Abstract
Background and aims
Spatial distribution of soil nutrients (soil heterogeneity) and availability have strong effects on above- and belowground plant functional traits. Although there is ample evidence on the tight links between functional traits and ecosystem functioning, the role played by soil heterogeneity and availability as modulators of such relationship is poorly known.
Methods
We conducted a factorial experiment in microcosms containing grasses, legumes and non-legume forbs communities differing in composition to evaluate how soil heterogeneity and availability (50 and 100 mg N) affect the links between traits and ecosystem functioning. Community-aggregated specific leaf area (SLAagg) and specific root length (SRLagg) were measured as both relevant response traits to soil heterogeneity and availability, and significant effect traits affecting ecosystem functioning (i.e., belowground biomass, β-glucosidase and acid phosphatase activities, and in situ N availability rate).
Results
SRLagg was negatively and significantly associated to β-glucosidase, phosphatase and N availability rate in the high nutrient availability and heterogeneous distribution scenario. We found a significant negative relationship between SLAagg and availability rate of mineral-N under low nutrient availability conditions.
Conclusions
Soil heterogeneity modulated the effects of both traits and nutrient availability on ecosystem functioning. Specific root length was the key trait associated with soil nutrient cycling and belowground biomass in contrasted heterogeneous soil conditions. The inclusion of soil heterogeneity into the trait-based response-effect framework may help to scale from plant communities to the ecosystem level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Abiotic filters such as resource availability and heterogeneity constrain the species and traits, from a regionally available pool, which can persist at a site (Díaz et al. 1999). Environmental factors pose selective pressures on species traits, and thus filter the composition and structure of local communities (Weiher and Keddy 1999). Following this model, we should be able to predict how plant community assembly is altered by environmental changes by taking into account the nature and strength of the different filters, and the response traits for each of these filters (Grime 2006). As a consequence, plant communities with contrasting trait profiles may impact ecosystem processes through differential abundance of ecosystem-effect traits (Suding et al. 2008). In this context, ecosystem processes will be predictable only if those effect traits involved in the response to environmental filters control ecosystem processes to a significant extent (Lavorel and Garnier 2002).
The availability of soil resources is a major determinant of plant productivity, and strongly influences plant community assembly (Tilman 1982; Grime 2006). Changes in resource availability have also strong effects on traits related with plant resource use strategies above- and belowground, such as specific leaf area and specific root length, respectively (Craine et al. 2001). However, soil resources such as nutrients are heterogeneously distributed in space (hereafter soil heterogeneity) in most terrestrial ecosystems (Jackson and Caldwell 1993; Gross et al. 1995). Plants have developed contrasting root foraging mechanisms to deal with such heterogeneity (Kembel et al. 2008; Mommer et al. 2011). These mechanisms have physiological, such as changes in nutrient uptake capacity (Jackson et al. 1990), and morphological, such as modifications in biomass allocation patterns (Fransen and de Kroon 2001), consequences at the plant level, which scale up to affect plant populations and communities (Maestre et al. 2005; Maestre and Reynolds 2006).
Interactions between nutrient availability and soil heterogeneity are frequent and conspicuous, as plants selectively place their roots in the highest nutrient availability patches (Maestre et al. 2005; Maestre and Reynolds 2007). Although the importance of this interaction goes beyond the functioning of plant modules and individuals (de Kroon et al. 2012), only few studies have approached it at the community level (e.g. Maestre et al. 2005; Wijesinghe et al. 2005). As soil heterogeneity has profound effects on plant processes such as productivity and community assembly, we may expect potential cascading effects on ecosystem process tightly linked with them, such as C and N cycling (Huber-Sannwald and Jackson 2001). How soil heterogeneity modulates the effects of plant communities on ecosystem functioning will likely depend on the traits of the dominant species (Grime 1998), especially those traits related with resource-use strategy (Rajaniemi and Reynolds 2004; Kembel et al. 2008). However, it is unknown how links between plant communities and ecosystem functioning are modified by soil heterogeneity and the root responses it promotes (de Kroon et al. 2012).
In an earlier study, García-Palacios et al. (2011a) used model plant communities to assess the joint influence of plant functional group identity, nutrient availability and soil heterogeneity on several surrogates of ecosystem functioning. In this study, we focus on how plant functional traits mediate the relationship between plant communities and ecosystem functioning, and most importantly, on how two abiotic filters, soil heterogeneity and nutrient availability, affect this functional link. We use a trait-based response-and-effect framework (see Suding et al. 2008 for details) to scale processes from plant individuals to ecosystems through the community level in the context of these two abiotic filters. Specific leaf area and specific root length were measured at the community level as: 1) relevant response traits to the availability and spatial heterogeneity of soil nutrients (Wahl and Ryser 2000; Craine et al. 2001); and 2) significant effect traits affecting key ecosystem processes (belowground biomass and carbon [C], nitrogen [N] and phosphorus [P] cycling; Garnier et al. 2004; Díaz et al. 2007). These two functional traits were incorporated to the dataset described in García-Palacios et al. (2011a) to address the following questions: (i) are community-aggregated plant functional traits associated with surrogates of ecosystem functioning?, and (ii) do changes in resource heterogeneity and availability affect the relationship between community-aggregated functional traits and surrogates of ecosystem functioning?
Materials and methods
Experimental design
We conducted a microcosm experiment in the plant growth facilities of the Rey Juan Carlos University, located in Móstoles, central Spain (40º18′48″N, 38º52′57″W, 632 m a.s.l.). The experimental setting was a completely randomized factorial design comprising three factors described in detail by García-Palacios et al. (2011a). Four levels of plant functional group compositions were used, all with the same species richness: three mono-functional groups (all species in each microcosms belonging to grasses, legumes or non-legume forbs) and 3-PFG mixture (equitative representation of each PFG). Two levels of nutrient availability (NA: 50 and 100 mg of N added as Lolium multiflorum shoots) and two levels of spatial distribution of the organic material (NH: homogeneous and heterogeneous) were added. Nine replicates were established for each of the 16 treatment combinations, providing 144 assemblages total. For this study we only focused on two of these factors (NA and NH), and used the different plant communities created with the PFG factor to evaluate how NA and NH differently constrained community mean trait values and the subsequent effects on ecosystem functioning.
For this experiment, we selected 27 herbaceous perennial plant species naturally occurring in semi-arid Mediterranean roadside grasslands and abandoned fields undergoing secondary succession. Three different plant functional group pools (grasses, legumes and non-legume forbs) were assembled, each one containing nine species (see Appendix A in Supplementary Material). The mono-functional groups were obtained by randomly selecting six different species from each grasses, legumes and non-legume forbs pool. The composition of each replicate was modified to guarantee a minimum of two species variation within each mono-functional group. The 3-PFG mixture was created with two randomly selected species of each pool, forming a six species community. This design allowed us to evaluate the effects of different plant community compositions with a constant number of species. Seeds were obtained from commercial suppliers (Intersemillas, Valencia, Spain). Germination time was tested in a pilot experiment and used to correct the date of sowing. The seeds of each species were sown by hand and allocated randomly at a density of 400 seeds m−2. Six weeks after sowing, some individuals were removed to correct species density to a final density per species of 60 individuals m−2. Weeds were regularly removed during the experiment. To simulate field conditions similar to those experienced by semi-arid grasslands in central Spain, all the microcosms were kept under ambient light, temperature and rainfall for 17 months, from February 2008 to June 2009 (Mean monthly temperature = 14.16 °C ± 1.48, mean monthly rainfall = 34.71 mm ± 6.68).
Microcosms consisted of PVC pots (depth 33 cm, diameter 24 cm) filled with a 60:40 mixture of soil and sand (7,600 and 5,000 cm3, respectively; see Fig. 1). We collected the soil from a roadside grassland located in the surroundings of the Rey Juan Carlos University. The resulting mix of soil and sand had very low fertility (0.143 ± 0.01 mg total N g−1 soil and 0.389 ± 0.02 mg total P g−1 soil; mean ± SE, n = 10). The organic material was generated by growing L. multiflorum in a greenhouse maintained at air temperatures of 16 °C, PAR of 148 μmol m−2 s−2, and an average relative humidity of 50 %. L. multiflorum was selected because of its dominance in the roadside grassland where the soil was collected (P. García-Palacios, personal observation). After 8 weeks of growth, L. multiflorum plants were harvested and their shoots air-dried at 60 °C to constant weight and cut into 4 cm length pieces. To generate the two nutrient availabilities, different amounts of L. multiflorum shoots were added: 2.24 g and 4.48 g in the low and high nutrient availability treatments, respectively, which is equivalent to 50 mg and 100 mg N per microcosm, respectively. Within each NA level, the organic material was either added as a patch (heterogeneous treatment) or homogeneously mixed with the background soil along the entire pot volume. In the heterogeneous microcosms, we mixed 100 cm3 of background soil with the organic material and introduced this mix into a plastic cylinder consisting of a light mesh (Fig. 1). A second (control) cylinder, filled only with background soil, was placed alongside the patch cylinder. In the homogeneous microcosms, two plastic cylinders were placed within the pot as in the heterogeneous treatments. These patches were filled up with the same mixture of organic material and background soil present in the rest of the homogeneous pot.
Schematic representation (not drawn to scale) of the microcosms used in the experiment. In the heterogeneous treatments, a plastic cylinder was filled with the organic material and the other (control) was filled only with the soil + sand mixture. In the homogeneous treatments, the whole pot and the two plastic cylinders were filled with a homogeneously distributed mixture of soil + sand + organic matter
Community-aggregated plant functional traits
Two different plant functional traits were calculated at the community level from leaf and root samples collected in June 2009: specific leaf area (SLA) and specific root length (SRL). Both traits encompass a different portion of the species ecological niche and hence are complementary to assess the resource use strategy of plant species (Maire et al. 2009). Furthermore, SRL is a key trait to study root foraging responses to environmental filters such as soil nutrient availability and heterogeneity, because it allows separating between root biomass investments and realised root length densities (Kembel et al. 2008; Mommer et al. 2011). Specific leaf area (cm2/g) is the light-intercepting area of a leaf per unit of investment in dry mass (Reich et al. 1992). Specific root length (m/g) is the ratio of root length to dry mass (Cornelissen et al. 2003).
At the end of the experiment (June 2009), one fully expanded, not senescent well-lit leaf was harvested from every individual in each microcosm and scanned immediately. The leaves were subsequently oven-dried at 70 °C to constant weight, and weighed to the nearest microgram using a microbalance (Micro UMX-MX, Metler Toledo, Barcelona, Spain). Using these data, we calculated the average SLA of each species in each microcosm. Although SLA was measured in the optimal phenological moment to evaluate plant biomass in the herbaceous communities studied (García-Palacios et al. 2010), we acknowledge some degree of bias since the peak biomass of species with varying functional traits can differ among and within functional groups. We used three randomly selected soil cores (5 × 20 cm) to measure SRL. Separation of mixed root samples into species has appeared possible only in exceptional cases where species differed considerably in root architectural traits like root diameter and color (Li et al. 2006). Although we could not visually trace back the mixed root samples to individual plant species in most of the microcosms, we were able to confidently trace back one individual from each species in at least one replicate from each factor combination (two NH × two NA levels). We used this data to calculate the species average SRL in each treatment combination, which was assigned to each individual belonging to that species in any of the four scenarios evaluated (two NH × two NA levels). Five fine roots fragments (<2 mm in diameter) were selected from each individual and kept cool and humid in the refrigerator. Roots were washed and carefully sieved (0.2 mm mesh size). One absorptive root (sensu Cornelissen et al. 2003) free of soil particles was selected from each root fragment and scanned. The roots were subsequently oven-dried at 60 °C to constant weight, and weighed to the nearest microgram. Scanned leaves and roots were processed with ImageJ software (http://rsb.info.nih.gov/ij/) to measure leaf area and root length.
To aggregate all the species trait values in the community into a single metric, we used a community weighted mean value (hereafter CWM) for each trait. This metric was calculated as the mean trait values in the community, weighted by the relative biomass of the species carrying each value (Garnier et al. 2004). It represents the expected functional trait value of a random community sample, often understood as the dominant trait value in a community (Díaz et al. 2007; Lavorel et al. 2008). According to the mass ratio hypothesis (Grime 1998), where ecosystem functioning is majorly determined by trait values of the dominant species, the surrogates of ecosystem functioning evaluated should be predictable from the CWM of SLA and SRL if they are significant effect traits affecting these surrogates. The aboveground biomass of each species in each microcosm was used to weight SLA. Aboveground biomass was also used to weight SRL due to the difficulty in assessing root biomass for each species in the community (Holdaway et al. 2011).
Surrogates of ecosystem functioning
The cores used for SRL measurements in June 2009 were also employed to estimate belowground biomass and measure several surrogates of ecosystem functioning. Additionally, total root biomass from 16 microcosms containing all possible treatment combinations was measured. In those microcosms, root density at the three soil cores was a good predictor of total root biomass (R2 = 0.67, P < 0.0001, n = 16). This relationship was used to compute total belowground biomass in all the microcosms of the experiment. After harvesting, the soil was bulked, sieved (2 mm mesh) and air-dried for 15 days prior to analyses. We measured the activity of β-glucosidase and acid phosphatase enzymes, and in situ N availability rate as surrogates of C, P and N cycling. Extracellular enzymes are the proximate agents of organic matter decomposition, deconstructing plant cell walls, and measures of these activities can be used as indicators of nutrient cycling (Sinsabaugh et al. 2008). The principal functions of these two enzymes in the C and P cycles are hydrolysis of cellobiose to glucose in the case of the β-glucosidase, and hydrolysis of phosphomonoesters to phosphates in the case of the acid phosphatase (Sinsabaugh et al. 2008). The activity of acid phosphatase and β-glucosidase was measured using p-nitrophenyl phosphate (Tabatabai and Bremner 1969) and p-nitrophenyl-b-D-glucopyranoside (Tabatabai 1982) as substrates, respectively. In situ Mineral N availability rate was measured with anionic and cationic exchange membranes as the sum of NH +4 -N and NO -3 -N extracted from the membranes divided by the time period (25 days). This rate is often related with the N mineralization rate (Subler et al. 1995) and provides one of the most reliable indices of plant nutrient availability (Ziadi et al. 1999). The laboratory techniques used to measure these soil variables are described in detail in García-Palacios et al. (2011a).
Statistical analyses
Pearson correlations were used to evaluate the relationships between the community-aggregated plant functional traits and the surrogates of ecosystem functioning. We modelled the network of relationships between the two community-aggregated plant functional traits (SLAagg and SRLagg hereafter) and the surrogates of soil functioning (β-glucosidase, phosphatase, Mineral-N and belowground biomass) using structural equation modelling (SEM, Grace 2006). SEM has been identified as an appropriate tool to investigate interactions between multiple traits and ecosystem functioning based on prior knowledge (Vile et al. 2006). We constructed a different model for each of the four surrogates of soil functioning in all the four scenarios evaluated (low and high nutrient availability × homogeneous and heterogeneous distribution of the organic material), totaling sixteen models. The effects of PFG were pooled into these two factors because we were interested in evaluating the relationships between plant traits and ecosystem functioning in plant communities differing in composition, regardless of the functional group considered.
We assessed the model fit of SEMs using the traditional χ 2 goodness-of-fit test, but because of its sensitivity to sample size, the Normed Fit Index (NFI) and the Root Mean Square Error of Approximation (RMSEA) index were also considered (Grace 2006). The RMSEA index is useful when the null hypothesis is not of exact fit, and the advantage of the NFI is its sensitivity to model misspecifications (Schermelleh-Engel and Moosbrugger 2003). Unlike many statistical tests, low probability values indicate that the covariance matrix implied by the model does not fit the covariance matrix derived directly from the data. P values higher than 0.05 and 0.01 in the RMSEA and χ 2 indices, respectively, are required to guarantee an acceptable fit (Schermelleh-Engel and Moosbrugger 2003). An acceptable fit indicates that the structure of the model is a reasonable explanation of the covariance structure among the variables. As the number of sample moments (3 sample covariances + 3 sample variances) was equal to the number of distinct parameters to be estimated (2 regression coefficients + 1 free covariance + 3 free variances), the number of degrees of freedom was zero. Consequently, no probability level could be assigned to the chi-square statistic, making the model untestable. To solve this problem, the free covariance weight between SLAagg and SRLagg was fixed, and the best solution was chosen through maximization of the maximum likelihood function. When a satisfactorily fitting model is developed, path coefficients estimates are obtained, using the maximum likelihood estimation technique (Grace 2006). Since multiple models were tested, we adjusted goodness-of-fit tests for possible Type I error with sequential Bonferroni corrections (Holm 1979), and assessed the fit following the recommendations for model evaluation proposed by Schermelleh-Engel and Moosbrugger (2003). Correlations and SEM analyses were carried out using SPSS version 14.0 and AMOS version 19.0 (SPSS Inc., Chicago, IL, USA), respectively.
Results
SLAagg was positively correlated with the activity of the two soil enzymes (Table 1), although this relation was only marginally significant in the case of the β-glucosidase (P = 0.064). In contrast, SLAagg was negatively correlated with the in situ availability rate of mineral-N and with belowground biomass. SRLagg was negatively associated with the activity of both enzymes and with the in situ availability rate of mineral-N (Table 1), but positively associated with belowground biomass (P < 0.001).
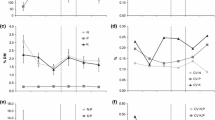
All the SEM evaluated, except two of the belowground biomass models, fitted the data acceptably, as indicated by the several goodness-of-fit statistics (Fig. 2). These models were able to explain ca. 20 % of the β-glucosidase variance in both high nutrient availability scenarios (Fig. 2b and d). However, they had little explanatory power for the phosphatase enzyme, except for the high nutrient availability plus heterogeneous distribution scenario (r 2 = 0.14; Fig. 2d). The models conducted were able to explain up to 20 % of the variation in mineral-N in all the scenarios evaluated, excepting the high nutrient availability plus heterogeneous distribution scenario (r 2 = 0.11; Fig. 2d). In the case of the belowground biomass, our SEM model was able to explain ca. 40 % of the belowground biomass variance in the high nutrient availability plus heterogeneous distribution scenario (Fig. 2d), but had smaller explanatory power in the others (Fig. 2a-c).
Structural equation models for the effects of the community-aggregated specific leaf area (SLAagg) and specific root length (SRLagg) on the surrogates of soil functioning (activity of β-glucosidase and phosphatase, in situ availability rate of mineral-N and belowground plant biomass) in each of the four scenarios evaluated. a low nutrient availability (NA), homogeneous spatial distribution of nutrients (NH), b high NA, homogeneous NH, c low NA, heterogeneous NH, and d high NA, heterogeneous NH. Single-headed arrows signify a hypothesized causal influence of one variable upon another. Double-headed arrows signify a correlation in which no direction is specified, possibly owing to shared causal influences. Numbers adjacent to arrows are path coefficients, analogous to regression weights and indicative of the effect size of the relationship. Width of arrows is proportional to the strength of path coefficients. r 2 indicates the proportion of variance explained. Goodness-of-fit statistics for each model are shown in the lower right corner. Significance levels are as follows: *P < 0.05, **P < 0.01 and ***P < 0.001. Sample size was 36 for the enzymes and belowground biomass models, and 24 for the mineral-N models
The correlation coefficient between SLAagg and SRLagg was ca. −0.30 in all the scenarios evaluated, although it was only significant in the high nutrient availability plus heterogeneous distribution scenario (r = −0.40 in the enzymes and belowground biomass models, and r = −0.49 in the mineral-N model; Fig. 2). We found a significant negative effect of SLAagg in the mineral-N models under low nutrient availability (Fig. 2a and c). When nutrients were supplied at a higher rate, SRLagg was negatively related with the activity of β-glucosidase (Fig. 2b and d). Interestingly, in the high nutrient availability and heterogeneous distribution scenario, we found strong and significant effects of SRLagg on all the surrogates of ecosystem functioning evaluated excepting the mineral-N (Fig. 2d). This trait had a negative effect on both soil enzymes and mineral-N, but had a positive effect on belowground biomass.
Discussion
Most of the studies relating functional traits with ecosystem functioning have evaluated surrogates that change slowly along the time, such as total soil nutrient pools (Lavorel and Garnier 2002; Garnier et al. 2004), instead of dynamic variables measuring real process rates. We found significant correlations between SLAagg and SRLagg and surrogates of C, N and P cycling, allowing us to scale from dominant resource-use strategies in plant communities to ecosystem functioning. The positive relationships between community-aggregated SLA and the activity of β-glucosidase and phosphatase found suggest that plant communities with high litter quality (high SLAagg), and hence fast decomposition rates (Garnier et al. 2004; Wardle 2002), enhance soil C and P cycling. This result supports previous studies where SLA was found to affect ecosystem processes such as litter decomposition rate, total soil carbon and nitrogen accumulation (Garnier et al. 2004; Díaz et al. 2007). However, this positive relationship could be also driven by the negative correlation found between SLAagg and SRLagg (ρ = −0.279, P = 0.001), since SRLagg is negatively associated with both enzymatic activities (Table 1). The negative correlation found between SLAagg and belowground biomass suggests a decrease in root production in the communities with an improved light harvesting strategy.
SRL is often positively related with efficiency of soil resource uptake per unit root mass, root turnover rates and root N concentrations (Cornelissen et al. 2003; Canadell et al. 1996), hence affecting root decomposition and soil nutrient cycling. The negative association found between SRLagg and the surrogates of C, N and P cycling evaluated could be due to a spurious relationship promoted by other traits associated with belonging to a specific functional group, as SRLagg and nutrient cycling are both highly determined by the identity of the functional group considered (Appendix B and García-Palacios et al. 2011a). Non-legume forb communities showed the highest values of SRLagg (Appendix B), which were related with an increased belowground biomass. García-Palacios et al. (2011a) found that non-legume forb communities were plastic enough to compensate their low root foraging precision with a high physiological ability to acquire N from the organic patches. This foraging strategy supports the positive association between SRLagg and belowground biomass, as high SRL is often related with high root system densities in nutrient-poor soil conditions, such as our model system (Mommer et al. 2011; but see Comas and Eissenstat 2004).
Trait variation in plant communities may have its greatest impact on ecosystem functioning in environments where limiting resources are spatially heterogeneous (Cardinale et al. 2000; Tylianakis et al. 2008). These impacts are especially strong when resource heterogeneity promotes an increase in resource availability (Lamb et al. 2004; Maestre et al. 2005; Maestre and Reynolds 2007). Our results allow us to move a step forward and provide a mechanistic explanation for the tight links between plant communities and ecosystem functioning observed in heterogeneous environments. Soil nutrient heterogeneity promotes a strong link between trait variation in plant communities and ecosystem functioning through effects on root traits such as SRL. Intense relations between SRLagg and ecosystem functioning surrogates were only found in the high nutrient availability plus heterogeneous distribution scenario.
The negative correlation between SLAagg and SRLagg was significant only in the high availability and heterogeneity distribution scenario. Although SRL and SLA are usually positively related (Wright and Westoby 1999; Grime 2006), resource availability and heterogeneity have been found to promote positive, negative and null evolutionary correlations between these traits at the community level (Reich et al. 2003; Kembel and Cahill 2011). In this scenario, SRLagg showed the strongest negative effect upon the three surrogates of nutrient cycling. However, plant communities with high SRL should have fast root decomposition rates and an efficient nutrient cycling (Craine et al. 2005), which is at odds with our results. Can we explain this counterintuitive pattern? Although we do not have data to support the following suggestion, we speculate that roots with high SRL, and hence thin diameters and high turnover rates (Craine et al. 2005; Comas and Eissenstat 2009), will have lower ability to associate with soil microbes promoting nutrient cycling. Ad example, a high mortality or turnover rate makes it harder for N-fixing bacteria and mycorrhizal fungi to colonize plant roots (Eissenstat et al. 2000; Menge et al. 2007). This link may be stronger in nutrient heterogeneous environments where species-specific mutualisms between plant roots and mycorrhizal fungi have been found to be potential key determinants of the soil heterogeneity effects on relationships between plants and nutrient cycling (Hodge et al. 2000, 2001). Plant communities experiencing the highest (non-legume forbs) and lowest (legumes and grasses) SRLagg could be inhibiting and promoting, respectively, soil nutrient cycling (Craine et al. 2002; Niklaus et al. 2006; McLaren and Turkington 2010). The enhancement of C cycling found by García-Palacios et al. (2011a) in plant communities with low SRL (legumes) and heterogeneous supply of soil nutrients supports this hypothesis.
Interestingly, SLAagg showed a negative relationship with the in situ availability rate of mineral N in the low nutrient availability scenario. This result suggests a link between litter quality and nitrogen mineralization rates in nutrient-poor soils (Wardle 2002). Similar effects have been found during the initial phases of succession on nutrient-poor substrates, where plant community composition alters the functioning of microbial communities, which in turn, changes the supply of soil N to plants (Berendse 1998; García-Palacios et al. 2011b). This feedback is likely to be most evident in ecosystems with low organic matter content, such as our microcosms, where existing substrate pools in the soil are small relative to the inputs of nutrients entering the soil from plant detritus (Zak et al. 2003). This effect supports our second hypothesis of nutrient availability increasing the strength of the relationship between plant traits and soil functioning.
Scaling from soil heterogeneity-plant responses to the ecosystem level is complicated, because it is difficult to distinguish between the effects of factors such as inter-annual variation in key resources or nutrient availability, and those of soil heterogeneity on ecosystem functioning (Huber-Sannwald and Jackson 2001). Here we provided an example on how introducing soil heterogeneity into a trait-based response-effect framework, as first suggested by Kembel et al. (2008), may help to disentangle the complex effects of soil heterogeneity on ecosystem functioning. The functional traits approach allowed us to get new insights that were missed when a simple plant functional group classification was used (García-Palacios et al. 2011a). As an example, the non-legume forb communities showed higher belowground biomass in the high nutrient availability plus heterogeneous distribution scenario (García-Palacios et al. 2011a). This result is in accordance with the positive relationship found between SRLagg and belowground biomass, but does not match when evaluating the surrogates of C, N and P cycling. Therefore, at fine spatial scales, the use of quantitative traits provides a more accurate description and allows identifying those plant attributes (e.g. root length density, diameter and turnover rate) influencing ecosystem responses to soil heterogeneity.
In conclusion, soil heterogeneity modulates the effects of traits on ecosystem functioning and articulates the role of nutrient availability on such effects. Specific root length in our nutrient-poor soil was the key trait associated with soil nutrient cycling and belowground biomass in contrasted heterogeneous soil conditions. The explicit consideration of soil heterogeneity offers great potential to fully disentangle the mechanisms underlying the effects of trait variation in plant communities on ecosystem functioning, particularly under the ongoing increases in nutrient availability being experienced by terrestrial environments worldwide (Lee et al. 2010).
References
Berendse F (1998) Effects of dominant plant species on soils during succession in nutrient-poor ecosystems. Biogeochemistry 42:73–88
Canadell J, Jackson RB, Ehleringer JR, Mooney HA, Sala OE, Schulze ED (1996) Maximum rooting depth of vegetation types at the global scale. Oecologia 108:583–595
Cardinale BJ, Nelson K, Palmer MA (2000) Linking species diversity to the functioning of ecosystems: on the importance of environmental context. Oikos 91:175–183
Comas LH, Eissenstat EM (2004) Linking root traits to maximum potential growth rate among eleven mature temperate tree species. Funct Ecol 18:388–397
Comas LH, Eissenstat DM (2009) Patterns in root trait variation among 25 co-existing North American forest species. New Phytol 182:919–928
Cornelissen JHC, Lavorel S, Garnier E, Diaz S, Buchmann N, Gurvich DE, Reich PB, ter Steege H, Morgan HD, van der Heijden MGA, Pausas JG (2003) Handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust J Bot 51:335–380
Craine JM, Froehle J, Tilman DG, Wedin DA, Chapin FS III (2001) The relationships among root and leaf traits of 76 grassland species and relative abundance along fertility and disturbance gradients. Oikos 93:274–285
Craine JM, Tilman DG, Wedin DA, Reich PB, Tjoelker MJ, Knops JMH (2002) Functional traits, productivity and effects on nitrogen cycling of 33 grassland species. Funct Ecol 16:563–574
Craine JM, Lee WG, Bond WJ, Williams RJ, Johnson LC (2005) Environmental constraints on a global relationship among leaf and root traits. Ecology 86:12–19
de Kroon H, Hendriks M, van Ruijven J, Ravenek J, Padilla FM, Jongejans E, Visser EJW, Mommer L (2012) Root responses to nutrients and soil biota: drivers of species coexistence and ecosystem productivity. J Ecol 100:6–15
Díaz S, Cabido M, Casanoves F (1999) Functional implications of trait–environment linkages in plant communities. In: Weiher E, Keddy P (eds) Ecological assembly rules – perspectives, advances, retreats. Cambridge University Press, Cambridge, pp 338–362
Díaz S, Lavorel S, de Bello F, Quétier F, Grigulis K, Robson TM (2007) Incorporating plant functional diversity effects in ecosystem service assessments. PNAS 104:20684–20689
Eissenstat DM, Wells CE, Yanai RD, Whitbeck JL (2000) Building roots in a changing environment: implications for root longevity. New Phytol 147:33–42
Fransen B, de Kroon H (2001) Long-term disadvantages of selective root placement: root proliferation and shoot biomass of two perennial grass species in a 2-year experiment. J Ecol 89:711–722
García-Palacios P, Soliveres S, Maestre FT, Escudero A, Castillo-Monroy AP, Valladares F (2010) Dominant plant species modulate responses to hydroseeding, irrigation and fertilization during the restoration of motorway slopes. Ecol Eng 36:1290–1298
García-Palacios P, Maestre FT, Gallardo A (2011a) Soil nutrient heterogeneity modulates ecosystem responses to changes in the identity and richness of plant functional groups. J Ecol 99:551–562
García-Palacios P, Bowker MA, Chapman SJ, Maestre FT, Soliveres S, Gallardo A, Valladares F, Guerrero C, Escudero A (2011b) Early-successional vegetation changes after roadside prairie restoration modify processes related with soil functioning by changing microbial functional diversity. Soil Biol Biochem 43:1245–1253
Garnier E, Cortez J, Billès G, Navas ML, Roumet C, Debussche M, Laurent G, Blanchard A, Aubry D, Bellmann A, Neill C, Toussaint JP (2004) Plant functional markers capture ecosystem properties during secondary succession. Ecology 85:2630–2637
Grace JB (2006) Structural equation modeling and natural systems. Cambridge University Press, New York
Grime JP (1998) Benefits of plant diversity to ecosystems: immediate, filter and founder effects. J Ecol 86:902–910
Grime JP (2006) Trait convergence and trait divergence in herbaceous plant communities: mechanisms and consequences. J Veg Sci 17:255–260
Gross KL, Pregitzer KS, Burton AJ (1995) Spatial variation in nitrogen availability in three successional plant communities. J Ecol 83:357–367
Hodge A, Robinson D, Fitter AH (2000) Are microbes more effective than plants at competing for nitrogen? Trends Plant Sci 5:304–308
Hodge A, Campbell CD, Fitter AH (2001) An arbuscular mycorrhizal fungus accelerates decomposition and acquires nitrogen directly from organic material. Nature 413:297–299
Holdaway RJ, Richardson SJ, Dickie IA, Peltzer DA, Coomes DA (2011) Species- and community-level patterns in fine root traits along a 120 000-year soil chronosequence in temperate rain forest. J Ecol 99:954–963
Holm S (1979) A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70
Huber-Sannwald E, Jackson RB (2001) Heterogeneous soil-resource distribution and plant responses-from individual-plant growth to ecosystem functioning. Prog Bot 62:451–476
Jackson RB, Caldwell MM (1993) Geostatistical patterns of soil heterogeneity around individual perennial plants. J Ecol 81:683–692
Jackson RB, Manwaring JH, Caldwell MM (1990) Rapid physiological adjustment of roots to localized soil enrichment. Nature 344:58–60
Kembel SW, Cahill JF Jr (2011) Independent evolution of leaf and root traits within and among temperate grassland plant communities. PLoS One 6:e19992
Kembel SW, de Kroon H, Cahill JF Jr, Mommer L (2008) Improving the scale and precision of hypotheses to explain root foraging ability. Ann Bot 101:1295–1301
Lamb EG, Haag JJ, Cahill JF Jr (2004) Patch-background contrast and patch density have limited effects on root proliferation and plant performance in Abutilon theophrasti. Funct Ecol 18:836–843
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol 16:545–556
Lavorel S, Grigulis K, McIntyre S, Garden D, Williams N, Dorrough J, Berman S, Quétier F, Thébault A, Bonis A (2008) Assessing functional diversity in the field –methodology matters! Funct Ecol 22:134–147
Lee M, Manning P, Rist J, Power SA, Marsh C (2010) A global comparison of grassland biomass responses to CO2 and nitrogen enrichment. Phil Trans R Soc B 365:2047–2056
Li L, Sun JH, Zhang FS, Guo T, Bao X, Smith FA, Smith SE (2006) Root distribution and interactions between intercropped species. Oecologia 147:280–290
Maestre FT, Reynolds JF (2006) Nutrient availability and atmospheric CO2 partial pressure modulate the effects of nutrient heterogeneity on the size structure of populations in grassland species. Ann Bot 98:227–235
Maestre FT, Reynolds JF (2007) Grassland responses to the heterogeneity and availability of two key resources. Ecology 88:501–511
Maestre FT, Bradford MA, Reynolds JF (2005) Soil nutrient heterogeneity interacts with elevated CO2 and nutrient availability to determine species and assemblage responses in a model grassland community. New Phytol 168:637–650
Maire V, Gross N, da Silveira Pontes L, Picon-Cochard C, Soussana JF (2009) Trade-off between root N acquisition and shoot N utilisation across 13 co-occurring pasture grass species. Funct Ecol 23:668–679
McLaren JR, Turkington R (2010) Ecosystem properties determined by plant functional group identity. J Ecol 98:459–469
Menge DNL, Levin SA, Hedin LO (2007) Evolutionary tradeoffs can select against nitrogen fixation and thereby maintain nitrogen limitation. PNAS 105:1573–1578
Mommer L, Dumbrell AJ, Wagemaker CAM, Ouborg NJ (2011) Belowground DNA based techniques: untangling the network of plant root interactions. Plant Soil 348:115–121
Niklaus PA, Wardle DA, Tate KR (2006) Effects of plant species diversity and composition on nitrogen cycling and the trace gas balance of soils. Plant Soil 282:83–98
Rajaniemi T, Reynolds HL (2004) Root foraging for patchy resources in eight herbaceous plant species. Oecologia 141:514–525
Reich PB, Walters MB, Ellsworth DS (1992) Leaf lifespan in relation to leaf, plant and stand characteristics among diverse ecosystems. Ecol Monogr 62:365–392
Reich PB, Wright IJ, Cavender-Bares J, Craine JM, Oleksyn J, Westoby M, Walters MB (2003) The evolution of plant functional variation: traits, spectra, and strategies. Int J Plant Sci 164:143–164
Schermelleh-Engel K, Moosbrugger H (2003) Evaluating the fit of structural equation models: tests of significance and descriptive goodness-of-fit measures. Meth Psych Res 8:23–74
Sinsabaugh RL, Lauber CL, Weintraub MN, Ahmed B, Allison SD, Crenshaw C, Contosta AR, Cusack D, Frey S, Gallo ME, Gartner TB, Hobbie SE, Holland K, Keeler BL, Powers JS, Stursova M, Takacs-Vesbach C, Waldrop M, Wallenstein M, Zak DR, Zeglin LH (2008) Stoichiometry of soil enzyme activity at global scale. Ecol Lett 11:1252–1264
Subler S, Blair JM, Edwards CA (1995) Using anion-exchange membranes to measure soil nitrate availability and net nitrification. Soil Bio Biochem 27:911–917
Suding KN, Lavorel S, Chapin FS, Cornelissen H, Diaz S, Garnier E, Goldberg D, Hooper DU, Jackson ST, Navas ML (2008) Scaling environmental change through the community-level: a trait-based response-and-effect framework for plants. Glob Chang Biol 14:1125–1140
Tabatabai MA (1982) Soil enzymes. In: Page AL, Miller RH, Keeney DR (eds) Methods of soil analysis. Part 2. Agronomical monograph No. 9. 2nd ed. American Society of Agronomy and Soil Science of America, Madison, pp 501–538
Tabatabai MA, Bremner JM (1969) Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol Biochem 1:301–307
Tilman D (1982) Resource competition and community structure. Monographs in population biology. Princeton University Press, New Jersey
Tylianakis T, Rand TA, Kahmen A, Klein AM, Buchmann N, Perner J, Tscharntke T (2008) Resource heterogeneity moderates the biodiversity-function relationship in real world ecosystems. PLOS Biol 6:947–956
Vile D, Shipley B, Garnier E (2006) A structural equation model to integrate changes in functional strategies during old-field succession. Ecology 87:504–517
Wahl S, Ryser P (2000) Root tissue structure is linked to ecological strategies of grasses. New Phytol 148:459–471
Wardle D (2002) Communities and ecosystems: linking the aboveground and belowground components. Monographs in population biology v. 34. Princeton University Press, Madison
Weiher E, Keddy P (1999) Ecological assembly rules: perspectives, advances, retreats. Cambridge University Press, Cambridge
Wijesinghe DK, John EA, Hutchings MJ (2005) Does pattern of soil resource heterogeneity determine plant community structure? An experimental investigation. J Ecol 93:99–112
Wright IJ, Westoby M (1999) Differences in seedling growth behaviour among species: trait correlations and shifts along nutrient compared to rainfall gradients. J Ecol 87:85–97
Zak DR, Holmes WE, White DC, Peacock AD, Tilman D (2003) Plant diversity, soil microbial communities, and ecosystem function: are there any links? Ecology 84:2042–2050
Ziadi N, Simard RR, Allard G, Lafond J (1999) Field evaluation of anion exchange membranes as a N soil testing method for grasslands. Can J Soil Sci 79:281–294
Acknowledgments
We thank Patricia Valiente, Jorge Papadopoulos, Becky Mou, Carlos Díaz, Rafael Sendra and Santiago Soliveres for their help during the greenhouse and laboratory work. PGP was supported by a postdoctoral contract from Comunidad de Madrid (REMEDINAL-2) and by a Fulbright fellowship from the Spanish Ministerio de Educación. FTM is supported by the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement n° 242658. RM was supported by the MICINN-Spain (grants AGL2010-10935-E and CGL2011-28778 and Ramón y Cajal contract). This research was supported by the EXPERTAL grant, funded by Fundación Biodiversidad-CINTRA.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Gerlinde De Deyn.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 376 kb)
Rights and permissions
About this article
Cite this article
García-Palacios, P., Maestre, F.T. & Milla, R. Community-aggregated plant traits interact with soil nutrient heterogeneity to determine ecosystem functioning. Plant Soil 364, 119–129 (2013). https://doi.org/10.1007/s11104-012-1349-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1349-6