Abstract
While it is well-established that the spatial distribution of soil nutrients (soil heterogeneity) influences the competitive ability and survival of individual plants, as well as the productivity of plant communities, there is a paucity of data on how soil heterogeneity and global change drivers interact to affect plant performance and ecosystem functioning. To evaluate the effects of elevated CO2, soil heterogeneity and diversity (species richness and composition) on productivity, patterns of biomass allocation and root foraging precision, we conducted an experiment with grassland assemblages formed by monocultures, two- and three-species mixtures of Lolium perenne, Plantago lanceolata and Holcus lanatus. The experiment lasted for 90 days, and was conducted on microcosms built out of PVC pipe (length 38 cm, internal diameter 10 cm). When nutrients were heterogeneously supplied (in discrete patches), assemblages exhibited precise root foraging patterns, and had higher total, above- and belowground biomass. Greater aboveground biomass was observed under elevated CO2. Species composition affected the below:aboveground biomass ratio and interacted with nutrient heterogeneity to determine belowground and total biomass. Species richness had no significant effects, and did not interact with either CO2 or nutrient heterogeneity. Under elevated CO2 conditions, the two- and three-species mixtures showed a clear trend towards underyielding. Our results show that differences among composition levels were dependent on soil heterogeneity, highlighting its potential role in modulating diversity–productivity relationships.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increases in the atmospheric concentration of CO2 ([CO2]) and changes in species composition and richness of communities are two key features of global environmental change, which is affecting natural ecosystems worldwide (Mendelson and Rosenberg 1994; Chapin et al. 2000). Hence, an enormous research effort has been devoted to understanding the ecological consequences of changes in both [CO2] and biodiversity (reviewed, among others by Bazzaz 1990; Körner and Bazzaz 1996; Loureau et al. 2002). Nevertheless, few studies have evaluated the interactive effects of [CO2] and biotic diversity on the productivity, biomass allocation patterns and composition of plant communities (Niklaus et al. 2001, Reich et al. 2001, 2004; He et al. 2002). The potential response of natural communities to both factors is likely to be nonadditive, which suggests the need for multifactorial experimental approaches when evaluating them (Shaw et al. 2002).
Another environmental variable that has attracted the attention of ecologists is the spatial heterogeneity in the availability of soil-based resources. Such heterogeneity (hereafter termed “soil heterogeneity”) has profound consequences for ecosystem composition, functioning and management (Huber-Sannwald and Jackson 2001). At small spatial scales, it promotes a variety of plant responses, including the proliferation of roots into resource-rich patches and the increase in nutrient uptake rates (Hodge 2004), which modify the competitive ability and survival of individuals, and the productivity of assemblages (Hutchings et al. 2003). Despite being the subject of an large number of studies conducted over the last few decades, soil heterogeneity is a factor that has been somewhat neglected in both biodiversity and elevated [CO2] research (but see Arnone 1997; Maestre et al. 2005, 2006). It has been recently shown that soil heterogeneity interacts separately with species diversity (Maestre et al. 2006) and with [CO2] (Maestre et al. 2005) to determine the biomass responses of grassland assemblages. These results suggest that soil heterogeneity, a ubiquitous feature of most terrestrial ecosystems (Hutchings et al. 2000), may modify the responses of plant individuals and assemblages to joint changes in biodiversity and [CO2].
To our knowledge, no study has evaluated how plant assemblages respond to joint changes in soil heterogeneity, [CO2] and biodiversity. Such studies are essential for advancing our understanding of how global environmental change may impact plant communities, and testing whether soil heterogeneity may modify observed responses to global change drivers. In order to fill this gap, we conducted a microcosm experiment to evaluate the joint effects of [CO2], soil heterogeneity, species richness and species composition (defined here as the list of species present in a particular assemblage) on the productivity, biomass allocation patterns and root foraging precision of grassland assemblages. Our assemblages were formed by monocultures, two- and three-species mixtures of Lolium perenne L., Plantago lanceolata L. and Holcus lanatus L. Using this model system, we tested the hypothesis that the response of assemblage productivity to soil heterogeneity, [CO2] and changes in species composition or richness is not predictable from the responses to any one of these single factors. Statistical interactions between these factors are expected because: (i) both soil heterogeneity and [CO2] have been shown to modify the productivity and biomass allocation patterns of assemblages (Niklaus and Körner 2004; Maestre et al. 2005), (ii) these responses are also affected by the number and identity of the species forming them (He et al. 2002; Reich et al. 2004), and (iii) the species employed differ in traits related to their ability to respond to both [CO2] and soil heterogeneity (Poorter 1993; Robinson and Van Vuuren 1998).
Materials and methods
Experimental design
We conducted a factorial microcosm experiment in the Duke University Phytotron between January and April 2005. The experiment consisted of two levels of [CO2] (37.5 and 70 Pa), two levels of spatial distribution of the organic material (homogeneous and heterogeneous), and seven species combinations (Lolium monocultures, Plantago monocultures, Holcus monocultures, Lolium + Plantago mixtures, Lolium + Holcus mixtures, Plantago + Holcus mixtures and Lolium + Plantago + Holcus mixtures).
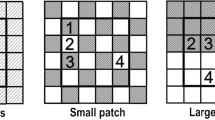
Microcosms consisted of PVC pipe (length 38 cm, internal diameter 10 cm) filled with, from the base, 5 cm of gravel (for drainage), and then 28 cm of a 50:50 mixture of soil and sand (hereafter referred to as “background soil”; Fig. 1). The soil, a sandy loam, was collected from the top 30 cm at a site in the Duke Forest (35°55′N, 78°52′W). On top of the background soil we placed a 2 cm layer of a 50:50 mixture of organic Duke Forest soil:peat to avoid the formation of physical crusts in the surface of the microcosms, and to recreate the typical accumulation of organic matter in the topsoil of temperate grasslands. To recreate realistic microbial communities, all microcosms were irrigated with 200 ml of a soil microbial “inoculum”. To obtain it, 5 kg of fresh soil from turf communities (containing Trifolium repens L., Plantago, Holcus, Lolium and Anthoxanthum odoratum L.) surrounding the Phytotron was mixed with 50 l of water and the mixture agitated every 8 h for two days. The resulting solution was filtered with a 106 μm sieve and added to the microcosms prior to addition of the organic soil. In addition, 50 ml of a 106 μm sieved solution derived from root macerations (roots were collected from the turf communities described above) were applied to all microcosms twice during the first ten days of growth.
To each microcosm we added 1.036 g of air-dried and ground (<2 mm) T. repens shoots (3.9% N, 10.8 C:N), equivalent to adding 40 mg of N per microcosm, collected from turf communities surrounding Duke University. This organic material was added homogeneously (homogeneous treatment) or as a patch (heterogeneous treatment). In both cases the same amount of nutrient was added in order to maintain the same overall nutrient availability. In the homogeneous treatment, we thoroughly mixed the organic material with the background soil before introducing it into the PVC pipe. In the heterogeneous treatments, the organic material was localized within discrete 31 cm3 volumes of soil (Fig. 1). To create one of these patches we mixed 25 cm3 (approximately) of background soil with the organic material and introduced the resulting mix into a 31 cm3 plastic cylinder (length 75 mm and internal diameter 23 mm) consisting of a light mesh with square pores 5 × 10 mm in size (we hereafter refer to this as the patch cylinder). A second (control) cylinder, filled only with background soil, was placed 2 cm apart from and alongside the patch cylinder. Both cylinders were placed 18 cm above the gravel layer. Both the microcosms and the patch cylinder receiving the organic material were marked, so all the cylinders receiving the nutrients were placed in the same position. In the homogeneous treatment, two plastic cylinders were introduced in the same way, but were filled with the background soil mixed with the organic material.
Seeds from the three species (obtained from commercial suppliers) were placed in trays with plant growth medium (Metro-Mix 200, Scotts Company, OH, USA) and germinated in a growth chamber (20 °C temperature and PAR of 350 μmol m−2 s−1 with a 14 h photoperiod). Owing to different germination times, as revealed by a previous test conducted with these seeds, the three species were germinated on different days to ensure that all the species had the same degree of development (one-leaf stage) and a similar size at the start of the experiment. Within each microcosm, the planting positions were allocated at random, but the same planting grid was maintained in each of the microcosms by using a wire grid pattern secured to the top of the containers. Monocultures contained six seedlings of a single species, two species mixtures three seedlings of each of two species, and three species mixtures two seedlings of each species. In this way, the total plant density across microcosms was kept constant (764 seedlings m−2). Seedlings that died during the first week of the experiment were replaced. After that period, no further mortality was observed.
We established four replicated microcosms for each of the 28 treatment combinations, resulting in a total of 112 microcosms (2 [CO2] levels × 2 spatial distributions of the organic material levels × 7 species composition levels × 4 replicates). The microcosms were placed in four walk-in growth chambers (two for each [CO2] level), within which air temperature and [CO2] were independently controlled. For each [CO2] level, half of the microcosms per treatment were randomly assigned to one of the chambers (28 microcosms per chamber), and were then randomly grouped in two wheeled trolleys. To minimize possible chamber effects, the [CO2] levels and trolleys were rotated between chambers every week. This process was repeated 12 times during the experiment, and at harvest all of the microcosms had spent the same amount of time in each chamber. After every rotation, the position of the microcosms within each chamber was randomized. Temperatures in the growth chambers ranged from 12 °C at night to 21 °C during the day, and this regime included a simulated dawn and dusk period, each of 2 h duration, where the temperature was gradually ramped up or down. Relative humidity followed a similar pattern, ranging from 85 to 70%, as did the lights. PAR was maintained at 500 μmol m−2 s−1 during the first week of the experiment, 750 μmol m−2 s−1 during the second week of the experiment, and at 1,000 μmol m−2 s−1 thereafter. This gradual ramping-up of light intensity was used to prevent high-light shock responses. Each microcosm was irrigated daily with 30 ml of distilled water during the first two weeks of the experiment, and with 50 ml hereafter. To reduce limitations on plant growth due to low overall soil fertility, all of the microcosms were watered with 50 ml of a nutrient solution containing 35 mg of Ca (added as CaCl2·2H20) and 29 mg of Mg (added as MgSO4·7H2O) twice during the course of the experiment (1 February and 1 March).
Harvest
Plants were grown in the chambers for 90 days, a period equivalent to a growing season. After this time, the aboveground biomass of all the microcosms was cut at the soil surface and sorted by species. Leaves and stems were dried at 60 °C until constant weight. Once the aboveground biomass was removed, volumetric soil moisture (0–12 cm depth) was measured in all the microcosms using a HydroSense probe (Campbell Scientific, Logan, UT, USA). After these measurements, the soil was carefully removed from the microcosm unit and the roots were harvested. We extracted the roots within each cylinder by cutting those outside it; the rest of the root system was also collected, and all the roots were dried as described above. In the mixtures it was not possible to separate the roots by species.
Precision of root foraging (i.e., the ability of a plant to proliferate roots into a nutrient patch) was measured in each of the microcosms with the index RII (Armas et al. 2004). In the heterogeneous treatments, RII was calculated as (RBp − RBc)/(RBp + RBc), where RBp and RBc are the root biomasses in the patch and control cylinders, respectively. RII ranges from −1 to +1, a value of zero indicating equal root growth in patches and background soil, and no precision of foraging. Increasingly positive values indicate increasing precision, and negative values the opposite (i.e., avoidance of nutrient patches by roots). In the homogeneous treatment, one of the cylinders was selected as a patch cylinder (the one located in the same place as the patch cylinder in the heterogeneous treatments), and the other as a control.
Two indices were used to compare the yields of the mixtures relative to their component monocultures: the relative yield total (RYT, de Wit 1960) and D max (Loreau 1998). Relative yield is the total species biomass in a mixture divided by its average monoculture biomass for the same combination of [CO2] and heterogeneity treatments. Since the RYT for a mixture is the sum of the relative yields for all species, a RYT value greater than 1 indicates “nontransgressive” overyielding (production in a mixture that exceeds the average biomass of its component monocultures; Roscher et al. 2005). D max was estimated as (TBM − MON)/MON, where TBM and MON are the total biomass of a given mixture and the total biomass of its component species with the largest monoculture value for the same combination of [CO2] and heterogeneity treatments, respectively. D max values higher than 0 indicate “transgressive” overyielding (production in a mixture that exceeds its most productive component monoculture; Roscher et al. 2005).
Statistical analyses
We evaluated the effects of species richness (SR, three levels), species composition (SC, seven levels), [CO2] (two levels) and soil heterogeneity (SH, two levels) on assemblage biomass (total, above- and belowground), below:aboveground biomass ratio and soil moisture with a four-way nested ANOVA, with SC nested within SR. In these analyses, SR terms were tested against the appropriate SC terms; all other terms were tested against the error term. This approach tests whether there is a significant effect of increasing species richness over and above possible effects of species composition (Giller et al. 2004). To control for differences in plant size when evaluating the patterns of biomass allocation (Reich 2002), we analyzed the residuals from a regression between the log-transformed below:aboveground ratio (dependent variable) and the log-transformed total biomass data (independent variable). Root foraging precision data in the heterogeneous treatments were analyzed with a three-way nested ANOVA (with [CO2], SC and SR as main factors) as described above. RYT and D max data were analyzed with a three-way ANOVA, with richness (two vs three species), [CO2] and SH as main factors; in these analyses, all the main effects and the interactions were considered fixed, and thus were tested against the residual term. To satisfy ANOVA assumptions, soil moisture data were transformed with a power function (x 1.3), aboveground biomass and RYT data were square-root transformed, and total biomass, belowground biomass and the below: aboveground biomass ratio data were log-transformed. All statistical analyses were performed using SPSS 10.0 (SPSS Inc., Chicago, IL, USA). Although we conducted a large number of statistical tests, given the ANOVA design we used, P values were not adjusted for multiple testing, as this approach is considered overly conservative (Gotelli and Ellison 2004).
Results
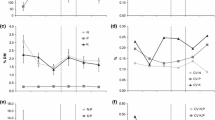
The assemblages had higher aboveground biomass under elevated [CO2] and when the nutrients were heterogeneously supplied (Fig. 2; Table 1). Aboveground biomass was also influenced by species composition (Fig. 2; Table 1). For belowground biomass, a significant SC × SH interaction was found (Fig. 2; Table 1). To investigate this interaction, we conducted separated ANOVAS for each SH level. These revealed that the differences in belowground biomass among composition levels were modified by SH (see Fig. S1 in the Electronic Supplementary Material). The analysis of total biomass yielded similar results, as a significant SC × SH interaction was found (Table 1). Total, above- and belowground biomass increased with the number of species included in the assemblages (see Fig. S2 in the Electronic Supplementary Material), but this increase was not significant in any case (Table 1). The below:aboveground biomass ratio was affected by SC, but not by the rest of the factors evaluated (Fig. 2; Table 1).
Aboveground biomass ratio (upper panels), aboveground biomass (central panels) and belowground biomass (lower panels) of the assemblages compared among composition, CO2 concentration and soil heterogeneity levels. Data are means +1 SE (n=4). Lo = Lolium perenne monoculture, Pl = Plantago lanceolata monoculture, Hl = Holcus lanatus monoculture, Lopl = Lolium + Plantago mixtures, Plhl = Plantago + Holcus mixtures, Lohl = Lolium + Holcus mixtures, and Plohl = Plantago + Lolium + Holcus mixtures
When the organic material was supplied heterogeneously, the assemblages demonstrated precise root foraging patterns (Fig. 3). [CO2], SC and SR had no significant effects on root proliferation in the heterogeneous treatment (P>0.106 in all cases), nor were any significant interactions found. We found little evidence for overyielding in the two- and three-species mixtures evaluated (Fig. 4). Both the RYT and D max indices were lower under elevated [CO2] (P=0.003 and P<0.001, respectively, no interactions), but were not affected by mixture richness or SH (see Table S1 in the Electronic Supplementary Material).
Root foraging precision into nutrient patches (RII index) compared among composition and soil heterogeneity levels. Data are mean ±95% confidence intervals (n=8). Significant foraging precision is indicated by confidence intervals that do not overlap zero. For an explanation of the x-axis labels see the legend for Fig. 1
Relative total yield and D max data for two- and three-species mixtures compared among CO2 concentration and soil heterogeneity levels. Data are mean ± 95% confidence intervals (n=12 and 4 for the two- and three-species mixtures, respectively). Significant over- or underyielding is shown by confidence intervals that do not overlap unity and zero for the RYT and D max indices, respectively
Discussion
Using model communities, we tested the hypothesis that the response of plant assemblage traits to soil heterogeneity, [CO2] and changes in species composition or richness is not predictable from the assemblage responses to any one of these single factors. Since we found no significant three-way interactions between these factors in any of the response variables evaluated, we must reject this hypothesis. However, we observed significant species composition × soil heterogeneity interactions for total and belowground biomass. These interactions indicate that the effects of species identity are dependent on soil heterogeneity, and suggest that soil heterogeneity may be potentially relevant in modulating diversity–productivity relationships.
Regardless of the identity and number of species forming them, all the assemblages had higher biomass when nutrients were heterogeneously supplied. Such a supply also elicited highly precise root foraging patterns at the assemblage level. It has been shown that when a fixed amount of nutrients is made available to a plant, their acquisition will be more efficient when the nutrients are spatially concentrated because of preferential root allocation in these areas (Jackson and Caldwell 1996). This more efficient nutrient acquisition results in a greater rate of biomass development (Hodge 2004; Maestre et al. 2005). Interestingly, we did not find any significant relationship between root foraging precision and aboveground biomass (data not shown). This suggests that other responses to soil heterogeneity, such as an increase in nutrient uptake (Hodge 2004), may have been relevant in our experiment.
As found in previous studies conducted with grassland species (e.g., Navas et al. 1999; Jones et al. 2000; Niklaus and Körner 2004), elevated [CO2] increased the aboveground biomass of the assemblages. These effects were independent of species richness/composition and soil heterogeneity, as indicated by the lack of interactions among these factors. It is possible that elevated [CO2] effects were mainly mediated by increased water availability because of the increase in the water use efficiency of the assemblages (e.g., Niklaus and Körner 2004). In spite of the fact that microcosms grown under elevated [CO2] had higher biomass than those grown under ambient [CO2], soil moisture at harvest was slightly higher in the former (see Fig. S3 in the Electronic Supplementary Material). In our experiment, the release of nutrients from the organic material added depends on mineralization by the soil microbial and faunal populations, a process that is typically accelerated with increasing water availability (Ebersberger et al. 2003). Thus, the increase in water availability observed under elevated [CO2] could have also increased nutrient mineralization rates and availability to plants in our experiment (Niklaus and Körner 2004).
Results for the RYT and the D max indices were qualitatively similar, although none of the mixtures significantly overyielded when using the latter metric (vs. a mixture when using the RYT). D max is a very demanding test of overyielding (Fridley 2002), and biodiversity studies usually report a higher degree of nontransgressive versus transgressive overyielding (e.g., Fridley 2002; Roscher et al. 2005). Interestingly, the mixtures evaluated showed a clear trend towards underyielding relative to their component monocultures under elevated [CO2]. These responses were driven by a strong biomass increment in the Holcus (+25%) and Plantago (+21%) monocultures relative to that of mixtures containing these species, and by negative interactions between Plantago and the other two species evaluated under elevated [CO2]. A detailed analysis of the relative yield of the different species (data not shown) revealed that the contribution of Lolium to the total aboveground biomass marginally increased (P=0.058) in the Holcus–Lolium mixtures, but remained unchanged and significantly decreased (P=0.047) in the Plantago–Lolium and three-species mixtures, respectively, under elevated [CO2]. Furthermore, the relative yield of Holcus remained unchanged in the Plantago–Holcus mixtures in response to elevated [CO2], despite its larger growth rate and responsiveness to [CO2], but increased (P=0.048) in the three-species mixtures. We note that both Holcus and Lolium were larger than Plantago in the mixtures (F. T. Maestre, personal observation) and, thus, neither aboveground competition for light nor differences in the responsiveness to [CO2] seemly likely to explain the effects of Plantago on both Lolium and Holcus. We speculate that these effects are driven by [CO2]-induced changes in the composition and abundance of rhizosphere microorganisms involved in nutrient cycling (Verhagen et al. 1994) and/or in negative feedbacks between Plantago and coexisting species (Bever 2002).
The lack of a significant [CO2] × richness interaction for the productivity of the assemblages and the trend towards underyielding under elevated [CO2] for the mixtures contrast with the results of previous studies. Using a wider range of richness levels than used here (from 1 to 12 species), Reich et al. (2001) and He et al. (2002) found that the enhancement of biomass accumulation in response to elevated levels of [CO2] was mostly achieved in species-rich assemblages. However, Reich et al. (2004) did not find strong evidence for [CO2] × species richness interactions when evaluating the productivity of assemblages of varying diversity (from 1 to 16 species). We also failed to find overall significant [CO2] × soil heterogeneity interactions when evaluating productivity patterns. However, Maestre et al. (2005) found that the aboveground biomass of assemblages formed by five species (Holcus, Lolium, Plantago, Anthoxanthum odoratum L. and Trifolium repens L.) was affected by [CO2] × soil heterogeneity interactions. These contrasting results are not fully unexpected given the importance of both the experimental context and intra- and interspecific interactions when evaluating the response of grass and forb species to factors such as [CO2] and soil heterogeneity (Navas et al. 1999; Hutchings et al. 2003).
Given that we used natural soil and organic material, the decomposition of the organic material in our experiment may most closely resemble that observed in the field (Hodge 2004). We acknowledge, however, that our experimental approach has limitations. Given the short duration of our experiment, the observed responses might be the result of a transitive effect of a community that is not fully established. This duration, together with the low number of species used and the size of the microcosm units, might not allow some potentially important aspects of niche filling to be expressed (Spehn et al. 2005), and thus effects of species richness on productivity may be underestimated. In addition, growing model communities in PVC tubes may alter root foraging responses due to physical restriction of lateral root growth (Hutchings et al. 2003). Lastly, the standardized growing conditions used may amplify plant responses to the treatments evaluated over those in the field. These limitations clearly indicate that the extrapolation of our results to the natural world must be done with caution. Nevertheless, we are confident that our findings demonstrate potential plant responses to simultaneous changes in [CO2], soil heterogeneity, and species diversity (Jones et al. 2000).
Most of the research conducted to date evaluating the effects of diversity on ecosystem processes in terrestrial ecosystems have used grassland species (Reich et al. 2001; Loreau et al. 2002; van Ruijven and Berendse 2003; Spehn et al. 2005; Roscher et al. 2005), which are known to be highly responsive to soil heterogeneity (Robinson and Van Vuuren 1998). Soil heterogeneity has been identified as a potential “hidden treatment” in biodiversity experiments (Huston and McBride 2002), but has barely been considered when evaluating community and ecosystem responses to changes in biodiversity (Lawton 2000; but see Maestre et al. 2006). Our results suggest that the interactions between soil heterogeneity and diversity attributes are potentially important when determining the productivity of grassland assemblages, and agree with the view that the effects of diversity on ecosystem processes may be dependent upon the environmental context evaluated (Reich et al. 2001; Fridley 2002).
References
Armas C, Pugnaire FI, Ordiales R (2004) Measuring plant interactions: a new comparative index. Ecology 85:2682–2686
Arnone JA III (1997) Temporal responses of community fine root populations to long-term elevated atmospheric CO2 and soil nutrient patches in model tropical ecosystems. Acta Oecol 18:367–376
Bazzaz FA (1990) The response of natural ecosystems to the rising global CO2 levels. Annu Rev Ecol Syst 21:167–196
Bever JD (2002) Negative feedback within a mutualism: host-specific growth of mycorrhizal fungi reduces plant benefit. Proc R Soc Lond B 269:2595–2601
Chapin FS III, Zavaleta ES, Eviner VT, Naylor RL, Vitousek PM, Reynolds HL, Hooper DU, Lavorel S, Sala OE, Hobbie SE, Mack MC, Diaz S (2000) Consequences of changing biodiversity. Nature 405:234–242
Ebersberger D, Niklaus PA, Kandeler E (2003) Long-term CO2 enrichment stimulates N-mineralisation and enzyme activities in calcareous grassland. Soil Biol Biochem 35:965–972
Fridley JD (2002) Resource availability dominates and alters the relationship between species diversity and ecosystem productivity in experimental plant communities. Oecologia 132:271–277
Giller PS, Hillebrand H, Berninger UG, Gessner MO, Hawkins S, Inchausti P, Inglis C, Leslie H, Malmqvist B, Monaghan MT, Morin PJ, O´Mullan G (2004) Biodiversity effects on ecosystem functioning: emerging issues and their experimental test in aquatic environments. Oikos 104:423–436
Gotelli NJ, Ellison AM (2004) A primer of ecological statistics. Sinauer Associates, Sunderland, MA
He JS, Bazzaz FA, Schmid B (2002) Interactive effects of diversity, nutrients and elevated CO2 on experimental plant communities. Oikos 97:337–348
Hodge A (2004) The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytol 162:9–24
Huber-Sannwald E, Jackson RB (2001) Heterogeneous soil-resource distribution and plant responses-from individual-plant growth to ecosystem functioning. Progr Bot 62:451–476
Huston MA, McBride AC (2002) Evaluating the relative strengths of biotic versus abiotic controls on ecosystem processes. In: Loreau M, Naeem S, Inchausti P (eds) Biodiversity and ecosystem functioning: synthesis and perspectives. Oxford University Press, Oxford, pp 47–60
Hutchings MJ, John EA, Stewart AJA (2000) The ecological consequences of environmental heterogeneity. Blackwell, Oxford
Hutchings MJ, John EA, Wijesinghe DK (2003) Toward understanding the consequences of soil heterogeneity for plant populations and communities. Ecology 84:2322–2334
Jackson RB, Caldwell MM (1996) Integrating resource heterogeneity and plant plasticity: modelling nitrate and phosphate uptake in a patchy soil environment. J Ecol 84:891–903
Jones TH, Bezemer TM, Körner Ch, Lawton JH, Thompson LJ (2000) Comparing studies of artificial and natural ecosystem responses to CO2 enrichments. Biotronics 29:1–7
Körner Ch, Bazzaz FA (eds)(1996) Carbon dioxide, population, and community. Academic, San Diego, CA
Lawton JH (2000) Concluding remarks: a review of some open questions. In: Hutchings MJ, John EA, Stewart AJA (eds) The ecological consequences of environmental heterogeneity. Blackwell, Oxford, pp 401–424
Loreau M (1998) Separating sampling and other effects in biodiversity experiments. Oikos 82:600–602
Loreau M, Naeem S, Inchausti P (eds)(2002) Biodiversity and ecosystem functioning: synthesis and perspectives. Oxford University Press, Oxford
Maestre FT, Bradford MA, Reynolds JF (2005) Soil nutrient heterogeneity interacts with elevated CO2 and nutrient availability to determine species and assemblage responses in a model grassland community. New Phytol 168:637–650
Maestre FT, Bradford MA, Reynolds JF (2006) Soil heterogeneity and community composition jointly influence grassland biomass. J Veg Sci 17:261–270
Mendelson R, Rosenberg NJ (1994) Framework for integrated assessments of global warming impacts. Clim Change 28:15–44
Navas ML, Garnier E, Austin MP, Gifford RM (1999) Effect of competition on the responses of grasses and legumes to elevated atmospheric CO2 along a nitrogen gradient: differences between isolated plants, monocultures and multi-species mixtures. New Phytol 143:23–331
Niklaus PA, Körner Ch (2004) Synthesis of a six-year study of calcareous grassland responses to in situ CO2 enrichment. Ecol Monogr 74:491–511
Niklaus PA, Leadley PW, Schmid B, Körner Ch (2001) A long-term field study on biodiversity × elevated CO2 interactions in grassland. Ecol Monogr 71:341–356
Poorter H (1993) Interspecific variation in the growth response of plants to an elevated ambient CO2 concentration. Vegetation 104/105:77–97
Reich PB (2002) Root-shoot relations: Optimality in acclimation and adaptation or the “Emperor’s new clothes”? In: Waisel Y, Eshel A, Kafkafi U (eds) Plant roots: the hidden half. Marcel Dekker, New York, pp 205–220
Reich PB, Knops J, Tilman D, Craine J, Ellsworth D, Tjoelker M, Lee T, Wedin D, Naeem S, Bahauddin D, Hendrey G, Jose S, Wrage K, Goth J, Bengston W (2001) Plant diversity enhances ecosystem responses to elevated CO2 and nitrogen deposition. Nature 410:809–812
Reich PB, Tilman D, Naeem S, Ellsworth DS, Knops J, Craine J, Wedin D, Trost J (2004) Species and functional group diversity independently influence biomass accumulation and its response to CO2 and N. Proc Natl Acad Sci USA 101:10101–10106
Robinson D, Van Vuuren MMI (1998) Responses of wild plants to nutrient patches in relation to growth rate and life-form. In: Lambers H, Poorter H, van Vuuren MMI (eds) Variation in plant growth. Backhuys, Leiden, pp 237–257
Roscher C, Temperton VM, Scherer-Lorenzen M, Schmitz M, Schumacher J, Schmid B, Buchmann N, Weisser WW, Schulze ED (2005) Overyielding in experimental grassland communities—irrespective of species pool or spatial scale. Ecol Lett 8:419–429
van Ruijven J, Berendse F (2003) Positive effects of plant species diversity on productivity in the absence of legumes. Ecol Lett 6:170–175
Shaw MR, Zavaleta ES, Chiariello NR, Cleland EE, Mooney HA, Field CB (2002) Grassland responses to global environmental changes suppressed by elevated CO2. Science 298:1987–1990
Spehn EM, Hector A, Joshi J, Scherer-Lorenzen M, Schmid B, Bazeley-White E, Beierkuhnlein C, Caldeira MC, Diemer M, Dimitrakopoulos PG, Finn JA, Freitas H, Giller PS, Good J, Harris R, Hogberg P, Huss-Danell K, Jumpponen A, Koricheva J, Leadley PW, Loreau M, Minns A, Mulder CPH, O’Donovan G, Otway SJ, Palmborg C, Pereira JS, Pfisterer AB, Prinz A, Read DJ, Schulze ED, Siamantziouras ASD, Terry AC, Troumbis AY, Woodward FI, Yachi S, Lawton JH (2005) Ecosystem effects of biodiversity manipulations in European grasslands. Ecol Monogr 75:37–63
Verhagen FJM, Hageman PEJ, Woldendorp JW, Laabroek HJ (1994) Competition for ammonium between nitrifying bacteria and plant roots in soil in pots—effects of grazing by flagellates and fertilization. Soil Biol Biochem 26:89–96
de Wit CT (1960) On competition. Verslagen Landbouwkundige Onderzoekingen 66:1–82
Acknowledgments
We thank María D. Puche, José L. Quero, Anne Rosenbarger and the Duke Phytotron staff for their help with logistics, and Michael Hutchings, Fernando Valladares, Justin Wright, Nina Buchmann, Christian Körner and three anonymous reviewers for comments on previous versions of the manuscript. FTM was supported by a Fulbright fellowship (FU2003–0398), and by a Ramón y Cajal contract from the Spanish Ministerio de Educación y Ciencia. This research was supported in part by USDA Specific Co-operative Agreement #58-1270-3-070, NSF-DEB−02-12123, NSF-IBN-99-85877 to the Duke Phytotron, and NSF-SBR-9521914 (Subcontract # 538819-55801 from Carnegie Mellon University), and complies with US laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Nina Buchmann.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Maestre, F.T., Reynolds, J.F. Biomass responses to elevated CO2, soil heterogeneity and diversity: an experimental assessment with grassland assemblages. Oecologia 151, 512–520 (2007). https://doi.org/10.1007/s00442-006-0577-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0577-y