrass prairie ecosystems. Southwest Nat 25:215–24.
Griffin JN, Jenkins SR, Gamfeldt L, Jones D, Hawkins SJ, Thompson RC. 2009. Spatial heterogeneity increases the importance of species richness for an ecosystem process. Oikos 118:1335–42.
Grime JP. 1998. Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J Ecol 86:902–10.
Hagerty T, Kingston M. 1992. The soils of Middlesex County. Ottawa, ON: Ontario Ministry of Agriculture and Food.
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setälä H, Symstad AJ, Vandermeer J, Wardle DA. 2005. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol Monogr 75:3–35. http://www.jstor.org/stable/4539083 [accessed 5 August 2014].
Horrocks CA, Heal KV, Harvie B, Tallowin JB, Cardenas LM, Dungait JAJ. 2016. Can species-rich grasslands be established on former intensively managed arable soils? Agric Ecosyst Environ 217:59–67. https://doi.org/10.1016/j.agee.2015.10.015.
Hough-Snee N, Long AL, Jeroue L, Ewing K. 2011. Mounding alters environmental filters that drive plant community development in a novel grassland. Ecol Eng 37:1932–6. https://doi.org/10.1016/j.ecoleng.2011.06.013.
Krause A, Pugh TAM, Bayer AD, Lindeskog M, Arneth A. 2016. Impacts of land-use history on the recovery of ecosystems after agricultural abandonment. Earth Syst Dyn 7:745–66.
Liu Y, Bortier MF, De Boeck HJ, Nijs I. 2017. Root distribution responses to three-dimensional soil heterogeneity in experimental mesocosms. Plant Soil 421:353–66.
Maestre F, Reynolds J. 2007a. Amount or pattern? Grassland responses to the heterogeneity and availability of two key resources. Ecology 88:501–11.
Maestre FT, Bradford MA, Reynolds JF. 2005. Soil nutrient heterogeneity interacts with elevated CO2 and nutrient availability to determine species and assemblage responses in a model grassland community. New Phytol 168:637–50.
Maestre FT, Bradford MA, Reynolds JF. 2006. Soil heterogeneity and community composition jointly influence grassland biomass. J Veg Sci 17:261–70. http://doi.wiley.com/10.1111/j.1654-1103.2006.tb02445.x.
Maestre FT, Quero JL, Valladares F, Reynolds JF. 2007. Individual vs. population plastic responses to elevated CO2, nutrient availability, and heterogeneity: a microcosm experiment with co-occurring species. Plant Soil 296:53–64.
Maestre FT, Reynolds JF. 2006a. Nutrient availability and atmospheric CO2 partial pressure modulate the effects of nutrient heterogeneity on the size structure of populations in grassland species. Ann Bot 98:227–35.
Maestre FT, Reynolds JF. 2006b. Spatial heterogeneity in soil nutrient supply modulates nutrient and biomass responses to multiple global change drivers in model grassland communities. Glob Chang Biol 12:2431–41.
Maestre FT, Reynolds JF. 2007b. Biomass responses to elevated CO2, soil heterogeneity and diversity: an experimental assessment with grassland assemblages. Oecologia 151:512–20.
McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, Groffman PM, Hart SC, Harvey JW, Johnston CA, Mayorga E, McDowell WH, Pinay G. 2003. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–12.
McKane RB, Johnson LC, Shaver GR, Nadelhoffer KJ, Rastetter EB, Fry B, Giblin AE, Kielland K, Kwiatkowski BL, Laundre JA, Murray G. 2002. Resource-based niches provide a basis for plant species diversity and dominance in Arctic tundra. Nature 415:68–71.
Naeth MA, Cohen Fernández AC, Mollard FPO, Yao L, Wilkinson SR, Jiao Z. 2018. Enriched topographic microsites for improved native grass and forb establishment in reclamation. Rangel Ecol Manag 71:12–18. https://doi.org/10.1016/j.rama.2017.08.004.
Neill C, Wheeler MM, Loucks E, Weiler A, Von Holle B, Pelikan M, Chase T. 2015. Influence of soil properties on coastal sandplain grassland establishment on former agricultural fields. Restor Ecol 23:531–8.
Phoenix GK, Johnson D, Grime JP, Booth RE. 2008. Sustaining ecosystem services in ancient limestone grassland: importance of major component plants and community composition. J Ecol 96:894–902.
Richardson PJ, MacDougall AS, Larson DW. 2012. Fine-scale spatial heterogeneity and incoming seed diversity additively determine plant establishment. J Ecol 100:939–49.
Sampson F, Knopf F. 1994. Prairie conservation in North America. Bioscience 44:418–21. http://bioscience.oxfordjournals.org/cgi/doi/10.2307/1312365.
Stein A, Gerstner K, Kreft H. 2014. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol Lett 17:866–80.
Stover HJ. 2018. Soil homogenization: plant species diversity, ecosystem properties and soil freezing effects during tallgrass prairie restoration. PhD dissertation, Department of Biology, Western University, London, Ontario.
Stover HJ, Henry HAL. 2018. Soil homogenization and microedges: perspectives on soil-based drivers of plant diversity and ecosystem processes. Ecosphere 9:e02289.
Suding KN, Ashton IW, Bechtold H, Bowman WD, Mobley ML, Winkleman R. 2008. Plant and microbe contribution to community resilience in a directionally changing environment. Ecol Monogr 78:313–29.
R Core Team. 2017. R: a language and environment for statistical computing. https://www.r-project.org/.
Tilman D, Pacala S. 1993. The maintenance of species richness in plant communities. In: Ricklefs RE, Schluter D, Eds. Species diversity in ecological communities: historical and geographical perspectives. 1st edn. Chicago: The University of Chicago Press. p 13–25.
Tylianakis JM, Rand TA, Kahmen A, Klein AM, Buchmann N, Perner J, Tscharntke T. 2008. Resource heterogeneity moderates the biodiversity-function relationship in real world ecosystems. PLoS Biol 6:0947–56.
Walker L, del Moral R. 2003. Primary succession and ecosystem rehabilitation. New York, NY: Cambridge University Press.
Williams BM, Houseman GR. 2014. Experimental evidence that soil heterogeneity enhances plant diversity during community assembly. J Plant Ecol 7:461–9. http://jpe.oxfordjournals.org/content/early/2013/10/25/jpe.rtt056.short. Last accessed 04/09/2014.
Zirbel CR, Bassett T, Grman E, Brudvig LA. 2017. Plant functional traits and environmental conditions shape community assembly and ecosystem functioning during restoration. J Appl Ecol 54:1070–9.
Acknowledgements
This research was supported by the Natural Sciences and Engineering Research Council of Canada (Discovery Grant to H.A.L.H. and a Vanier Canada Graduate Scholarship to H.J.S.) and Research Western (doctoral scholarship to H.J.S.). We thank the research assistants who helped with field and laboratory work and the Nature Conservancy of Canada for a research permit to collect grass leaf litter from one of their properties.
Author information
Authors and Affiliations
Corresponding author
Additional information
Authors’ Contribution
HJS and HALH conceived of and designed the study. HJS performed research and analyzed data. HJS and HALH wrote the paper.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stover, H.J., Henry, H.A.L. Soil Homogenization Modifies Productivity, Nitrogen Retention and Decomposition in Restored Grassland. Ecosystems 23, 264–277 (2020). https://doi.org/10.1007/s10021-019-00401-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-019-00401-w