Abstract
We conducted an experiment to evaluate the plastic phenotypic responses of individuals, growing under intra-specific competition, and populations of three co-occurring grassland species (Lolium perenne, Plantago lanceolata, and Holcus lanatus) to joint variations in atmospheric CO2 partial pressure (P CO2; 37.5 vs. 70 Pa), nutrient availability (NA; 40 vs. 120 mg N added as organic material), and the spatial pattern of nutrient supply (SH; homogeneous vs. heterogeneous nutrient supply). At both the population and individual levels, the aboveground biomass of the three species significantly increased when the nutrients were heterogeneously supplied. Significant two- (SH × NA) and three-term (P CO2 × NA × SH) interactions determined the response of traits measured on populations (aboveground biomass and below: aboveground biomass ratio, BAR) and individuals (aboveground biomass and specific leaf area). The combination of a high SH and NA elicited the highest plasticity of aboveground biomass in populations and individuals of the three species evaluated, and of BAR in Holcus. Soil heterogeneity and elevated P CO2 elicited the highest plasticity in the SLA of Plantago and Lolium individuals. Our results show that populations, and not only individuals, respond to soil heterogeneity in a plastic way, and that plastic responses to elevated P CO2 are complex since they vary across traits and species, and are influenced by the availability of nutrients and by their spatial distribution. They also emphasize the importance of soil heterogeneity as a modulator of plant responses to global change drivers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The analysis of the ecological and evolutionary consequences of phenotypic plasticity, i.e. the ability of an organism to adjust its performance by altering its morphology and/or physiology in response to varying environmental conditions, in plants has a long tradition among ecologists, geneticists, and evolutionary biologists (Pigliucci 2001; Valladares et al. 2006). Under natural conditions, plants are arranged in populations and communities, and are exposed to simultaneous changes in the availability and heterogeneity of environmental factors such as light, water, and nutrients, all of which can elicit plastic responses in plants (von Caemmerer et al. 2001; Hodge 2004; Baer et al. 2004). However, studies evaluating plastic responses of plants growing in competition and under varying conditions of more than a single resource are virtually lacking (Urbas and Zobel 2000). Such studies are critical to accurately predict the ecological and evolutionary consequences of phenotypic plasticity, as it has often been found that plastic responses of individually grown plants are a poor predictor of those of populations and assemblages (Navas et al. 1999; Poorter and Navas 2003).
Increases in the atmospheric CO2 partial pressure (P CO2) and in nutrient availability are two key components of the environmental change being currently faced by terrestrial ecosystems worldwide (Houghton et al. 2001). Important research efforts have been devoted to explore how plants respond to such increases, which have been shown to promote plastic responses in a wide variety of plant traits (for a review see Pritchard et al. 1999). Plant responses to P CO2 are often dependent on the availability of nutrients such as nitrogen and phosphorus (Berntson and Bazzaz 1997; Stöcklin and Körner 1999). However, it is poorly known whether such responses are also modified by the spatial pattern of nutrient supply (hereafter named soil heterogeneity), since very few studies have explicitly incorporated it as a factor when evaluating plant responses to global change drivers (Arnone 1997; Maestre et al. 2005; Maestre and Reynolds 2006a,b, 2007). Soil heterogeneity is common place in most terrestrial ecosystems (Hutchings et al. 2000). When it occurs at spatial scales perceptible by the root system of individual plants, it promotes plastic responses such as the proliferation of roots into the nutrient patches, which in turn increases nutrient uptake and changes the specific root length (Hodge 2004). These responses have the potential to modify the size of competing individuals within populations (Maestre and Reynolds 2006a) and the composition and productivity of multi-species assemblages (Wijesinghe et al. 2005; Maestre et al. 2005, 2006).
Using a series of microcosm experiments, we have recently evaluated the effects of joint changes in P CO2, nutrient availability, soil heterogeneity, and biotic diversity (species composition, richness, and evenness) on the productivity of grassland assemblages formed by Lolium perenne L., Holcus lanatus L., and Plantago lanceolata L., as well as on the size-symmetry of populations of these species (Maestre and Reynolds 2006a,b, 2007). In this article, we present new data from these experiments to evaluate how the simultaneous changes in P CO2, nutrient availability, and soil heterogeneity affected phenotypic plasticity of populations and individuals growing under intra-specific competition. Specifically, we tested the hypothesis that, at these two organization levels, responses to soil heterogeneity, P CO2, and nutrient availability can only be predicted from the interaction of these factors, rather than from any of these factors alone, as suggested by the high number of significant interactive effects among factors found in previous studies (Maestre et al. 2005; Maestre and Reynolds 2006b).
Materials and methods
Experimental design
We conducted a factorial microcosm experiment in the Duke University Phytotron between 3 January and 4 April 2005. The experiment had four factors: two atmospheric P CO2 (37.5 and 70 Pa), two fertilization levels (40 and 120 mg of N added as organic material), two levels of spatial distribution of the organic material (homogeneous and heterogeneous), and three species (Lolium, Holcus, and Plantago).
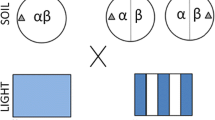
Microcosms consisted of PVC pipe filled with, from the base, gravel (for drainage), a 50:50 mixture of soil and sand (hereafter named ‘background soil’), and a 50:50 mixture of organic soil: peat (hereafter named ‘organic soil’; Fig. 1). The soil was a sandy loam of the White Store series, collected from the top 30 cm of mineral horizon in the Duke Forest (35°55′N, 78°52′W; http://www.env.duke.edu/forest/), and was thoroughly mixed with the sand using a cement mixer. The soil was neither sieved nor dried prior to the mixing with the sand, but rocks, logs, and big woody root fragments were removed before mixing. The organic soil was placed to avoid the formation of physical crusts in the surface of the microcosms, and to simulate the typical accumulation of organic matter in the topsoil of temperate grasslands. All microcosms were irrigated with 200 mL of a grassland soil microbial inoculum. To obtain it, 3 kg of fresh soil from turf communities (dominated by Trifolium repens L., Plantago, and Anthoxantum odoratum L.) surrounding the Phytotron (35°55′N, 78°52′W) were mixed with 30 l of water and the mixture was agitated every 8 h for 2 days. The resulting solution was filtered with a 106 μm sieve and added to the microcosms prior to the addition of the organic soil.
To generate the two levels of overall nutrient availability we added 1.036 and 3.108 g of finely cut (2 mm) dried Trifolium shoots (3.9% N, 10.8 C:N) in the low- and high-nutrient availability factors, respectively (equivalent to 40 and 120 mg of N per microcosm, respectively). Within each of these nutrient availability levels, the T. repens shoots were added homogeneously (homogeneous treatment) or as a patch (heterogeneous treatment). In both cases the same amount of nutrient was added, and thus we maintained the same overall nutrient availability. In the homogeneous treatment, we thoroughly mixed the organic material with the background soil before introducing it into the PVC pipe. In the heterogeneous treatment, the organic material was localized within discrete 31 cm3 volumes of soil (Fig. 1). To create one of these patches we mixed 25 cm3 of background soil with the organic material and introduced the resulting mix into a 31 cm3 plastic cylinder (length 75 mm; internal diameter 23 mm) consisting of a light mesh with square pores 5 × 10 mm in size. A second cylinder, filled only with background soil, was placed 2 cm apart and parallel to the patch cylinder. Cylinders were located 12 cm below the surface of the organic soil. In the homogeneous treatments, both cylinders were filled with the mixture of background soil and organic material.
Seeds from the three species used were obtained from commercial suppliers. Due to different germination times and growth rates, as revealed by previous tests, the different species were germinated in different days to ensure that all the species had the same degree of development (one-leaf stage) and a similar size at the starting of the experiment. Each microcosm contained a monoculture formed by six seedlings of a single species. The planting positions of the six seedlings were allocated at random, but the same planting grid was maintained in all the microcosms using a wire grid pattern secured to the top of the containers (Fig. 1). Seedlings that died during the first week of the experiment were replaced. After that period, no further mortality was observed. One week after planting, all microcosms were watered with 100 ml of a 106 μm sieved solution derived from root macerations (roots were collected from the turf communities surrounding the Phytotron) just after planting.
We established four replicated microcosms for each of the 24 factor combinations, resulting in 96 microcosms in total (2 P CO2 levels × 2 nutrient availability levels × 2 spatial distribution of the organic material levels × 3 species levels × 4 replicates). The microcosms were introduced in four walk-in growth chambers (two for each P CO2), within which atmospheric temperature and P CO2 were independently controlled. For each P CO2, half of the microcosm units per combination of factors were randomly assigned to one of the chambers (24 microcosms per chamber), and then were randomly grouped in two-wheeled trolleys. To minimize possible chamber effects, the P CO2 and trolleys were rotated between chambers every week. This process was repeated 12 times during the experiment, and at harvest all the microcosms spent the same amount of time in each chamber. In addition, the position of the microcosms within each chamber was randomized once a week. The chambers were maintained at a day/night air temperature of 21/12°C, a 15 h photoperiod, and an average relative humidity of 70% during the day and 85% during the night. The photoperiod included a simulated dawn and dusk period, each of 2 h duration, where the temperature, the relative humidity, and the lights were gradually ramped up or down. PAR was maintained at 500 μmol/m2/s during the first week of the experiment, 750 μmol/m2/s during the second week of the experiment, and at 1000 μmol/m2/s1 thereafter.
Each microcosm was irrigated daily with 30 ml of distilled water during the first two weeks of the experiment, and with 50 ml amount thereafter. To reduce limitations to plant growth due to low overall soil fertility, all the microcosms were watered with 50 ml of a nutrient solution containing 35 mg of Ca (added as CaCl2·2H20) and 29 mg of Mg (added as MgSO4·7H2O) twice during the course of the experiment (1 February and 1 March).
Measurements and harvest
Plants were grown in the chambers for 90 days, a period equivalent to a growing season. After 90 days of growth, the plants were harvested. At this time, we selected in each individual the three youngest and fully expanded leaves without any symptoms of damage or senescence (1728 leaves in total) for measuring specific leaf area (SLA) according to Garnier et al. (2001). Leaf area was measured immediately after collection with an LI-3100 leaf area meter (Li-Cor Inc., Lincoln, NE, USA). After this measurement, the remaining of the aboveground biomass of each individual plant was clipped at the soil surface and then dried at 60°C to constant mass. After aboveground harvesting, the soil was carefully removed from the microcosm unit and the roots were harvested and dried as above. They were so large and entangled that it was not logistically feasible to separate them by individuals.
Statistical analyses
We evaluated the effects of P CO2 (37.5 vs. 70 Pa), nutrient availability (40 vs. 120 mg N) and soil heterogeneity (homogeneous vs. heterogeneous nutrient supply) on traits measured on populations (aboveground biomass and below: aboveground biomass ratio, BAR) and individuals (aboveground biomass and SLA) with a three-way analysis of variance (ANOVA) and multivariate analysis of variance (MANOVA), respectively, with all the factors being fixed. In the MANOVA analyses, data from the six individuals sampled were included. Our data did not meet the homogeneity of variance–covariance matrices assumption of MANOVA (Box´s M test, P < 0.05), even after square-root, log-, and arcsine-transformations. Thus, we used the semi-parametric permutational multivariate analysis of variance (PERMANOVA) approach (Anderson 2001). It is based on the use of permutation tests to obtain P-values, does not rely on the assumptions of MANOVA (multivariate normality and homogeneity of variances–covariances), and can handle complex experimental designs. Separate ANOVA/PERMANOVA analyses were conducted for each trait measured and species.
We estimated the plastic response of individuals and populations to the factors evaluated with the Relative Distances Plasticity Index (RDPI; Valladares et al. 2006). This was done by comparing responses (aboveground biomass, SLA, and BAR) observed in the treatment with homogeneous nutrient supply, P CO2 = 37.5 Pa and 40 mg of N added (hereafter named control treatment) with those observed in the rest of treatments. RDPI was calculated as |(B t − B c)|/(B t + B c), where B c is the response variable in the control treatment and B t the biomass measured for a given combination of factors. RDPI ranges from 0 to 1; the higher the value of the index, the more plastic is the response of the individual/population to a given combination of factors. It was obtained from phenotypic distances estimated for all pair-wise combinations among replicates from different environmental conditions (Valladares et al. 2006); as n = 4 per treatment, for every comparison between the control and the rest of treatments we obtained 16 relative phenotypic distances that were used in the statistical analyses. Separate RDPI values were obtained for each individual. RDPI data were analyzed separately for each trait and species with a one-way ANOVA (populations) or MANOVA (individuals), with treatment combination as the main factor. As the RDPI data obtained for individuals did not meet the homogeneity of variance–covariance matrices assumption of MANOVA (Box’s M test, P < 0.05), data were analyzed using PERMANOVA. To visualize the effects of factors at the individual level, we conducted a principal coordinate analysis (PCO) of the RDPI data (Quinn and Keough 2002).
PERMANOVA and PCO analyses were performed using the programs PERMANOVA 1.6 (Anderson 2005) and CAP (Anderson 2004), respectively (both can be freely downloaded from http://www.stat.auckland.ac.nz/∼mja/Programs.htm). We used the Euclidean distance for both analyses. We conducted PERMANOVA analyses using 10,000 permutations (of residuals under the reduced model; Anderson and Ter Braak 2003). ANOVA analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL, USA). In order to control for differences in plant size (Reich 2002) when analyzing BAR data, we used total biomass as a covariate. We did not adjust P values for multiple testing because this approach is considered overly conservative (Gotelli and Ellison 2004).
Results
Morphological responses at the population and individual levels
Populations of the three species evaluated had higher aboveground biomass under heterogeneous conditions of nutrient supply and with the addition of nutrients (Fig. 2, Table 1). Such increases were non-additive in Holcus and Lolium, as indicated by significant soil heterogeneity (SH) × nutrient availability (NA) interactions. The same interaction was found for the BAR in Plantago. Increases in P CO2 led to higher biomass in Holcus populations. Individuals of the three species evaluated had, in most cases, higher aboveground biomass under high nutrient supply and when the nutrients were added heterogeneously (Table 2, Fig. A1 in Electronic Supplementary Material); in the case of Plantago, increases in response to both factors were non-additive, as indicated by a significant NA × SH interaction (Table 2). There were significant interactions among all three factors when evaluating the SLA of Plantago individuals. This variable increased in Lolium under heterogeneous nutrient supply (Table 2, Fig. A2 in Electronic Supplementary Material).
Phenotypic plasticity of populations and individuals
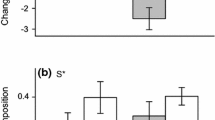
When compared to the control, the different combinations of factors evaluated elicited plastic responses in the three species at both the population and individual levels (i.e., RDPI > 0; Figs. 3 and 4). In both cases, the magnitude of these responses differed among traits within each species, and between species for a given trait. At the population level, the increase of nutrients in a heterogeneous manner (combinations of factors 1 and 4 in Fig. 3) promoted the highest plasticity in traits such as the aboveground biomass in all the species and BAR in Holcus (Fig. 3). At the individual level, significant differences among factor combinations were found for the three species in aboveground biomass (PERMANOVA; F Plantago = 4.88, P < 0.001; F Lolium = 4.08, P < 0.001; F Holcus = 21.03, P < 0.001), and for Lolium and Plantago in SLA (PERMANOVA; F Plantago = 4.51, P < 0.001; F Lolium = 3.97, P < 0.001; F Holcus = 1.02, P = 0.423). The first two PCO axes explained 46–68% of the variation observed in the RDPI data obtained at the individual level, and clearly separated some combination of factors in the three species for aboveground biomass, and in Plantago and Lolium for SLA (Fig. 4). The combination of SH and high NA elicited the highest plasticity in the aboveground biomass of the three species evaluated (post hoc results after PERMANOVA, P < 0.001; see Fig. A3 in the Electronic Supplemental Material for raw RDPI data). Soil heterogeneity and elevated P CO2 elicited the highest plasticity in the SLA of Plantago and Lolium (post hoc results after PERMANOVA, P < 0.001; see Fig. A4 in the Electronic Supplemental Material for raw RDPI data).
Effects of atmospheric P CO2, nutrient availability, and soil heterogeneity on the phenotypic plasticity, as measured with the relative distances plasticity index (RDPI), of aboveground biomass and below: aboveground biomass ratio of Plantago lanceolata, Lolium perenne, and Holcus lanatus populations. Different lowercase letters indicate significant differences between combination of factors within each species (P < 0.05; Tukey’s HSD test after one-way ANOVA). Data are means ± SE (n = 16). Combinations of factors as follows: (1) 37.5 Pa P CO2, heterogeneous supply, 120 mg of N added; (2) 37.5 Pa P CO2, heterogeneous nutrient supply, 40 mg of N added; (3) 37.5 Pa P CO2, homogeneous nutrient supply, 120 mg of N added; (4) 70 Pa P CO2, heterogeneous nutrient supply, 120 mg of N added; (5) 70 Pa P CO2, heterogeneous nutrient supply, 40 mg of N added; (6) 70 Pa P CO2, homogeneous nutrient supply, 120 mg of N added; (7) 70 Pa P CO2, homogeneous nutrient supply, 40 mg of N added
Results of a principal coordinate analysis summarizing the effects of atmospheric P CO2, nutrient availability, and soil heterogeneity on the phenotypic plasticity, as measured with the RDPI, of aboveground biomass and specific leaf area (SLA) of Plantago lanceolata, Lolium perenne, and Holcus lanatus individuals. Data represent means ± SE (n = 16). See Figs. A3 and A4 in Electronic Supplementary Material for raw RDPI data
Discussion
A series of two- and three-term interactions between the factors evaluated determined different allocation and morphological responses of populations and individuals in both Holcus and Plantago. Thus, our working hypothesis, i.e., population- and individual-level responses to the environmental factors evaluated cannot be predicted from any of them in isolation, was supported by our results.
Populations of the three species allocated less biomass to roots as nutrient availability increased, as commonly observed in herbaceous plants (Poorter and Nagel 2000). Soil heterogeneity and elevated P CO2 also promoted a similar response in Holcus. Responses to P CO2 may be partially explained by the significant increase in soil moisture observed under elevated (CO2) in our experiment (Maestre and Reynolds 2006a). Decreases in water availability not only promote a decrease in water uptake per unit of root mass, but also a reduced uptake of nutrient with high soil mobility because of the delivery of nutrients by mass flow is impeded in dry soils (Marschner 1995). These factors are expected to increase biomass allocation of roots, a view supported by Poorter and Nagel (2000). Previous studies evaluating the effects of soil heterogeneity on the BAR of plant populations have found an increase (Maestre et al. 2006) or no change (Day et al. 2003) on this variable. We suggest, but cannot confirm, that the decrease in BAR observed in response to soil heterogeneity is the result of increasing the nutrient use efficiency under heterogeneous nutrient supply (Jackson and Caldwell 1996).
For those traits where significant differences among factors were found, the magnitude of the plastic response to both soil heterogeneity and nutrient availability was higher than that of P CO2. Nonetheless, it must be noted that P CO2 elicited plastic responses in all the traits measured (i.e., RDPI values higher than 0) at both the individual and population levels, as found in previous studies (see Pritchard et al. 1999; Curtis and Wang 1998 for reviews). When elevated P CO2 was combined with high nutrient availability, the magnitude of plastic responses increased substantially compared to those in response to elevated P CO2 alone for most traits, indicating that plastic responses to this factor were constrained by low nutrient availability (Stöcklin and Körner 1999; Grünzweig and Körner 2003; Maestre et al. 2005). A similar response was found when elevated P CO2 was combined with soil heterogeneity, regardless of the amount of nutrients. To our knowledge, this study is the first one to report such plastic responses in either individuals or populations.
Soil heterogeneity significantly increased aboveground biomass at both the population and individual levels in the three species evaluated. Compared to studies evaluating the effects on individually grown plants, few studies so far have evaluated the effects of soil heterogeneity on plant populations. These studies have reported either increases (Facelli and Facelli 2002; Day et al. 2003; Maestre et al. 2006) or no changes (Casper and Cahill 1996, 1998; Fransen et al. 2001) in the aboveground biomass of populations grown under heterogeneous nutrient supply. Although the limited work conducted so far does not allow for a full understanding of the mechanisms underlying these discrepancies, they are likely to be promoted by differences in the experimental approach and growing conditions among studies. It has been shown that factors such as the scale of the nutrient patches relative to the size of the plants (Day et al. 2003), the identity of the neighbors (Maestre and Reynolds 2006b), and the characteristics of the material used to create nutrient patches (Hodge 2004) modify the magnitude of observed responses to soil heterogeneity. As found in studies evaluating responses of the studied species in the absence of competition (Fransen et al. 1998; Grime and Mackey 2002), the individuals forming the populations studied responded positively to soil heterogeneity when growing under intra-specific competition. This factor also interacted with nutrient availability and P CO2 to determine the SLA of Plantago individuals. To our knowledge, this is the first time that such a result is reported. Previous studies have suggested that the increase in nutrient uptake, as a result of the combination of root proliferation into nutrient patches and/or adjustments in nutrient uptake efficiency, is the primary process leading to increasing biomass under heterogeneous nutrient supply (Hutchings et al. 2003; Hodge 2004). The potential role of plant architectural traits on this process is, however, virtually unknown. Further studies are needed to: (i) assess the generality of the effects of soil heterogeneity on SLA found in this study; and (ii) elucidate the relative importance of changes in architectural traits vs. processes such as root foraging precision as drivers of plant responses to soil heterogeneity.
Our results indicate that both populations and individuals respond to soil heterogeneity in a plastic way. Previous studies evaluating the effects of soil heterogeneity on plant populations have reported a significant effect of soil heterogeneity on the above- and belowground growth of individuals that, however, was not reflected on either the productivity or the structure of populations (Casper and Cahill 1996, 1998; but see Day et al. 2003; Maestre et al. 2006). It has been suggested that such results could be promoted by phenotypic plasticity in response to soil heterogeneity, which can fully compensate for nutrient patchiness (Fitter et al. 2000). Our results do agree with this affirmation, as plastic responses of populations to soil heterogeneity were clearly evident for the traits evaluated. It must be noted that populations of the three species strongly proliferated roots in response to soil heterogeneity in our experiment (Maestre and Reynolds 2006a), a morphologically plastic response commonly found with individuals (see Hodge 2004 for a review). The question whether plastic responses to soil heterogeneity are adaptive, as suggested by Fitter et al. (2000), cannot be answered with the results from our study because we did not measure nutrient capture from the nutrient patches and the background soil. However, they clearly show that populations, and not only individuals, respond to soil heterogeneity in a plastic way.
The extrapolation of our results to the natural world must be done with caution because of the limitations of our microcosm approach (see discussion in Maestre et al. 2005). They do indicate, however, that plastic responses of individuals and populations to increases in P CO2 are complex, since not only they vary across species and are affected by the availability of nutrients but also they are influenced by their spatial distribution. Therefore, soil heterogeneity is a potentially important modulator of plant responses to global change drivers, and future studies should consider it explicitly when evaluating such responses.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Aust Ecol 26:32–46
Anderson MJ (2004) CAP: a FORTRAN computer program for canonical analysis of principal coordinates. Department of Statistics, University of Auckland, New Zealand
Anderson MJ (2005) PERMANOVA: a FORTRAN computer program for permutational multivariate analysis of variance. Department of Statistics, University of Auckland, New Zealand
Anderson MJ, Ter Braak CJF (2003) Permutation tests for multi-factorial analysis of variance. J Stat Comp Simul 73:85–113
Arnone JA III (1997) Temporal responses of community fine root populations to long-term elevated atmospheric CO2 and soil nutrient patches in model tropical ecosystems. Acta Oecol 18:367–376
Baer SG, Blair JM, Collins SL, Knapp AK (2004) Plant community responses to resource availability and heterogeneity during restoration. Oecologia 139:617–629
Berntson GM, Bazzaz FA (1997) Nitrogen cycling in microcosms of yellow birch exposed to elevated CO2: Simultaneous positive and negative feedbacks. Global Change Biol 3:247–258
Casper BB, Cahill JF Jr. (1996) Limited effects of soil nutrient heterogeneity on populations of Abutilon theophrasti (Malvaceae). Am J Bot 83:333–341
Casper BB, Cahill JF Jr. (1998) Population-level responses to nutrient heterogeneity and density by Abutilon theophrasti (Malvaceae): an experimental neighborhood approach. Am J Bot 85:1680–1687
Curtis PS, Wang XZ (1998) A meta-analysis of elevated CO2 effects on woody plant mass, form, and physiology. Oecologia 113:299–313
Day KJ, Hutchings MJ, John EA (2003) The effects of spatial pattern of nutrient supply on yield, structure and mortality in plant populations. J Ecol 91:541–553
Facelli E, Facelli JM (2002) Soil phosphorus heterogeneity and mycorrhizal symbiosis regulate plant intra-specific competition and size distribution. Oecologia 133:54–61
Fitter A, Hodge A, Robinson D (2000) Plant response to patchy soils. In: Hutchings MJ, John EA, Stewart AJA (eds) The Ecological Consequences of Environmental Heterogeneity. Blackwell Science, Cambridge, pp 71–90
Fransen B, de Kroon H, Berendese F (1998) Root morphological plasticity and nutrient acquisition of perennial grass species from habitats of different nutrient availability. Oecologia 115:351–358
Fransen B, de Kroon H, Berendese F (2001) Soil nutrient heterogeneity alters competition between two perennial grass species. Ecology 82:2534–2546
Garnier E, Shipley B, Roumet C, Laurent G (2001) A standardized protocol for the determination of specific leaf area and leaf dry matter content. Funct Ecol 15:688–695
Gotelli NJ, Ellison AM (2004) A Primer of Ecological Statistics. Sinauer Associates, Sunderland
Grime JP, Mackey JML (2002) The role of plasticity in resource capture by plants. Evol Ecol 16:299–307
Grünzweig JM, Körner Ch (2003) Differential phosphorus and nitrogen effects drive species and community responses to elevated CO2 in semi-arid grassland. Funct Ecol 17:766–777
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
Houghton JT, Ding Y, Griggs DJ, Noguer M, van der Linden PJ, Xiaosu D (2001) Climate Change: The Scientific Basis. Cambridge University Press, Cambridge
Hutchings MJ, John EA, Stewart AJA (2000) The Ecological Consequences of Environmental Heterogeneity. Blackwell Science, Oxford
Hutchings MJ, John EA, Wijesinghe DK (2003) Toward understanding the consequences of soil heterogeneity for plant populations and communities. Ecology 84:2322–2334
Jackson RB, Caldwell MM (1996) Integrating resource heterogeneity and plant plasticity: Modelling nitrate and phosphate uptake in a patchy soil environment. J Ecol 84:891–903
Maestre FT, Bradford MA, Reynolds JF (2005) Soil nutrient heterogeneity interacts with elevated CO2 and nutrient availability to determine species and assemblage responses in a model grassland community. New Phytol 168:637–650
Maestre FT, Bradford MA, Reynolds JF (2006) Soil heterogeneity and community composition jointly influence grassland biomass. J Veg Sci 17:261–270
Maestre FT, Reynolds JF (2006a) Nutrient availability and atmospheric CO2 partial pressure modulate the effects of nutrient heterogeneity on the size structure of populations in grassland species. Ann Bot 98:227–235
Maestre FT, Reynolds JF (2006b) Spatial heterogeneity in nutrient supply modulates plant nutrient and biomass responses to multiple global change drivers in model grassland communities. Global Change Biol 12:2431–2441
Maestre FT, Reynolds JF (2007) Biomass responses to elevated CO2, soil heterogeneity and diversity: an experimental assessment with grassland assemblages. Oecologia 151:512–520
Marschner H (1995) Mineral Nutrition of Higher Plants. Academic Press, London
Navas ML, Garnier E, Austin MP, Gifford RM (1999) Effect of competition on the responses of grasses and legumes to elevated atmospheric CO2 along a nitrogen gradient: differences between isolated plants, monocultures and multi-species mixtures. New Phytol 143:323–331
Pigliucci M (2001) Phenotypic Plasticity: Beyond Nature and Nurture. Johns Hopkins University Press, Baltimore
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol 27:595–607
Poorter H, Navas ML (2003) Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytol 157:175–198
Pritchard SG, Rogers HH, Prior SA, Peterson CM (1999) Elevated CO2 and plant structure: a review. Global Change Biol 5:807–837
Quinn GP, Keough MJ (2002) Experimental Design and Data Analysis for Biologists. Cambridge University Press, Cambridge
Reich PB (2002) Root-shoot relations: Optimality in acclimation and adaptation or the “Emperor´s new clothes”? In: Waisel Y, Eshel A, Kafkafi U (eds) Plant Roots: The Hidden Half. Marcel Dekker, New York, pp 205–220
Stöcklin J, Körner Ch (1999) Interactive effects of elevated CO2, P availability and legume presence on calcareous grassland: results of a glasshouse experiment. Funct Ecol 13:200–209
Urbas P, Zobel K (2000) Adaptive and inevitable morphological plasticity of three herbaceous species in a multi-species community: field experiment with manipulated nutrients and light. Acta Oecol 21:139–147
Valladares F, Sánchez-Gómez D, Zavala MA (2006) Quantitative estimation of phenotypic plasticity: bridging the gap between the evolutionary concept and its ecological applications. J Ecol 94:1103–1116
von Caemmerer S, Ghannoum O, Conroy JP, Clark H, Newton PCD (2001) Photosynthetic responses of temperate species to free air CO2 enrichment (FACE) in a grazed New Zealand pasture. Aust J Plant Physiol 28:439–450
Wijesinghe DK, John EA, Hutchings MJ (2005) Does pattern of soil resource heterogeneity determine plant community structure? An experimental investigation. J Ecol 93:99–112
Acknowledgements
We thank María D. Puche, Andrea Castillo, and Anne Rosenbarger for their help during the different phases of the study, and Angela Hodge and two anonymous reviewers for useful comments and suggestions on an earlier version of the manuscript. F.T.M. was supported by a Fulbright fellowship (FU2003-0398), by a Ramón y Cajal contract from the Spanish Ministerio de Educación y Ciencia (MEC), and by an Early Career Project Grant from the British Ecological Society (ECPG 231/607). F.V. was supported by the MEC grants RASINV (CGL2004-04884–CO2–02/BOS) and PLASTOFOR (AGL2004–00536/FOR). J.L.Q. was supported by an FPI fellowship from MEC (BES-2003–1716). This research was supported by USDA Specific Co-operative Agreement #58–1270–3–070, NSF-DEB-02–12123, NSF-IBN-99–85877 to the Duke Phytotron, and NSF-SBR-9521914 (Subcontract # 538819–55801 from Carnegie Mellon University).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Angela Hodge
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maestre, F.T., Quero, J.L., Valladares, F. et al. Individual vs. population plastic responses to elevated CO2, nutrient availability, and heterogeneity: a microcosm experiment with co-occurring species. Plant Soil 296, 53–64 (2007). https://doi.org/10.1007/s11104-007-9289-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9289-2