Abstract
An ideal biomarker must meet several parameters to enable its successful adoption; however, the nature of glioma makes it challenging to discover valuable biomarkers. While biomarkers require simplicity for clinical implementation, anatomical features and the complexity of the brain make it challenging to perform histological examination. Therefore, compared to biomarkers from general histological examination, liquid biomarkers for brain disease offer many more advantages in these minimally invasive methods. Ideal biomarkers should have high sensitivity and specificity, especially in malignant tumors. The heterogeneous nature of glioma makes it challenging to determine useful common biomarkers, and no liquid biomarker has yet been adopted clinically. The low incidence of brain tumors also hinders research progress. To overcome these problems, clinical applications of new types of specimens, such as extracellular vesicles and comprehensive omics analysis, have been developed, and some candidate liquid biomarkers have been identified. As against previous reviews, we focused on and reviewed the sensitivity and specificity of each liquid biomarker for its clinical application. Perusing an ideal glioma biomarker would help uncover the common underlying mechanism of glioma and develop new therapeutic targets. Further multicenter studies based on these findings will help establish new treatment strategies in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
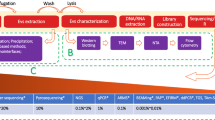
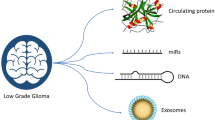
Gliomas account for 25% of all central nervous system (CNS) tumors. Gliomas are the most common primary brain tumors, and most patients with glioblastoma (GBM), which account for about 60% of all types of glioma, die within 2 years [1]. However, some clinical problems make it difficult to overcome gliomas. One cause is the rarity of brain tumors; approximately, only 24.7 per 100,000 people suffer from CNS tumors annually [1]. Although a small number of reports suggest the possibility of a predictive biomarker or mathematical model to be used before the onset of glioma [2, 3], the gold-standard diagnosis of brain tumors still remains to be imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI). Since the rarity of gliomas raises the threshold for these tests, nearly all patients were admitted to a specialized hospital with some neurological symptoms and already had a brain tumor of a specific size that was detected in CT or MRI studies. Another problem is accurately diagnosing the disease despite numerous imaging modalities. In the course of the standard treatment of GBM with chemoradiotherapy, some patients experienced tumor recurrence, and some patients experienced pseudoprogression, which was caused by an inflammatory response, edema, and radiation-induced reaction [4]. These complicated clinical situations interfere with clinical decisions. Therefore, studies on biomarkers for gliomas have been conducted, some of which were based on brain tissue specimens [5]. In contrast to other organs, a biopsy of brain tumor tissue is more hazardous and invasive because of the anatomical features surrounding the skull and complicated brain structures, including vessels and fiber fascicules [6]. Liquid biomarkers, such as those found in blood and cerebrospinal fluid, have advantages in their minimally invasive nature. In this review, we focused on liquid biomarkers and categorized them into types of specimens: blood, cerebrospinal fluid (CSF), urine, and others. Each category was divided according to the type of candidate, such as circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), micro RNA (miRNA), peptide, and extracellular vesicles (EVs). The sensitivity and specificity of each biomarker were reviewed. We also discussed current problems and future perspectives.
Liquid biomarkers
Blood
Blood consistently circulates throughout the body. Previous studies have identified blood as a promising specimen for identifying marker candidates for many diseases, including malignancies [7]. Similar studies have been performed for gliomas, some of which have calculated the sensitivity and specificity, which would directly contribute to the clinical value (Table 1). Previous studies have reported the usefulness of CTC identification. The detection of CTCs is associated with prognosis in some types of cancers [7], and the detection rate of CTCs in GBM patients is the same regardless of recurrence [8]. Despite the low rate of extracranial metastasis [9], 20–40% of patients who suffered from GBM had CTCs in their blood specimens [10, 11]. CTCs overexpress some genes associated with the mesenchymal phenotype [10, 12], which is known to contribute to tumor dissemination [8, 12]. Other studies have revealed that the expression of some genes, such as telomerase reverse transcriptase (TERT) and epidermal growth factor receptor (EGFR), can help detect CTCs [11, 13]. The combination of gene mutation and fusion has been indicated as the utility for detecting GBM from healthy controls (HC) [14]. Detection of CTCs, which focuses on polyploidy of chromosome 8, would contribute to distinguishing between radiation necrosis and true glioma recurrence [15].
Previous studies have reported the utility of plasma ctDNAs in blood samples. Methylation of the O6-methylguaniate-DNA methyltransferase (MGMT) promoter, one of the most important prognostic markers for glioma patients, can be detected through ctDNA extraction [16]. Other studies have indicated the amplification and mutation of ERBB2, MET, EGFR, and PTEN, which are known to be specific genetic mutations in gliomas [17]. Another study focused on the malignant nature of GBM demonstrated the utility of Ras association domain family 1 isoform A (RASSF1A) and death-associated protein kinase (DAPK) in ctDNA from blood specimens [18]. RASSF1A was first isolated from other malignancies, such as lung and breast cancers [19]. Further studies revealed its utility in detecting primary malignant brain tumors by brain tumor specimen analysis [20]. DAPK is associated with neuronal cell death. Recent studies have indicated that its deregulation causes abnormal neuronal loss in Alzheimer’s disease [21].
Some miRNAs have been identified as biomarkers of GBM. miRNA is a type of non-coding small RNA that typically range from 18 to 22 base pairs [22]. Recent studies have indicated that miRNAs regulate gene expression and contribute to proliferation, differentiation, apoptosis, invasion, and carcinogenesis [23,24,25]. Previous basic research has revealed that some miRNAs are deregulated in glioma tissues compared to normal brain tissues. The deregulation of miRNAs contributes to immune suppression, which is controlled by GBM [26]. The expression levels of some miRNAs are sustained for over 6 months after surgery [26]. miR-128 is one of the major candidates as a biomarker of GBM; however, its biochemical effect remains unclear. Some previous studies mentioned that this molecule is downregulated in patients with glioma. Additional receiver-operating characteristic (ROC) analysis revealed its utility as a diagnostic marker to discriminate World Health Organization (WHO) grade 1 from other grades [27]. In contrast, another study detected that this candidate molecule was upregulated in GBM patients [26]. A study investigated the clinical role of miRNAs as diagnostic biomarkers. The expression levels of these miRNAs in blood samples were significantly higher in GBM patients than in healthy individuals. They were detected with high sensitivity and specificity, showing an association with patient prognosis [28,29,30]. Serum miR-205 and miR-100 have also been identified as valuable diagnostic and prognostic biomarkers, which are downregulated in patients with glioma [31, 32]. In particular, miR-100 has been suggested as a diagnostic marker of GBM in HC with sensitivity and specificity of 77.89 and 83.33%, respectively [32]. The serum level of miR-182, which is upregulated in glioma patients, was also detected as a valuable candidate prognostic biomarker and the determination of grading based on WHO classification [33, 34]. Other studies have investigated genome-wide serum miRNA expression and identified multiple combinations of miRNAs that could detect malignant astrocytoma with high sensitivity and specificity [35, 36], none of which have been used in clinical practice.
Previous studies have identified peptides as candidate liquid biomarkers for gliomas. Three serum proteins, BMP2, HSP70, and CXCL10, identified from the data bank on astrocytoma gene expression, could differentiate GBM from HC with high sensitivity and specificity [37]. A comparison of blood samples from GBM patients and HC revealed eight peptides, leucine-rich alpha-2-glycoprotein (LRG1), complement component C9 (C9), C-reactive protein (CRP), alpha-1-antichymotrypsin, apolipoprotein B-100, gelsolin (GSN), Ig alpha-1 chain C region, and apolipoprotein A-IV, which were detected as candidate biomarkers of GBM by quantitative comparisons of the plasma proteasome [38]. Some of these molecules (LRG1, C9, and CRP) were positively correlated with tumor size, and GSN was negatively correlated with PFS and OS in the Kaplan–Meier curve analysis [38]. Upon further analysis of some of these biomarker candidates, the expression level of LRG1 in GBM was found to be higher than other diffuse gliomas [39], and GSN, which is known as a calcium-adjusted actin filament protein and negatively controlled by miR-654-5p and miR-450b-5p, was found to suppress glioma cell proliferation and invasion [40]. Filamin C (FLNC), which also contributes to actin function, is expressed in glioma tissue in a grade-dependent manner. The serum anti-FLNC autoantibody was detected as a biomarker for early diagnosis of lower-grade glioma (LGG) [41]. The association between cancers and changes in the coagulation system has been noted previously [42], and similar findings have been reported in glioma. Cathepsin D, a protease, showed high expression in the serum of patients with HGG than in those with LGG [43]. The serum expression level of plasminogen activator inhibitor-1 (PAI-1), a primary inhibitor of urokinase-type plasminogen activator (PA) and tissue-type PA, is higher in HGG and is reduced by treatment. Therefore, PAI-1 may contribute to preoperative diagnostic markers and post-operative markers for glioma recurrence [44]. Other studies have also focused on coagulation, angiogenesis, and inflammation markers; none has been associated with clinical outcomes [45]. Some studies have investigated molecules associated with the hallmark of tumors, such as proliferative activity. A high expression level of osteopontin in serum specimens is associated with poor prognosis [46]. Focusing on the invasive nature of glioma, the expression level of tissue inhibitor of metalloproteinase-1 in plasma specimens is associated with the diagnosis and prognosis of patients with glioma [46, 47]. Another study indicated that YKL-40, a mesenchymal marker for glioma, is higher in GBM patients than in HC and is associated with the surgical resection rate and prognosis [48]. The serum expression level of YKL-40 was substantially elevated in GBM patients compared to that in other glioma patients [49]. Previous studies have revealed that YKL-40 is associated with cell proliferation, escape apoptosis, and extracellular matrix remodeling [49], and could be used as a diagnostic and surrogate biomarker. Another study investigated diagnostic serum biomarkers, including glial fibrillary acidic protein (GFAP). It concluded that none of the investigated markers was suitable for histological diagnosis [50]. Previous studies have investigated prognostic biomarkers for gliomas. Insulin-like growth factor binding protein 2 (IGFBP-2), a malignancy-associated protein, is associated with the prognosis of patients with GBM. At 650 ng/mL, the preoperative plasma concentration of IGFBP-2 can predict the prognosis for 12 months or less with a sensitivity and specificity of 62 and 80%, respectively [51].
EVs, which are composed of a hydrophilic aqueous core and hydrophobic outer lipid bilayer, are associated with glioma proliferation, migration, and invasion by transporting intracellular materials [5, 52]. Recent studies have revealed that some EVs can be used as biomarkers for gliomas. Some genetic mutations specific for glioma, such as isocitrate dehydrogenase 1 (IDH1) and EGFR variant III (EGFRvIII), were successfully detected in blood samples [53, 54]. Some of these studies revealed that genetic mutations of EGFR variant III (EGFRvIII) from EV could help detect HGG from HC at a sensitivity and specificity of 81.58 and 79.31% [55]. One characteristic of EVs is their high permeability. Basic research has found that EVs from glioma cells cross the intact blood–brain barrier (BBB) and could be detected in the peripheral blood of xenograft models [56]. Another basic research revealed that the endothelial recycling endocytic pathways, a type of transcytosis, contribute to EV translation from the intact BBB [57].
Cerebrospinal fluid
CSF is produced continuously and is recycled as blood and lymph fluid [58]. CSF is collected during lumbar puncture and brain tumor surgery and can potentially provide evidence for critical molecular biomarkers of brain tumors. Tumor-associated biomarkers may be more prominent in body fluids close to the disease site, as tumor cells usually coexist with their microenvironment. In particular, the presence of the BBB in the brain may prevent the release of putative biomarkers into the systemic circulation, including the blood [59]. Nevertheless, CSF is expected to be a significant source for detecting brain tumor biomarkers due to its contact with brain tissue and proximity to most tumor masses [60]. The primary analysis of CSFs involves the circulation of cell-free nucleic acids, including cell-free DNA and RNA. This not only detects somatic mutations, insertions, deletions, and copy number variations but also aids in evaluating methylation statuses and regulatory nucleic acids, such as miRNAs and long non-coding RNAs (lncRNAs). The analysis of proteins and EVs also supports the evaluation of RNA expression [61]. CSF is an ideal biofluid for ctDNA detection because of the low non-tumor background of circulating cell-free DNA (cfDNA) and its anatomic proximity [62]. ctDNA derived from tumors in the CNS is more abundant in the CSF than in plasma [63]. In this chapter, we focus on the biomarkers in CSF reported in the literature, especially ctDNA, microRNAs, and proteins. We also reviewed studies that discussed the sensitivity and specificity of these candidate biomarkers (Table 2).
While ctDNA is highly specific and provides important genomic information about tumors, ctDNA levels in plasma are low and must be detected with high sensitivity [64]. Wang et al. [65] analyzed 29 cases of glioma and found that the detection rate of ctDNA from CSF collected during surgery for primary CNS malignancies was 74%. CSF-ctDNA levels and detection rates in their study were significantly higher when the tumor was in contact with the CSF space, and in high-grade tumors; tumor size was not a statistically significant factor. Miller et al. [66] evaluated the glioma genome in CSF collected via lumbar puncture and found that ctDNA was detected in 49.4% of the patients and was associated with disease burden and adverse outcomes. In the same report, a combination of mutations in IDH, TP53, 1p/19q co-deletion, and alterations in the gene encoding alpha-thalassemia/mental-retardation-syndrome-X-linked (ATRX), which define the LGG subtype, were consistent between CSF and glioma. Further detailed genomic analysis of gliomas in CSF revealed mutations within the TERT promoter, the protein-coding region of TP53, the catalytic domain of IDH1, deletions of CDKN2A and CDKN2B, amplification of the EGFR gene, and in-frame deletions of EGFR-mutant III. In a recent report, multigene cancer genome panel sequencing showed that mutations in the ctDNA of CSF were highly consistent with those in tumor DNA. In terms of tumor mutation burden (TMB), which has shown promise as a biomarker for immune checkpoint inhibitor therapy in several types of cancer, ctDNA TMB in the CSF was strongly correlated with tumor DNA TMB, especially for GBM [67]. Similar attempts have been made for brainstem and cerebral hemispheric gliomas. Pan et al. [68] analyzed 57 CSF-ctDNA samples and found nine medullary and 23 diffuse intrinsic pontine gliomas (DIPG), H3F3A, HIST1H3B, TP53, ATRX, PDGFRA, FAT1, PPM1D, IDH1, NF1, PIK3CA, ACVR1, and other mutations, all of which were detected in > 83% of cases. At least half of the alterations were also detected in the CSF in 91.9% (34/37) of cases. These findings suggest that deep sequencing of ctDNA can help detect tumor-specific mutations in both cerebral hemispheres and in brainstem gliomas.
In gliomas, changes in the biosynthesis and expression of miRNAs play an important role in key signaling pathways associated with various tumor characteristics, such as glioma development, invasion, and malignant transformation. In recent years, significant levels of miRNAs have been found in CSF samples, making them valuable biomarker candidates [69]. Furthermore, given the presence of the BBB, the expression and function of miRNAs can more accurately reflect pathological conditions in the CSF than in plasma [70]. Several miRNAs have been identified as candidate biomarkers for differentiating gliomas from other brain tumors. Teplyuk et al. [70] reported that miR-10b and miR-21 levels were significantly increased in the CSF of patients with GBM and brain metastases from breast and lung cancer compared to tumors in remission and various non-neoplastic diseases. Baraniskin et al. [71] compared CSF samples from controls with several neurological diseases (e.g., CNS lymphoma and cancerous brain metastases) and reported that miR-15b and miR-21 were highly expressed in glioma patients and at a sensitivity of 90% and specificity of 100%, patients were diagnosed with glioma. Measuring the members of the miR-200 family that are aberrantly expressed in the CSF of patients with brain metastases can discriminate GBM from metastatic brain tumors, and quantifying seven miRNAs can be used to distinguish GBM from metastatic brain tumors with high accuracy (91–99%). In addition, Kopkova et al. [72] reported that the expression levels of five miRNAs (miR-30e, miR-140, let-7b, mR-10a, and miR-21-3p) could be combined to differentiate between the brain and non-brain tumors as well as GBMs, low-grade gliomas, meningiomas, and brain metastases in patients with suspected brain tumors. Akers et al. [73] found that nine miRNAs (miR-21, 218-5p, 193b-3p, 331-3p, 374a-5p, 548c-3p, 520f-3p, 27b-3p, and 30b-3p) can help diagnose GBM, and that their properties differ between CSF-derived from cisterns, such as the brain and ventricles, and that from the lumbar region. Interestingly, the combined expression levels of miR-10b and miR-196b in the CSF of GBM patients were prognostic factors. A meta-analysis demonstrated high sensitivity (85%) and specificity (90%) of miRNAs for glioma diagnosis, and further studies are expected in the future [74].
Tumor-specific protein biomarkers can be detected in the CSF owing to the presence of proteins secreted or leaked by tumor tissues or resulting from abnormal BBB function. The discovery of biomarkers is particularly urgent for differentiating high-grade gliomas that require early therapeutic interventions. Khwaja et al. [75] found that Attractin is a protein secreted in the CSF specific to patients with WHO grade 3 and 4 malignant gliomas and plays a role in glioma migration. Sampath et al. [76] found that vascular endothelial growth factor (VEGF), an important mediator of angiogenesis and malignant transformation of glial tumors, was detected in 89% of cases of anaplastic astrocytoma and GBM and in only 27% of non-glioma cases such as medulloblastoma, lymphoma, and metastatic tumors, and was not detected in normal CSF samples. Furthermore, VEGF expression levels were significantly higher in patients with high-grade astrocytoma than in those without glioma, suggesting its potential as a differential marker for malignant gliomas. In their review of the literature on proteomic screening of glioma-related protein biomarkers in CSF, Shen et al. [77] identified 19 differentially expressed proteins and found several important protein networks, including IL-6/STAT-3 and four novel proteins of IL-6, galanin (GAL), HSPA5, and WNT4 in functional analysis. Recent reports have shown that chitinase-3-like protein 1 (CHI3L1) and GFAP are valuable GBM markers in both retrospective and confirmatory prospective observational studies [78]. Further advances in the proteomic analysis of CSF in brain tumors will likely lead to the identification of biomarkers for diagnostic and therapeutic monitoring.
Despite recent advances, there has not been much detailed validation of CSF-derived EVs as biomarkers; however, many studies have shown that EVs can be found and isolated from the CSF of patients with brain tumors [79] and may help detect tumor-specific biomarkers in the brain. Chen et al. [54] showed that EVs collected from the CSF of glioma patients contained mutant IDH1 transcripts and that detection and quantification of mutant and wild-type IDH1 RNA transcripts are possible. In a prospective study [80], the expression level of miR-21 in EVs isolated from the CSF of GBM patients was tenfold higher on average than in controls, allowing for the diagnosis of GBM with a sensitivity and specificity of 87% and 93%, respectively. Figueroa et al. [81] also demonstrated the detection of EGFRvIII in CSF-derived EVs in GBM patients with EGFRvIII-positive tissue. The detection rate of EGFRvIII positivity of CSF-derived EVs in EGFRvIII-positive GBM patients was 61% sensitivity and 98% specificity, and the high specificity enabled the accurate determination of EGFRvIII tumor positivity status. Further detection of CSF-derived EVs is required in the future.
Other liquid biomarkers
Previous studies have focused on urine as a promising liquid biomarker for cancer [82]. VEGF, which contributes to tumor growth, has been investigated as a predictive biomarker for the progression of some types of cancers. Previous studies have reported the utility of VEGF concentration in urine samples to predict tumor progression in GBM patients [83]. In basic research using a xenograft GBM rat model, some particular proteins were extracted from urine before GBM was manifested in the rat brain by MRI studies [84]. These studies suggest that urine biomarkers could help detect brain tumors in the early phase. Another study revealed that the miRNAs extracted from EVs in urine samples reflected the pattern of miRNAs from patient-derived organoids. The diagnostic model of miRNA expression pattern, which was constructed from the GBM patient dataset, could differentiate between patients and noncancer individuals with a sensitivity and specificity of 100 and 97%, respectively, in an individual dataset [85].
Other fluid samples that may serve as liquid biomarkers include cystic fluid and saliva. Biomarkers in cyst fluids have been investigated for some time but remain largely unknown. In 1997, Jallo et al. [86] reported that Tenascin-C is expressed in the cyst fluid of glioma samples and is highly expressed in proportion to the grade. VEGF is an important regulatory protein in angiogenesis, strongly expressed in malignant gliomas, and highly expressed in cystic fluid [87, 88]. In their evaluation of VEGF levels in the serum and cyst fluid of 14 patients with primary brain tumors (six GBMs, three low-grade gliomas, and one ependymoma), Stockhammer et al. [89] reported that VEGF detected in the cyst fluid of recurrent GBMs had the highest level, and that serum VEGF levels did not differ from those in healthy individuals, suggesting that VEGF may be produced by the tumor in an immunoreactive manner and released into the cyst fluid. L1 cell adhesion molecule (L1CAM), a member of the immunoglobulin-like cell adhesion molecule family, is present at high levels in the cyst fluid of GBM and metastatic brain tumors and may be a potential biomarker [90]. In a recent study [91], GBM cyst fluid contained hormones such as insulin-like growth factor 1, insulin, erythropoietin, growth hormone, testosterone, estradiol, and triiodothyronine. There was a correlation between cyst fluid concentrations of growth hormone and testosterone and tumor volume and a negative correlation between cyst fluid concentrations of erythropoietin and survival rate. In the same study, proteomic analysis of GBM cyst fluid revealed the presence of SPARCL1, IGF-BP2, osteopontin, FAM3C, TREM2, CD166, and prosaposin, which promote tumor cell proliferation, migration, and invasion; kininogen-1/bradykinin, which promotes glioma cell migration; Clusterin, CD5L, HYOU1, and prosaposin, which are involved in anti-apoptotic effects; and hepatocyte growth factor activator. Despite the limited number of studies on liquid biomarkers in saliva samples, García-Villaescusa [92] reported significant increases in the metabolites leucine, valine, isoleucine, propionic acid, alanine, acetic acid, ethanolamine, and sucrose compared to those in the control group. With recent improvements in biomarker retrieval technology, including next-generation sequencing, body fluid samples, including cyst fluid and saliva, can now be retrieved and evaluated in the future.
Perspective
The utility and possibilities of developing liquid biomarkers of glioma have been mentioned; however, this research still has some limitations. First, the low detection rate of biomarkers for glioma is difficult compared to other types of cancers. In cfDNA analysis using multiple types of cancers, the detection rate of somatic mutations in GBM was the lowest, with only approximately 50% at best [93,94,95]. Other cfDNA analyses of brain tumors have indicated similar detection rates of somatic mutations [17]. These previous studies suggested that the lower detection rate of liquid markers is associated with physical obstacles such as the blood–brain barrier (BBB), which prevents candidates from entering circulation [94].
Second, some previous papers mentioned chronological changes in the values of candidate molecules and difficulties in sample management [83]. Previous studies have indicated the utility of ctDNA and cfDNA; these biomarkers have a half-life of only a few minutes to hours [96]. Thus, these short half-times are not in line with actual clinical practice. The clinical timing of sample collection differs for each study design, and it is challenging to aggregate the results and compare the effectiveness of these biomarkers. The detection rate of biomarkers also depends on the specimen type. Some previous reports have indicated that the detection rate of the same candidate markers differed according to the type of sample [16].
Third, the detection rate of these liquid biomarkers depends on the type of tumor and its grading. Diagnosing tumors in the CNS is more diverse than cancer in other organs [97], and discovering the common candidate marker is difficult. Some studies have indicated that LGGs have lower cell proliferation rates, lack necrotic tissue, and have normal BBB, making it difficult to detect candidate liquid biomarkers [96].
Finally, one of the biggest challenges associated with gliomas is their heterogeneous nature. The ideal biomarker has high sensitivity and specificity, especially in malignant tumors, making it difficult to identify a common glioma biomarker. If we could define a common biomarker for glioma, this candidate molecule would subserve the underlying mechanism of glioma. In other words, this research strategy can potentially be a new therapeutic target for gliomas. Some of these candidate molecules were focused on targeted therapy of GBM, such as chimeric antigen receptor T-cell therapy [98], and further studies in this area can lead to promising outcomes for discovering new treatments for patients with gliomas.
Data Availability
Data sharing is not applicable to this article as no new data were created or analyzed in this review article.
Abbreviations
- ATRX:
-
Alpha-thalassemia/mental-retardation-syndrome-X-linked
- BBB:
-
Blood–brain barrier
- C9:
-
Complement component 9
- CHI3L1:
-
Chitinase-3-like protein 1
- CNS:
-
Central nervous systems
- CRP:
-
C-reactive protein
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computed tomography
- CTC:
-
Circulating tumor cell
- ccfDNA:
-
Circulating cell-free DNA
- ctDNA:
-
Circulating tumor DNA
- DIPG:
-
Diffuse intrinsic pontine glioma
- EGFR:
-
Epidermal growth factor receptor
- EGFRvIII:
-
Epidermal growth factor receptor variant III
- EV:
-
Extracellular vesicle
- FLNC:
-
Filamin C
- GAL:
-
Galanin
- GBM:
-
Glioblastoma
- GFAP:
-
Glial fibrillary acidic protein
- GSN:
-
Gelsolin
- HC:
-
Healthy control
- HGG:
-
High-grade glioma
- IDH1:
-
Isocitrate dehydrogenase 1
- IGFBP-2:
-
Insulin-like growth factor binding protein 2
- L1CAM:
-
L1 cell adhesion molecule
- LGG:
-
Lower grade glioma
- lncRNA:
-
Long non-coding RNA
- LRG1:
-
Leucine-rich alpha-2-glycoprotein
- miRNA:
-
MicroRNA
- MGMT:
-
O6-Methylguanine-DNA methyltransferase
- MRI:
-
Magnetic resonance imaging
- PA:
-
Plasminogen activator
- PAI-1:
-
Plasminogen activator inhibitor-1
- RASSF1A:
-
Ras association domain family 1 isoform A
- ROC:
-
Receiver-operating characteristic
- TERT:
-
Telomerase reverse transcriptase
- TMB:
-
Tumor mutation burden
- VEGF:
-
Vascular endothelial growth factor
- WHO:
-
World Health Organization
References
Ostrom QT, Price M, Neff C et al (2022) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2015–2019. Neuro Oncol 24(Suppl 5):v1–v95
Gartner W, Ilhan A, Neziri D et al (2010) Elevated blood markers 1 year before manifestation of malignant glioma. Neuro Oncol 12(9):1004–1008
Sturrock M, Hao W, Schwartzbaum J et al (2015) A mathematical model of pre-diagnostic glioma growth. J Theor Biol 380(7):299–308
Kucharczyk MJ, Parpia S, Whitton A et al (2017) Evaluation of pseudoprogression in patients with glioblastoma. Neurooncol Pract 4(2):120–134
Silantyev AS, Falzone L, Libra M et al (2019) Current and future trends on diagnosis and prognosis of glioblastoma: from molecular biology to proteomics. Cells 8(8):863
Hall JW 3rd, Buss E, Grose JH (2016) Factors affecting the development of speech recognition in steady and modulated noise. J Acoust Soc Am 139(5):2964
Zhang L, Riethdorf S, Wu G et al (2012) Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res 18(20):5701–5710
Alix-Panabières C, Pantel K (2014) Challenges in circulating tumour cell research. Nat Rev Cancer 14(9):623–631
Tamai S, Kinoshita M, Sabit H et al (2019) Case of metastatic glioblastoma with primitive neuronal component to the lung. Neuropathology 39(3):218–223
Sullivan JP, Nahed BV, Madden MW et al (2014) Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer Discov 4(11):1299–1309
Müller C, Holtschmidt J, Auer M et al (2014) Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med 6(247):247ra101
Chistiakov DA, Chekhonin VP (2018) Circulating tumor cells and their advances to promote cancer metastasis and relapse, with focus on glioblastoma multiforme. Exp Mol Pathol 105(2):166–174
Macarthur KM, Kao GD, Chandrasekaran S et al (2014) Detection of brain tumor cells in the peripheral blood by a telomerase promoter-based assay. Cancer Res 74(8):2152–2159
Palande V, Siegal T, Detroja R et al (2022) Detection of gene mutations and gene-gene fusions in circulating cell-free DNA of glioblastoma patients: an avenue for clinically relevant diagnostic analysis. Mol Oncol 16(10):2098–2114
Gao F, Cui Y, Jiang H et al (2016) Circulating tumor cell is a common property of brain glioma and promotes the monitoring system. Oncotarget 7(44):71330–71340
Wang Z, Jiang W, Wang Y et al (2015) MGMT promoter methylation in serum and cerebrospinal fluid as a tumor-specific biomarker of glioma. Biomed Rep 3(4):543–548
Piccioni DE, Achrol AS, Kiedrowski LA et al (2019) Analysis of cell-free circulating tumor DNA in 419 patients with glioblastoma and other primary brain tumors. CNS Oncol 8(2):Cns3
Balaña C, Ramirez JL, Taron M et al (2003) O6-methyl-guanine-DNA methyltransferase methylation in serum and tumor DNA predicts response to 1,3-bis(2-chloroethyl)-1-nitrosourea but not to temozolamide plus cisplatin in glioblastoma multiforme. Clin Cancer Res 9(4):1461–1468
Dammann R, Li C, Yoon JH et al (2000) Epigenetic inactivation of a RAS association domain family protein from the lung tumour suppressor locus 3p21.3. Nat Genet 25(3):315–319
Horiguchi K, Tomizawa Y, Tosaka M et al (2003) Epigenetic inactivation of RASSF1A candidate tumor suppressor gene at 3p21.3 in brain tumors. Oncogene 22(49):7862–7865
Chen D, Zhou XZ, Lee TH (2019) Death-associated protein kinase 1 as a promising drug target in cancer and Alzheimer’s disease. Recent Patents Anticancer Drug Discov 14(2):144–157
Khristov V, Lin A, Freedman Z et al (2022) Tumor-derived biomarkers in liquid biopsy of glioblastoma. World Neurosurg. https://doi.org/10.1016/j.wneu.2022.11.012
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116(2):281–297
Ambros V (2004) The functions of animal microRNAs. Nature 431(7006):350–355
Plasterk RH (2006) Micro RNAs in animal development. Cell 124(5):877–881
Roth P, Wischhusen J, Happold C et al (2011) A specific miRNA signature in the peripheral blood of glioblastoma patients. J Neurochem 118(3):449–457
Sun J, Liao K, Wu X et al (2015) Serum microRNA-128 as a biomarker for diagnosis of glioma. Int J Clin Exp Med 8(1):456–463
Swellam M, Ezz El Arab L, Al-Posttany AS et al (2019) Clinical impact of circulating oncogenic MiRNA-221 and MiRNA-222 in glioblastoma multiform. J Neurooncol 144(3):545–551
Swellam M, Bakr NM, El Magdoub HM et al (2021) Emerging role of miRNAs as liquid biopsy markers for prediction of glioblastoma multiforme prognosis. J Mol Neurosci 71(4):836–844
Lai NS, Wu DG, Fang XG et al (2015) Serum microRNA-210 as a potential noninvasive biomarker for the diagnosis and prognosis of glioma. Br J Cancer 112(7):1241–1246
Yue X, Lan F, Hu M et al (2016) Downregulation of serum microRNA-205 as a potential diagnostic and prognostic biomarker for human glioma. J Neurosurg 124(1):122–128
Zhang H, Wang J, Wang Z et al (2019) Serum miR-100 is a potential biomarker for detection and outcome prediction of glioblastoma patients. Cancer Biomark 24(1):43–49
Jiang L, Mao P, Song L et al (2010) miR-182 as a prognostic marker for glioma progression and patient survival. Am J Pathol 177(1):29–38
Xiao Y, Zhang L, Song Z et al (2016) Potential diagnostic and prognostic value of plasma circulating MicroRNA-182 in human glioma. Med Sci Monit 22:855–862
Yang C, Wang C, Chen X et al (2013) Identification of seven serum microRNAs from a genome-wide serum microRNA expression profile as potential noninvasive biomarkers for malignant astrocytomas. Int J Cancer 132(1):116–127
Ohno M, Matsuzaki J, Kawauchi J et al (2019) Assessment of the diagnostic utility of serum MicroRNA classification in patients with diffuse glioma. JAMA Netw Open 2(12):e1916953
Elstner A, Stockhammer F, Nguyen-Dobinsky TN et al (2011) Identification of diagnostic serum protein profiles of glioblastoma patients. J Neurooncol 102(1):71–80
Miyauchi E, Furuta T, Ohtsuki S et al (2018) Identification of blood biomarkers in glioblastoma by SWATH mass spectrometry and quantitative targeted absolute proteomics. PLoS ONE 13(3):e0193799
Furuta T, Sugita Y, Komaki S et al (2020) The Multipotential of Leucine-Rich α-2 glycoprotein 1 as a clinicopathological biomarker of glioblastoma. J Neuropathol Exp Neurol 79(8):873–879
Zhang J, Furuta T, Sabit H et al (2020) Gelsolin inhibits malignant phenotype of glioblastoma and is regulated by miR-654-5p and miR-450b-5p. Cancer Sci 111(7):2413–2422
Adachi-Hayama M, Adachi A, Shinozaki N et al (2014) Circulating anti-filamin C autoantibody as a potential serum biomarker for low-grade gliomas. BMC Cancer 14:452
Falanga A, Marchetti M, Vignoli A (2013) Coagulation and cancer: biological and clinical aspects. J Thromb Haemost 11(2):223–233
Fukuda ME, Iwadate Y, Machida T et al (2005) Cathepsin D is a potential serum marker for poor prognosis in glioma patients. Cancer Res 65(12):5190–5194
Iwadate Y, Hayama M, Adachi A et al (2008) High serum level of plasminogen activator inhibitor-1 predicts histological grade of intracerebral gliomas. Anticancer Res 28(1B):415–418
Reynés G, Vila V, Martín M et al (2011) Circulating markers of angiogenesis, inflammation, and coagulation in patients with glioblastoma. J Neurooncol 102(1):35–41
Sreekanthreddy P, Srinivasan H, Kumar DM et al (2010) Identification of potential serum biomarkers of glioblastoma: serum osteopontin levels correlate with poor prognosis. Cancer Epidemiol Biomarkers Prev 19(6):1409–1422
Lin Y, Wang JF, Gao GZ et al (2013) Plasma levels of tissue inhibitor of matrix metalloproteinase-1 correlate with diagnosis and prognosis of glioma patients. Chin Med J (Engl) 126(22):4295–4300
Bernardi D, Padoan A, Ballin A et al (2012) Serum YKL-40 following resection for cerebral glioblastoma. J Neurooncol 107(2):299–305
Tanwar MK, Gilbert MR, Holland EC (2002) Gene expression microarray analysis reveals YKL-40 to be a potential serum marker for malignant character in human glioma. Cancer Res 62(15):4364–4368
Ilhan-Mutlu A, Wagner L, Widhalm G et al (2013) Exploratory investigation of eight circulating plasma markers in brain tumor patients. Neurosurg Rev 36(1):45–55 (discussion 55-46)
Lin Y, Jiang T, Zhou K et al (2009) Plasma IGFBP-2 levels predict clinical outcomes of patients with high-grade gliomas. Neuro Oncol 11(5):468–476
Chen C, Skog J, Hsu CH et al (2010) Microfluidic isolation and transcriptome analysis of serum microvesicles. Lab Chip 10(4):505–511
Al-Nedawi K, Meehan B, Micallef J et al (2008) Intercellular transfer of the oncogenic receptor EGFRvIII by microvesicles derived from tumour cells. Nat Cell Biol 10(5):619–624
Chen WW, Balaj L, Liau LM et al (2013) BEAMing and droplet digital PCR analysis of mutant IDH1 mRNA in glioma patient serum and cerebrospinal fluid extracellular vesicles. Mol Ther Nucleic Acids 2(7):e109
Manda SV, Kataria Y, Tatireddy BR et al (2018) Exosomes as a biomarker platform for detecting epidermal growth factor receptor-positive high-grade gliomas. J Neurosurg 128(4):1091–1101
García-Romero N, Carrión-Navarro J, Esteban-Rubio S et al (2017) DNA sequences within glioma-derived extracellular vesicles can cross the intact blood-brain barrier and be detected in peripheral blood of patients. Oncotarget 8(1):1416–1428
Morad G, Carman CV, Hagedorn EJ et al (2019) Tumor-derived extracellular vesicles breach the intact blood-brain barrier via transcytosis. ACS Nano 13(12):13853–13865
Patel AS, Allen JE, Dicker DT et al (2011) Identification and enumeration of circulating tumor cells in the cerebrospinal fluid of breast cancer patients with central nervous system metastases. Oncotarget 2(10):752–760
Xiao F, Lv S, Zong Z et al (2020) Cerebrospinal fluid biomarkers for brain tumor detection: clinical roles and current progress. Am J Transl Res 12(4):1379–1396
Samuel N, Remke M, Rutka JT et al (2014) Proteomic analyses of CSF aimed at biomarker development for pediatric brain tumors. J Neurooncol 118(2):225–238
Bertero L, Siravegna G, Rudà R et al (2019) Review: peering through a keyhole: liquid biopsy in primary and metastatic central nervous system tumours. Neuropathol Appl Neurobiol 45(7):655–670
Jones J, Nguyen H, Drummond K et al (2021) Circulating biomarkers for glioma: a review. Neurosurgery 88(3):E221-e230
De Mattos-Arruda L, Mayor R, Ng CKY et al (2015) Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun 6:8839
Merker JD, Oxnard GR, Compton C et al (2018) Circulating tumor DNA analysis in patients with cancer: American Society of Clinical Oncology and College of American Pathologists Joint Review. Arch Pathol Lab Med 142(10):1242–1253
Wang Y, Springer S, Zhang M et al (2015) Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci USA 112(31):9704–9709
Miller AM, Shah RH, Pentsova EI et al (2019) Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature 565(7741):654–658
Guo W, Jin L, Liang J et al (2022) Detection of mutation profiles and tumor mutation burden of cerebrospinal fluid circulating DNA by a cancer genomic panel sequencing in glioma patients. Clin Chim Acta 534:81–92
Pan C, Diplas BH, Chen X et al (2019) Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol 137(2):297–306
Tumilson CA, Lea RW, Alder JE et al (2014) Circulating microRNA biomarkers for glioma and predicting response to therapy. Mol Neurobiol 50(2):545–558
Teplyuk NM, Mollenhauer B, Gabriely G et al (2012) MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro Oncol 14(6):689–700
Baraniskin A, Kuhnhenn J, Schlegel U et al (2012) Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro Oncol 14(1):29–33
Kopkova A, Sana J, Machackova T et al (2019) Cerebrospinal fluid MicroRNA signatures as diagnostic biomarkers in brain tumors. Cancers (Basel) 11(10):1546
Akers JC, Hua W, Li H et al (2017) A cerebrospinal fluid microRNA signature as biomarker for glioblastoma. Oncotarget 8(40):68769–68779
Zhou Q, Liu J, Quan J et al (2018) MicroRNAs as potential biomarkers for the diagnosis of glioma: a systematic review and meta-analysis. Cancer Sci 109(9):2651–2659
Khwaja FW, Duke-Cohan JS, Brat DJ et al (2006) Attractin is elevated in the cerebrospinal fluid of patients with malignant astrocytoma and mediates glioma cell migration. Clin Cancer Res 12(21):6331–6336
Sampath P, Weaver CE, Sungarian A et al (2004) Cerebrospinal fluid (vascular endothelial growth factor) and serologic (recoverin) tumor markers for malignant glioma. Cancer Control 11(3):174–180
Shen F, Zhang Y, Yao Y et al (2014) Proteomic analysis of cerebrospinal fluid: toward the identification of biomarkers for gliomas. Neurosurg Rev 37(3):367–380
Schmid D, Warnken U, Latzer P et al (2021) Diagnostic biomarkers from proteomic characterization of cerebrospinal fluid in patients with brain malignancies. J Neurochem 158(2):522–538
Saugstad JA, Lusardi TA, Van Keuren-Jensen KR et al (2017) Analysis of extracellular RNA in cerebrospinal fluid. J Extracell Vesicles 6(1):1317577
Akers JC, Ramakrishnan V, Kim R et al (2013) MiR-21 in the extracellular vesicles (EVs) of cerebrospinal fluid (CSF): a platform for glioblastoma biomarker development. PLoS ONE 8(10):e78115
Figueroa JM, Skog J, Akers J et al (2017) Detection of wild-type EGFR amplification and EGFRvIII mutation in CSF-derived extracellular vesicles of glioblastoma patients. Neuro Oncol 19(11):1494–1502
Yan L, Borregaard N, Kjeldsen L et al (2001) The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem 276(40):37258–37265
Kirk MJ, Hayward RM, Sproull M et al (2008) Non-patient related variables affecting levels of vascular endothelial growth factor in urine biospecimens. J Cell Mol Med 12(4):1250–1255
Ni Y, Zhang F, An M et al (2018) Early candidate biomarkers found from urine of glioblastoma multiforme rat before changes in MRI. Sci China Life Sci 61(8):982–987
Kitano Y, Aoki K, Ohka F et al (2021) Urinary MicroRNA-based diagnostic model for central nervous system tumors using nanowire scaffolds. ACS Appl Mater Interfaces 13(15):17316–17329
Jallo GI, Friedlander DR, Kelly PJ et al (1997) Tenascin-C expression in the cyst wall and fluid of human brain tumors correlates with angiogenesis. Neurosurgery 41(5):1052–1059
Takano S, Yoshii Y, Kondo S et al (1996) Concentration of vascular endothelial growth factor in the serum and tumor tissue of brain tumor patients. Cancer Res 56(9):2185–2190
Weindel K, Moringlane JR, Marmé D et al (1994) Detection and quantification of vascular endothelial growth factor/vascular permeability factor in brain tumor tissue and cyst fluid: the key to angiogenesis? Neurosurgery 35(3):439–448 (discussion 448-439)
Stockhammer G, Obwegeser A, Kostron H et al (2000) Vascular endothelial growth factor (VEGF) is elevated in brain tumor cysts and correlates with tumor progression. Acta Neuropathol 100(1):101–105
Wachowiak R, Krause M, Mayer S et al (2018) Increased L1CAM (CD171) levels are associated with glioblastoma and metastatic brain tumors. Medicine (Baltimore) 97(38):e12396
Dahlberg D, Rummel J, Distante S et al (2022) Glioblastoma microenvironment contains multiple hormonal and non-hormonal growth-stimulating factors. Fluids Barriers CNS 19(1):45
García-Villaescusa A, Morales-Tatay JM, Monleón-Salvadó D et al (2018) Using NMR in saliva to identify possible biomarkers of glioblastoma and chronic periodontitis. PLoS ONE 13(2):e0188710
Bettegowda C, Sausen M, Leary RJ et al (2014) Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 6(224):224ra24
Schwaederle M, Husain H, Fanta PT et al (2016) Detection rate of actionable mutations in diverse cancers using a biopsy-free (blood) circulating tumor cell DNA assay. Oncotarget 7(9):9707–9717
Zill OA, Banks KC, Fairclough SR et al (2018) The landscape of actionable genomic alterations in cell-free circulating tumor DNA from 21,807 advanced cancer patients. Clin Cancer Res 24(15):3528–3538
Bagley SJ, Nabavizadeh SA, Mays JJ et al (2020) Clinical utility of plasma cell-free DNA in adult patients with newly diagnosed glioblastoma: a pilot prospective study. Clin Cancer Res 26(2):397–407
Louis DN, Perry A, Wesseling P et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Ooncol 23(8):1231–1251
Zhang C, Burger MC, Jennewein L et al (2016) ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. J Natl Cancer Inst 108(5):djv375
Funding
This work was supported by AMED under Grant Number JP20cm0106463 and JP21ck0106663 (to MN), JSPS KAKENHI Grant Number 22H00484 (to MN) and intramural clinical research grant from Kanazawa University Hospital (to MN).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tamai, S., Ichinose, T. & Nakada, M. Liquid biomarkers in glioma. Brain Tumor Pathol 40, 66–77 (2023). https://doi.org/10.1007/s10014-023-00452-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-023-00452-x