Abstract
Noninvasive examination is an emerging area in the field of neuro-oncology. Liquid biopsy captures the landscape of genomic alterations of brain tumors and revolutionizes the traditional diagnosis approaches. Rapidly changing sequencing technologies and more affordable prices put the screws on more application of liquid biopsy in clinical settings. In the past few years, extensive application of liquid biopsy has been seen throughout the whole diagnosis and treatment process of brain tumors, including early and accurate detection, characterization and dynamic monitoring. Here, we summarized and compared the most advanced techniques and target molecules or macrostructures related to brain tumor liquid biopsy. We further reviewed and emphasized recent progression in different clinical settings for brain tumors in blood and CSF. The preferred protocol, potential novel biomarkers and future development are discussed in the last part.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain and other CNS tumors encompass a broad range of different histology, molecular types and subtypes. Gliomas account for the most common primary cancer originated from neuroepithelium, among which glioblastoma (GBM) and medulloblastoma, respectively, make up the first leading type of adult and pediatric malignant brain tumors, with high intratumoural heterogeneity and low 5-year survival of 36% for GBM patients overall (Miller et al. 2021). Moreover, brain metastases (BMs) represent one of the most frequent and adverse events during or after the treatment of their extracranial primary tumors, and the top three types harboring intracranial metastatic tendency are lung cancer, breast cancer and melanoma. As one of the top ten cancer death causes, especially for men less than 40 years and women less than 20 years in the USA in 2018, brain and other CNS tumors are estimated to cause 18,600 cases of death in 2021 (Siegel 2021).
Most brain tumors are diagnosed at an advanced stage, thus making it crucial for secondary prevention when the tumor remains benign and significant mutations can be identified. The clinical diagnosis of brain tumor is highly dependent on radiological outcomes or histology of tumor tissue after resection, which either lacks sensitivity and specificity or is often delayed. Emerging nuclear medicine and magnetic resonance can resolve the early evaluation of the lesion localization, morphology and intensity; however, information at the molecular level remains limited. Other problems such as tumor evolution, heterogeneity and pseudoprogression (PsP), which presents as expanded and/or new regions of edema and enhancement on images, largely resembling tumor recurrence and radiation necrosis, also hamper the accurate evaluation and characterization by biopsy and radiology. As significant mutations like isocitrate dehydrogenase (IDH), BRAF or histone K27M have been discovered since 2008 and fundamentally changed the diagnostic approach in neuropathology to an integrated diagnosis that combines histological diagnosis and molecular profile (Capper et al. 2010, 2011; Khuong-Quang et al. 2012), early and accurate diagnosis of brain tumors on a molecular level becomes essential to reduce patients’ mortality and extend survivorship.
While genotyping of informative alterations is becoming more and more frequently involved in routine clinical oncology practice, the availability of cancer tissue is largely limited. The emergence of liquid biopsy opened up the potential of noninvasive sampling and dynamic genotyping. Liquid biopsy was first introduced in a meeting as an approach for the analysis of therapeutic or prognostic tumor biomarkers like circulating tumor cells (CTCs) in blood sample (Lianidou et al. 2010; Scher et al. 2009; Pantel and Alix-Panabières 2010). According to the NCI Dictionary of Cancer Terms, liquid biopsy refers to a test done on a sample of blood to search for cancer cells from a tumor that are circulating in the blood or for DNA fragments from tumor cells released into blood. Actually, liquid biopsy can be performed using other molecules and macrostructures (e.g., RNAs and extracellular vesicles (EVs)), from other tissue (e.g., premalignant lesions that are often precursors for malignancy) and in other body fluid, including cerebrospinal fluid (CSF), urine, saliva and even breast milk (Serrano et al. 2020).
Here, we briefly exemplify the procedure and techniques of liquid biopsy, and then focus on targets and clinical studies of liquid biopsy.
General procedure and analysis techniques
The general procedure of liquid biopsy typically involves the acquisition of body fluid, sample centrifugation, target isolation, extraction, quantification and a series of subsequent analyses. Subsequent procedure of different analysis platforms is quite parallel, usually involving three stages: library construction, sequencing, and data analysis.
Analysis techniques are mainly divided into PCR-based methods and sequencing-based methods. The next-generation sequencing (NGS)-based broad panel combining hundreds of genes and whole-genome approaches are optimal sequencing methods for liquid biopsy, since they are high throughput and time efficient, and can detect unknown mutations with high single base resolution and coverage (Kilgour et al. 2020).
PCR-based methods have advantages in sensitivity, cost, operation and analysis difficulty; however, incapability in detecting unknown mutations and PCR artifacts are shared drawbacks. Common artifacts can be divided into two main categories: amplicon biased variants (PCR bias) and mis-paired primers (PCR errors) (Acinas et al. 2005). PCR also participates in enriching targeted regions before library construction and is a known source of false positives in some sequencing-based methods.
Since the workflow of EVs liquid biopsy is the most complex and challenging, we use it as an example as shown in Fig. 1. In Table 1 we compared more detailed characteristics of various assays.
The general workflow of liquid biopsy to examine extracellular vesicles (Evs). A The techniques of extracting Evs: ultracentrifugation is recommended, but it is time-consuming and requires higher standard for techniques. Spin column-based methods are to filter by size or membrane affinity of EVs. B Four steps to evaluate the quality of extracted Evs. (i) Western blotting is to detect the specific protein markers such as CD81/CD63. (ii) TEM (transmission electron microscopy) allows direct EV characterization of structure and size of Evs. (iii) NTA (nanoparticle tracking analysis) can visualize particles ranging from 10 to 1000 nm to show the concentration and size of Evs. (iv) Flow cytometry supplements the information towards Evs’ size and markers. C The sequencing or PCR techniques ranked by sensitivity: sequencing of point mutation, epigenetic changes, copy number variations, structural changes or rearrangements. ARMS amplification refractory mutation system, BEAMing beads, emulsification, amplification and magnetics, PAP Pyrophosphorolysis-activated polymerization, EFIRM electric field induced release and measurement. * Targeted mutation, - unknown mutation
Liquids
Due to the special anatomical structure of the brain and other CNS tumors, most studies utilize blood (serum and plasma) and/or CSF. Serum and plasma are the most common source of liquid biopsy, and investigation on novel techniques usually start from blood. Martignano et al. recently conducted the first successful third-generation sequencing (Nanopore-seq) for copy number profiling from six lung cancer patients’ plasma DNA (Pittella-Silva et al. 2020). CSF may be more meaningful for primary brain tumor. Blood-to-CSF comparison by liquid biopsy is also critical for identifying tumor heterogeneity (Table 2).
Serum and plasma
Serum and plasma are completely different blood components that have distinct isolation stages. Several studies have agreed that plasma samples are more desirable than serum samples regarding cfDNA in liquid biopsy (Wong et al. 2016; Messaoudi et al. 2013; Thierry et al. 2010). The main point is that although a larger quantity of cfDNA can be extracted from serum samples (Jung et al. 2003), there is a higher percentage of DNA derived from white blood cell lysates, which lessens the purity of ctDNA (Umetani et al. 2006; Lui et al. 2002; Chan et al. 2005; Gautschi et al. 2004; Pittella-Silva et al. 2020). Before centrifugation, serum samples generally need clotting at room temperature, which adds to lysis of cells and even the degradation of ctDNA.
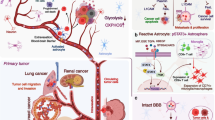
The existence of the blood–brain barrier (BBB) restricts the releasing of biomarkers from a primary brain tumor into circulation and subsequently reduces the accuracy of liquid biopsy for genomic alteration detection. Transcranial MR-guided focused ultrasound (MRgFUS) is capable of temporarily discharging the BBB with safety, resulting in enrichment of brain-derived cfDNA in circulation. Meng et al. applied MRgFUS to compare biomarkers in whole blood samples from nine GBM patients and healthy controls, and found acutely enhanced plasma cfDNA, neuron-derived EVs, and brain-specific protein S100b (Meng 2021).
When detecting ctDNAs in low abundance, the blood volume is another important consideration. To target de novo a single mutation with cumulative variant allele frequency (VAF) of 0.01% with 95% confidence would require 150–300 ml blood (Haque and Elemento 2017). However, the blood sample volume only required 15–30 ml for detecting any of ten independent VAF = 0.01% mutations or a single VAF = 0.1% mutation. Therefore, the sensitivity of a specific ctDNA test to local cancer patients depends not only on the amount of blood analyzed, but also on the number of mutations screened (McDonald et al. 2019).
Cerebrospinal fluid (CSF)
CSF appears to have an advantage over blood, perhaps partly due to the reduced background cells, for example, less EVs from leukocytes when analyzing tumor-derived EVs. Compared to plasma and serum, CSF is in direct contact with primary brain tumors or brain metastases (BMs), thus harboring a larger proportion of positive patients and more abundant source for liquid biopsy (Pan et al. 2019; Panditharatna et al. 2018; Jiang et al. 2017a; Romero et al. 2021; Aldea et al. 2020). For advanced tumors with leptomeningeal metastases (LM), CSF is the most representative liquid and CSF cytology has been the diagnostic gold standard. Wang et al. examined 35 primary CNS malignancies and found detectable levels of ctDNA in 74% cases of patients’ CSF, as all medulloblastomas, ependymomas, and high-grade gliomas that existed directly adjacent to a CSF reservoir, indicating that CSF could be useful in analyzing primary CNS tumor (Wang et al. 2015a). In another study exploring the role of CSF as a source of liquid biopsy in patients with LM, Li et al. enrolled 28 patients with EGFR-mutated non‐small cell lung cancer (NSCLC) and compared the performance of detecting driver gene by liquid biopsy in 26 patients, yielding 100% (26/26) of CSF cfDNA, 84.6% (22/26) of CSF lysis, and 73.1% (19/26) of plasma cfDNA (Li et al. 2018). The highest number of copy number variations (CNVs), EGFR T790M and MET were detected in CSF cfDNA, with the major CNVs being exclusive to CSF cfDNA rather than primary tissue or plasma (Table 3).
Limitations of CSF analysis mainly lie in the acquisition and purification. The acquisition of CSF sample is usually performed during surgery to obtain maximal levels of biomarkers or in routine lumbar puncture with radiological surveillance. Though CSF liquid biopsy has shown minimal invasiveness, symptoms of tumor-induced CNS space occupation, or abnormal coagulation are contraindicated for lumbar puncture due to risk of herniation. Contamination by blood in the CSF sample is another technical issue to be solved (Seoane et al. 2019).
Target molecules or macrostructures
Circulating cell-free DNA (cfDNA)/ circulating tumor DNA (ctDNA)
Due to cellular turnover, active secretion, apoptotic and necrotic cells surrounding or in the tumor tissue, a mixture of nucleic acids, which is termed cfDNAs, released in a tumor patient’s blood is elevated. A small tumor-derived fraction is held in cfDNA that carries cancer-specific genetic and epigenetic aberrations, which is termed ctDNA and can provide actionable information toward subsequent diagnosis and treatment (Diaz and Bardelli 2014). Identification methods of cfDNA usually involve one or more aspects of common DNA-based changes, including cfDNA levels, cfDNA integrity, copy number variations (CNV), tumor-specific DNA mutations, gene methylation, and gene fusions, reflecting the status of DNA in the tumor cells (Meo et al. 2017; Wu et al. 2020). The increase of cfDNA level and copy number in tumor patients are explained by the raised number of apoptotic and necrotic carcinoma cells. High cfDNA integrity (> 400 bp) usually results from normal tissue necrosis in addition to the typical oligonucleosomal DNA fragments, whereas shorter DNA fragments (< 400 bp) indicate ctDNA due to apoptosis, which allows selection for the shorter cfDNA to increase ctDNA sensitivity (Mouliere 2011, 2021; Underhill 2016; Jiang et al. 2015). Human GBM ctDNA identified in xenograft rat plasma was typically shorter than the background rat cfDNA (134–144 bp vs. 167 bp, respectively) (Underhill 2016). Size difference was also identified in CSF, plasma, and urine of glioma patients (median size of 148 bp for mutant cfDNA vs 169 bp for non-mutant cfDNA; 160 vs 169 bp; and 101 vs 133 bp, respectively) (Mouliere 2021).
Standard methods optimized for cfDNA isolation include column-based and magnetic bead-based methods, and many DNA isolation kits are practical for both serum/plasma and CSF cfDNA with different pre-processes. The column-based approach refers to using spin columns with usually a silica matrix to bind DNA fragments during the pre-process, and subsequently applying a vacuum pump or a minicentrifuge to remove contaminants. The magnetic bead-based approach is based on nano-beads with magnetic coating able to bind nucleic acids that can be regulated under a magnetic field. Automatable manipulation is highlighted, which allows large-scale processing and omits manual centrifugation that may damage DNAs due to shear forces (Martignano and Cell-Free 2019; Ali et al. 2017).
CtDNAs contain small fragments between 70 and 200 base pairs, and large fragments up to 21 kilobases in size (Jahr et al. 2001). The concentration of cfDNA and ctDNA is influenced by anatomical location, volume, and vascularity of the tumor body, which is, in general, lower in patients with early-stage disease than in patients with metastatic disease. For example, the detection rate of ctDNA is over half of most cancer types, even almost 100% of certain primary cancers (bladder, colorectal, and ovarian) and as low as 10% of glioma cases (Butler et al. 2017). For patients with extracranial localized tumors, only 49–78% of them present detectable ctDNA levels, while the proportion is 86–100% in patients with metastatic colorectal, gastroesophageal, pancreatic, and breast cancers (Bettegowda et al. 2014). The amount of cfDNA and ctDNA is also influenced by clearance, degradation and other physiological filtering events of the blood and lymphatic circulation. Their clearance from the circulation goes through the liver and kidney and their half-life in the blood varies from 15 min to several hours (Fleischhacker and Schmidt 2007).
Circulating free RNAs (cfRNA)
While mRNAs code for proteins, microRNAs (miRNA) are usually 21–24 nucleotides in length, representing small single-stranded non-coding RNA molecules that participate in post-transcriptional regulation. MiRNAs are more predominant species of cfRNA and a number of studies on CNS tumor liquid biopsy have revealed certain informative types of miRNAs. For instance, overexpression of miR-15b and miR-21 was found correlated with the presence of gliomas by Baraniskin et al. (Baraniskin et al. 2012), and enrichment of miR-10b and miR-21 in patients’ CSF might indicate GBM (Teplyuk et al. 2012), while assessing miR-21, miR-19, and miR-92a CSF levels led to a combined diagnostic accuracy with 95.7% sensitivity and 96.7% specificity for primary central nervous system lymphoma (PCNSL) diagnosis (Baraniskin et al. 2011).
Circular RNAs (circRNAs) mainly function as miRNA sponges or peptide-coding RNAs and regulate alternative splicing of parental genes, which are characterized by their covalently closed structure and high tolerance to exonucleases (Meng et al. 2017; Li et al. 2015; Hansen et al. 2013). An increasing number of studies on circRNA deep sequencing have shown a correlation between a global reduction of circRNAs and different types of cancers compared to their normal tissues, such as GBM and colon rectal carcinoma (Zhang et al. 2018; Xu et al. 2018; Yin and Liu 2020). Differential expression levels of certain circRNAs have been found to be in gliomas, indicating their potential function in tumorigenesis (Zhang et al. 2018; Xu et al. 2018). From newly pathologically diagnosed high-grade astrocytoma (HGA) samples, Li et al. identified abundant circRNAs in both HGA cells and corresponding cell-derived exosomes, with a higher level in HGA cells (Li 2021). Additionally, three serum exosome circRNAs were chosen as circulating biomarkers screening of HGA and were promising in distinguishing HGA patients from healthy individuals (Fig. 2).
Although cancer is believed to be mainly driven by changes in DNA, changes in some driving factors may be manifested in RNA rather than mutations in DNA sequences (Calabrese et al. 2020). Therefore, RNA analysis may exhibit changes in cancer-related pathways that have not been detected by pure DNA methods. Another benefit of RNA-based sequencing is the ability to detect fusion genes, which refer to hybrid of two or more coding gene sequences in a common regulatory region that harbors a distinct function from the two pre-fusion genes. They are pathognomonic rearrangements which are commonly reported in about 30–50% of GBM patients (Shah et al. 2013). Wang et al. applied targeted RNA sequencing and unveiled two novel oncogenic fusion transcripts (FGFR3-TACC3 and VTI1A-TCF7L2) in EVs extracted from GBM tissues, and five novel fusions including two tissue-derived fusion transcripts in the paired GBM patients’ plasma (n = 9) (Wang et al. 2020).
Compared to RNA in the lipid bilayer enclosed structures such as circulating tumor cells (CTCs), exocellular vesicles (EVs) or tumor-educated platelets (TEPs), cfRNA is single stranded and exposed to nucleases in bodily fluids with low abundance and integrity; thus, it possesses limited reliability. Moreover, a large part of RNA in cells comes from ribosomes and mitochondria. This limits the number of reads of other RNAs and the accuracy of the expression levels of these RNAs (Raz 2011).
Circulating tumor cells (CTCs)
CTCs refer to a crowd of tumor cells detached from a solid tumor lesion into the peripheral circulation. As a direct source of ctDNA, they not only represent a relatively easily available sample of tumor tissue, but also a group of dynamic metastatic precursors such as epithelial and mesenchymal markers (Alix-Panabières and Pantel 2013; Aceto et al. 2015). It has been reported that approximately 39% of GBM patients harbor CTCs, and defining its ctDNA could help reveal the EGFR amplification status of the primary tumor (Sullivan et al. 2014).
CTCs may be found held together by intercellular cell–cell junctions traveling together through the bloodstream, which are called CTC clusters. Clustered formation hinders immunological surveillance and protects tumor cells from reactive oxygen species (ROS), thus possessing greater metastatic potential (Sprouse et al. 2019). For liquid biopsy, CTCs clusters exhibit dissimilar genetic profiles and metastatic characteristics from single CTCs. GBM can release CTC clusters, which can overcome the BBB and reach the peripheral circulation (Krol et al. 2018). Though the incidence of extracranial metastatic GBM is low (0.4 to 0.5% of patients), mostly to bone, lymph nodes, lungs, and liver (Pasquier, et al. 1980; Kalokhe, et al. 2012; Fonkem et al. 2011), it is reported that CTCs are positive in 20.6–39% GBM patients (Sullivan et al. 2014; Müller et al. 2014).
Enrichment or isolation is a crucial step in the liquid biopsy of CTCs. The technology approaches are divided into two methods, respectively, based on immunological or physical characteristics. To evaluate the quality of enriched CTCs, different characteristics from the normal cell are compared by means of immunofluorescence, FISH (fluorescence in situ hybridization), PCR-based detection methods, and/or high-throughput single-cell sequencing. However, there are some special occasions when CTC enrichment may be difficult to complete. For instance, platelet-covered CTCs can shield their surface antigen and enhance the interaction between CTCs and leukocytes, which possess more metastatic potential (Weber and Springer 1997; Kitamura et al. 2015). To solve the problem, Jiang et al. performed efficient depletion of free plates in blood, then implemented herringbone CTC chip (HB-Chip), a microvortex-generating device, to enhance cell-capture ability. As a result, they observed the presence of CTCs extensively covered by platelets compared to conventional EpCAM-based capture (Jiang et al. 2017b).
Conventional CTCs positive sorting methods mainly target epithelial markers such as EpCAM, which may lead to fewer variety of molecular types of CTCs (Kulasinghe et al. 2017). GFAP is an activated protein marker located in the cytoplasm of astrocytes that is commonly expressed among brain tumors, with higher levels reported in gliomas compared to other types (Bodegraven et al. 2019). Zhao et al. utilized a novel CTCs positive sorting marker targeting the endocytic GFAP and framed an isolation system on CTCs of brain tumors derived from GFAP antibody immunomagnetic liposome beads (GFAP-IMLs), which was further combined with an EGFR immunofluorescent assay (Zhao et al. 2020). Through subsequent validation of Sanger sequencing, q-PCR and NGS, the system was shown to be feasible toward identifying CTCs from tumor tissues, CSF and peripheral blood samples. GFAP markers also outperformed those of just EpCAM or EGFR for CTC capture. One of these challenges lies in the separation of CTC, which usually requires fresh blood samples with immediate sample processing. This may limit the initial application of CTCs in small centers that do not have the ability to process fresh samples in a short time (Shankar et al. 2017).
Exocellular vesicles (EVs)
Both cancer and non-cancer cells may release EVs into the extracellular space to transport messages between cells, by means of budding or intracellular vesicles merging with the plasma membrane (Colombo et al. 2014; Kros et al. 2015). It is intriguing that double-stranded DNA in exosomes, the smallest group of EVs, represents the entire genome and reflects the mutational status of parental tumor cells (Thakur et al. 2014). Compared to the rarity of ctDNAs and CTCs, EVs are more abundant per milliliter of blood and are present in almost all kinds of body fluids. Lipid bilayer wrapped structures also protect the contents from degradation. Cargo information is available in EV and exosome databases, such as ExoCarta, Vesiclepedia, and EVpedia (Lin 2021).
EVs play an important part in transporting messages to promote tumor progression. The tumor tissue secrete EVs and then neighboring stromal cells may take EVs and alter the cell program. For instance, glioblastoma stem cells (GSCs) derive EVs that induce non-conventional angiogenic pathways, which are free from classic anti-angiogenic drugs inhibiting the vascular endothelial growth factor (VEGF) or VEGF receptors (Geraldo et al. 2019; Kane 2019). Lucero et al. examined the RNA transfer by EVs from GSCs to human brain microvascular endothelial cells (HBMVECs) on cell lines in vitro through RNA-seq and DNA methylation profiling plus computational epigenomic deconvolution in vivo in the TCGA collection. The study provided multi-dimensional evidence that morphologic vascularization patterns of GSC EVs-mediated anagenesis were visually similar to that of VEGF, but stimulated distinct transcriptional and epigenomic alterations in HBMVECs, and that the downregulation of angiostatic genes could be mediated by miRNAs, like export of miR-9–5p inducing therapy resistance in GSCs (Lucero et al. 2020). Gliomas may produce EVs containing miRNAs, such as miR-21, miR-222, miR-124-3p, miR-221, miR-451, miR-23a, miR-29a, miR-30a, and miR-92b, which may motivate proliferation and suppress apoptosis (Colombo et al. 2014; Li et al. 2013; Godlewski et al. 2010). There is evidence that analyzing the expression of miR-21, mir-222 and miR-124-3p from serum-isolated exosomes potentially enables discrimination between HGGs and LGGs (Santangelo et al. 2018). If the tumor-secreted EVs are taken by the immune system, it may lead to immunosuppression, possibly resulting in abnormally activated myeloid-derived suppressor cells (MDSC) and regulatory T (Treg) cells (Kahlert and Kalluri 2013). Additionally, in GSC-derived EVs, PD-L1 has been identified to be involved in this process which can be up-regulated in response to IFN-γ, as by PD1 blockade Ricklefs et al. significantly reversed the EV-mediated inhibition of T cell activation (Ricklefs et al. 2018).
Besides apoptotic bodies, the other two major classes of EVs, exosomes and microvesicles (MVs), overlap in their size and mimic surface proteins, but there is a lack a specific marker or cargo associated with MVs distinctive from exosomes (Lawson et al. 2016) and so it is challenging to isolate them. MVs are small vesicles ranging from 100 to 1000 nm that fall off the cell membrane after cell activation, injury or apoptosis (Lee et al. 2012). Exosomes are small saucer-shaped vesicles ranging from 40 to 100 nm in diameter, usually excessively produced by tumor cells, composed of proteins, lipids, DNA and RNA, which share large similarity to their original cell or tissue and harbor more diagnostic potentiality (Théry et al. 2002). In an exosome characterization study, Fernando et al. suggested that exosome-derived cfDNA makes up 90% of cfDNA and 93% of amplifiable plasma cfDNA in plasma (Fernando 2017). Thakur et al. exploited a novel nano-biosensor (SAM-AuNIs) which enables direct detection of biophysical difference, membrane property differentiation and distinguish between exosomes and MVs (Thakur et al. 2017). In conclusion, the current isolation and purification of EVs from plasma are technically more difficult, mainly concerning specificity compared to directly from cfDNA or CTCs, but harbor more sensitivity.
EVs from cancer cells and healthy cells are difficult to distinguish. The 5-ALA oral administration is performed before glioma surgery to promote tumor visualization and maximal resection, and its metabolite, PpIX, may be accumulated in gliomas-derived EVs. Maas investigated the feasibility of identifying PpIX-positive EVs from GBM patients orally administered 5-ALA (Maas et al. 2020). High-resolution flow cytometric on sorting EVs was conducted using plasma, and ddPCR analysis further presented the existence of GBM-associated miRNAs in PpIX-positive EVs, like miR-21 and miR-10b. Jalali et al. reported using a plasmonic microfluidic device with nanostructure to discriminate tumor-derived EV subgroups from different GBM cell lines, EVs from common glial cells and artificial liposomes (e.g., DOPC/Chol) that is homogenous to EVs demonstrated high sensitivity (Jalali 2021). TiN-NH-LSPR biosensor developed by Thakur et al. could detect and quantify CD44 and CD133 enhanced by GBM-secreted lactate, thus distinguishing that secreted exosomes sensitively form malignant cells (Thakur 2021).
Tumor-educated platelets (TEPs)
Platelets are blood cells forming blood coagulation in response to vascular endothelium damage. However, platelets also respond to a series of inflammatory events, cancer genesis and metastasis. When tumor-associated biomolecules are transferred into platelets, they become “educated”. For years high platelet count and expression of coagulation factors by cancer cells have been recognized as negative prognostic markers in a variety of cancers (Nash et al. 2002; Erpenbeck and Schön 2010; Gay and Felding-Habermann 2011). Firstly, platelets can regulate the tumor vasculature through which circulating tumor cells (CTCs) disseminate by releasing angiogenic and vascular remodeling factors in granules upon activation by thrombin triggered by tumor tissue-associated factors (Gay and Felding-Habermann 2011; Sabrkhany et al. 2011). For instance, GBM stem cells can transdifferentiate into endothelial cells (GECs) which are aberrantly responsive to increased levels of VEGF released by TEPs and boost tumor progression and angiogenesis (Vito et al. 2017). Increased expression of its receptors VEGFR1 and VEGFR2, VWF, and S1P was also identified in another study as predictive and monitoring biomarkers (Campanella et al. 2020). Next, platelets can generate cloaks covering tumor cells, which together with fibrin can provide a physically protective shield (Nieswandt et al. 1999; Palumbo et al. 2005) or MHC class I that confers a pseudonormal phenotype that disrupts recognition of tumor cell, to shelter tumor cells from cytotoxic activity of natural killer cells (Placke et al. 2012). Finally, platelets also play a role in the establishment of metastases by enhancing interaction of tumor cell with vascular endothelium under hemodynamic shear environment of circulation (Konstantopoulos and Thomas 2009) and recruit granulocytes to tumor cells to form early metastatic niches (Labelle et al. 2014).
Since platelets themselves do not have a nucleus, all RNA in platelets comes from the original megakaryocytes, interacted cells and circulation. Nilsson et al. demonstrated that cancer cells can transfer mutant RNA through EVs-like membrane vesicles into blood platelets, and identified a distinct group of cancer-associated RNA alterations including EGFR-vIII mutations in the TEPs from GBM patients compared to normal controls by gene-expression profiling (Nilsson et al. 2011). By liquid biopsy, the detection of tumor-related proteins, cfDNA and RNA sequestered by platelets enables blood-based cancer diagnostics and treatment monitoring. RNAs in platelets advance in their abundance, easy isolation, high quality, and platelets’ capacity to take up EVs comprising RNAs originated from tumors (Best et al. 2018; Sol and Wurdinger 2017). What is more, the life span of a common platelet is 7–10 days, thus it can be a promising source of RNA analysis, revealing a real-time status of tumor dynamics.
Recently, Best et al. reported a different liquid biopsy approach called thromboSeq by detecting TEP-absorbed tumor RNA and swarm-intelligence-enhanced algorithms to optimize biomarker gene lists, with its accuracy up to 90% (Best et al. 2017). Researchers investigated blood samples from patients diagnosed with advanced non-small cell lung cancer (n = 518), patients in early stage (n = 106), and controls without known cancers (n = 155). In the study, thromboSeq scanned about 5000 different RNA molecules found in platelets, continuously optimized its RNA gene panel, and found a few RNAs that can predict tumors. The results confirmed that the accuracy of thromboSeq in diagnosing early cancer was 81%, and the accuracy in diagnosing advanced cancer was 88%. In the matched group controlling age, smoking status and blood storage time, the accuracy rate was as high as 91%. The protocol and details of thromboSeq were updated in 2019 and it is promising in future pan-cancer and multiclass tumor diagnostics (Best et al. 2019).
There are some limitations that platelet mRNA profiles may be affected by non-cancerous systematic factors such as inflammatory or cardiovascular diseases; therefore, it makes it difficult to differentiate between TEPs and normal platelets and requires follow-up studies for further evaluation (Best et al. 2015). During the platelet-isolation procedure, residual cfDNA contamination can potentially introduce concomitant sequencing of reads mapping to intergenic regions, thus reducing the number of spliced intron-spanning platelet-RNA sequencing reads (Best et al. 2019).
Metabolites and proteins
In the absence of CSF cytology findings, analyzing patients’ CSF metabolites may help diagnose and distinguish different types of CNS tumors. However, identifying specific protein markers for CNS cancer is more challenging than cfDNA or cfRNA sequencing. Elevated protein levels may attribute to inflammation, unrelated diseases or responses to stress/hypoxia, making it hard to enter clinical practice.
Metabolomics and proteomics are promising in combination with liquid biopsy to identify novel biomarkers, thus providing disease screening and diagnosis. Ballester et al. examined 43 metabolites derived from CSF in primary or metastatic brain tumors patients by utilizing different spectrometries (Ballester et al. 2018). Furthermore, they found that IDH-mutant gliomas exhibited metabolite alterations of the tricarboxylic acid cycle, such as higher concentration of D-2-hydroxyglutarate, malic acid and succinate in CSF, when compared with healthy controls and other types of tumors. More potential protein biomarkers are summarized in a systematic review of eight proteomics articles (Shen et al. 2014). Out of these 19 differentially expressed proteins (B2M, CA2, CA12, CALD1, DDAH1, MYCN, PPIA, SPP1, VEGFB, ALB, MAPT, SERPINA3, SPARCL1), only attractin was identified twice among these 8 proteomic studies on glioma CSF, which may function as a mediator in malignant gliomas, representing a novel biomarker as well as a potential therapeutic target (Khwaja et al. 2006, 2007).
Intracranial germinomas occur in the pineal and suprasellar region, arising from abnormal migration and differential of various germ cell layers during embryogenesis. β-Human chorionic gonadotropin (bHCG) and alpha-fetoprotein (AFP) are relevant proteins representing germ cell origination, and the levels of both proteins have been shown to be significantly elevated in germinomas patients’ CSF. Both bHCG and AFP have been used to diagnose malignant germ cell tumors, monitor response to treatment and predict recurrence (Nishizaki et al. 2001; Qaddoumi et al. 2012). Placental alkaline phosphatase (PLAP) holds clinical potentiality for the diagnosis and monitoring of intracranial germinomas as a biomarker. Watanbe tested PLAP in 28 patients’ CSF with a chemiluminescent enzyme assay using antibodies, which presented high sensitivity and specificity of 94% and 97% (Watanabe et al. 2012).
Clinical applications
Detection
Non-invasive and convenient “biopsy”
As for tumor tissue pathological biopsy, which is still the golden standard for tumor diagnosis, there are many limitations. Patients with CNS tumor usually undergo surgery and the resected tumor tissue can be utilized for pathological examination, though brainstem glioma biopsy is not routinely performed, which ensures the quantity of tumor cells regardless of biopsy area and size. For those who do not meet the criteria of surgery, such as with disseminated systemic disease, the biopsy is costly and may induce adverse events. Surgical biopsy adds inconvenience from a scheduling perspective and there are difficulties in sample preservation. Tumor heterogeneity is another problem hampering the biopsy. For primary CNS tumor, there is intratumoral heterogeneity, and for BM, there is intermetastatic heterogeneity within the same patient.
Compared to tissue biopsy, liquid biopsy is noninvasive and convenient, allowing for early assessment and follow-up. Furthermore, since it is easy to acquire the samples, the preservation of samples can be omitted, and fresh samples enable monitoring the progression in real time through molecular and epigenetic changes profiling. Liquid biopsy samples contain synonymous or even more comprehensive genetic information than biopsy, thus providing more insights into molecular mutation status, intratumoral and intermetastatic heterogeneity, even predating changes seen on MRI.
Early detection in asymptomatic patients
For those without clinical symptoms or radiological findings, liquid biopsy helps evaluate the tumor existence or progression risk, consequently implementing early intervention to reduce the risk. The majority of cancer genes mutate at moderate rates (2–20%) or lower, despite some genetic changes happen at high incidence (Lawrence et al. 2014), and mutation rate is not perfectly correlated with driver gene potency. Though genetic mutations and chromosomal changes are seemingly random, they may actually follow specific patterns. Early monitoring of known patterns may predict the tumor onset in healthy ones or progression in diagnosed ones. Moreover, by revealing unknown patterns, it is capable to successively link mutant genotypes to cellular and clinical genotypes and obtain deep understanding of the tumor evolution and its mechanisms (Wang et al. 2015b).
A recent study by Fanfani et al. suggested that germline and somatic mutation information may help explain cancer heritability in the more comprehensive population with higher tumor risk, and could provide guidance on formulating plans for early detection and progression surveillance (Fanfani 2021).
For instance, Kammesheidt et al. successfully detected TP53 and KRAS mutations by liquid biopsy using cfDNA in healthy volunteers (Kammesheidt et al. 2016), which showed the ability of liquid biopsy in early-stage tumor detection.
Reducing the cost
There are occasions that early diagnosis definitely leads to better overall prognosis (Lennon et al. 2020), either because of currently incurable cancer types, or indolent cancers that would never progress. In both occasions, the early diagnosis has very limited impacts on improving treatment effects, but causes adverse economic, physical and psychological burdens, which is termed “overdiagnosis” (Srivastava et al. 2019).
As liquid biopsy is simple, rapid, sensitive and specific for low levels of target molecules or macrostructures to achieve early detection, low incidence of cancer occurrence in the general population is a parallel problem since current screening methodology and many analysis techniques are not cost-effective. Based on the analysis of thermodynamic and kinetic parameters reflecting complex molecular interactions in biological fluids, the DSC (differential scanning calorimetry) analysis has made promising progress in discriminating healthy from glioma patients (Tsvetkov et al. 2018; Engh 2011; Chagovetz et al. 2013). When DSC thermograms are applied in liquid biopsy, it is termed “thermal liquid biopsy”. Compared to sequencing liquid biopsy, thermal liquid biopsy has shown approximate performance but is more economical (Velazquez-Campoy et al. 2018; Hermoso-Durán et al. 2020; Rodrigo et al. 2019).
Likewise, FTIR (Fourier transform infrared) spectroscopy, nanoDSF (differential scanning fluorimetry) or CCP-based FRET (cationic conjugated polymer-based fluorescence resonance energy transfer) may substitute sequencing to reduce the cost, yet requiring more abundant studies to validate sensitivity and specificity (Tsvetkov et al. 2021; Sala et al. 2020; Ma et al. 2021). A new liquid biopsy technique, CancerSEEK [NCT04213326], not only detects mutant genes in the blood, but also locks on abnormal proteins in the blood, with an average price lower than 500 dollars. In about 1,000 cancer patients comprising common extracranial tumors like ovarian cancer and liver cancer, its accuracy rate reached 70%, which holds promise in expanding to screen brain tumors (Cohen et al. 2018). Other few commercial assays are also under development, such as GRAIL [NCT03085888], focusing on early detection.
Mouliere et al. implemented untargeted shallow whole‐genome sequencing (sWGS, < 0.4 ×) of ctDNA in CSF derived from 13 patients (Mouliere et al. 2018). Through cfDNA size profiles analysis, shorter DNA fragments (145 bp) were shown to be in positive association with the presence of tumor‐derived somatic copy number alterations (SCNAs) in CSF. Combining cfDNA SCNAs screening and fragmentation analysis using sWGS is feasible to provide information on the tumor genome at lower cost and more rapidly, which can also prepare for further sequencing in larger scales, such as whole-exome sequencing or targeted sequencing.
Characterization
Differential diagnosis
N6-methyladenosine (m6A) modification was widely found in peripheral blood cfRNA and the dysregulation of m6A modification level was closely linked with tumor occurrence and progression (Patil et al. 2016; Zhang et al. 2020). Based on 14,965 serum samples (gliomas = 185) of 12 cancer types, Zhang et al. screened a total of 228 m6A target miRNAs and selected 18 candidate miRNAs for the construction of s diagnostic signature (Zhang et al. 2021). The signature was proven to have no interference by gender, age or benign disease and yielded excellent performance, with an AUC of 0.979 in distinguishing cancer samples from non-cancer controls, and an AUC of 0.703 in distinguishing GBM samples from all the mixed samples in the training cohort.
Shen et al. developed an approach called cell-free methylated DNA immunoprecipitation and high-throughput sequencing (cfMeDIP-seq), which is bisulfite free and only requires 1–100 ng of cfDNA input (Nassiri et al. 2020). By profiling cfDNA of early-stage cancers from various body liquids, researchers were able to identify methylation patterns unique to each cancer type. Then with the help of big data and machine learning, they could build a classifier to determine the cancer type. Subsequent discovery cohort consisted of 189 samples from seven different tumor types to classify them, respectively, from healthy controls with an AUC > 0.9 for each cancer type. For further validation of plasma-based cfMeDIP-seq in CNS primary tumor, Nassiri et al. tested 220 patients with brain tumor and built a machine learning classifier (Nassiri et al. 2020). The classifier showed good performance (AUC = 0.99) in recognizing differentially methylated regions (DMRs) and distinguishing gliomas from BMs with extracranial primary tumor types along with healthy controls, and revealed highly specific signatures discriminating different primary brain tumors.
Sol et al. investigated the extended diagnostic power of thromboSeq (Best et al. 2017) to differentiate several neurological diseases (GBM, BMs and active multiple sclerosis lesions) (Sol 2020). By comparing the spliced RNA profile of TEPs, they successfully identified GBM patients from healthy controls (AUC = 0.97), and other neurological diseases (AUC = 0.81). They further developed a machine learning algorithm called digitalSWARM that scores on TEP to improve monitoring of individual GBM patients, which correlates with tumor behavior and allows distinguishing false positive progression from true progression (AUC = 0.86).
To discriminate tumor and non-tumor diseases and cross-compare different types of brain tumor, Wang et al. enrolled 163 patients with either nontumorous brain diseases, systemic lymphoma involving CNS, and metastatic or non-metastatic lung adenocarcinomas and analyzed their CSF using liquid chromatography–quadrupole time-of-fight spectrometric (LC-Q/TOF–MS) (Wang et al. 2020). As a result, 27 representative metabolites were proven diagnostic in discriminating different brain tumor types and yielded good performance with the highest AUC of 0.91 for the metastatic status of lung adenocarcinomas.
The WHO classification of tumors of the CNS
For those with unidentified brain mass, liquid biopsy helps ascertain the malignancy and correctly classify the tumor in time. In 2016. WHO published a new guideline on classifying CNS tumors; the molecular markers were especially spotlighted to describe brain tumor entities histology features, which were further refined especially in pediatric subtypes in 2021 (Louis et al. 2016). Furthermore, target therapies and treatment strategies for malignant brain tumor patients are predominantly dependent on specific molecular markers, emphasizing the importance of precision oncology. Recent clinical studies using liquid biopsy below approved the feasibility.
Wang et al. found using serum EVs for assessing the expression level of EGFR provided accurate prediction on HGG and LGG differentiation, and found positive correlation with the EGFR level and ki-67 labeling index, by implementing a flow cytometry method with microbead-based EV enrichment for detection, isolation and analysis of proteins and mRNAs in EVs. The analysis of NLGN3 and PTTG1 mRNA in EVs also revealed their potentiality as diagnostic markers (Wang et al. 2019).
Syndecan-1 (SDC1) mRNA can be detected in several GBM cell lines, but remains undetectable in normal brain tissues (Watanabe et al. 2006), and its expression level is related to a stroma hyperplastic, hypoxic and pro-angiogenic phenotype. Chandran firstly identified SDC1 as a plasma EV component which can help classify gliomas into GBM or LGG in a cohort (n = 82) (AUC: 0.81) and further in an independent cohort from TCGA (AUC: 0.91). They also provided robust evidence that the EVs containing SDC1 mRNA originated from GBM cells (Indira Chandran et al. 2019).
Huang et al. studied 11 patients with pediatric gliomas, including diffuse midline gliomas (DMGs) and successfully assessed H3 gene mutations in their CSF ctDNA for the first time, through targeted H3F3A and HIST1H3B via Sanger sequencing, and targeted H3.3K27M mutant H3F3A allele detection via nested PCR (Huang et al. 2017).
Billard et al. proposed a novel approach to classify diffuse gliomas by detecting telomeric DNA in blood samples and revealing telomere maintenance mechanisms (TMM) (sensitivity: 100%; specificity: 97.3%) (Billard et al. 2021). They further combined TMM with IDH mutation status and histological grading to develop a simple and rapid classification tool called the TeloDIAG, which efficiently defines five glioma subtypes corresponding to five different classes by the 2016 WHO classification (sensitivity of 56% and specificity of 97% for IDHwt astrocytoma).
Medulloblastoma (MB) is the most common form of malignant pediatric brain tumor, with a much lower frequency of oncogenic genomic mutations than other pediatric cancers or adult cancers. During MB progression and clinical treatment, distinct epigenetic signatures and altered DNA methylation were found by Northcott et al. who epigenetically analyzed 1256 cases (Northcott et al. 2017). Different subtypes display distinct transcriptional and epigenic profiles (WNT and SHH, driven by Wingless and Sonic hedgehog signaling pathways, and Group 3 and Group 4, with less clear underlying genetic and biological mechanism). The prognosis of MB is correlated with the subtype, for example, approximately 30% of patients with Group 3 or 4 MBs presented signs of metastasis at initial diagnosis (Srinivasan et al. 2016), and relapsed MB still maintains the original molecular subtype. Furthermore, they integrated this data with large public datasets (training cohort: 438; validation cohort: 189) and set up a reliable survival score for each patient.
Profiling genetic mutation for targeted therapy
Gliomas of the brainstem are molecularly heterogeneous and difficult for resection. Pan et al. accessed alterations in 47 brainstem glioma patients by NGS with ctDNA in CSF for potential targeted therapies, which include mutations in H3F3A/HIST1H3B, IDH, BRAF, PDGFRA, PPM1D and MET. Among all CSF ctDNA samples, 82.5% (47/57) yielded at least one tumor-specific mutation. Subsequently, the patient cohort went through surgical resection or open biopsy, and the matched DNAs were sequenced to verify the sensitivity and specificity of liquid biopsy. The results showed all primary tumor alterations by biopsy were detected in over 83% (31/37) of matched CSF ctDNA samples. For those with primary tumor DNA biopsy negative for mutations, they also found 30% (3/10) exhibited detectable mutations exclusively by CSF ctDNA sequencing. Finally, they carried out liquid biopsy using cfDNA from plasma, which showed lower sensitivity than matched CSF ctDNA (plasma: 38%, CSF: 100%) (Pan et al. 2019).
While previous study has exhibited that TERT promoter mutations are among initial genetic alterations in IDH-WT GBM (Barthel et al. 2018), they commonly happen and act as a precondition for rapid growth. C228T and C250T account for two of the most frequent alteration subtypes in TERT promoter mutations, but technically difficult in amplification due to the GC-rich regions in the promoter loci. Recently, Muralidharan and his colleagues developed a ddPCR assay to assess gliomas patients with TERT promoter mutations in cfDNA from plasma, independently in discovery and blinded validation (n = 157) cohorts from multiple centers (sensitivity: 62.5%; specificity 90%) (Muralidharan et al. 2021).
BRAF V600E mutation activates the MAPK/ERK pathway (Carlino et al. 2015) and contributes to a 10-year progression-free survival rate decrease by 33% compared to wild type in patients with pediatric LGGs (Lassaletta et al. 2017). As one of the inhibitors of the kinase BRAF, which mainly functions in MAPK/ERK signal paths, dabrafenib holds excellent effectivity against BRAF in vitro and the best diffusivity in brain tissues, thus harboring the most popularity in clinical practice. However, the use of dabrafenib is contraindicated in patients with wild-type BRAF due to the risk of tumor progression in these patients (Sanchez et al. 2018). To detect and quantify BRAF V600E mutation with a high efficiency, García-Romero et al. sequestered cfDNA and extracted EV-derived DNA from 29 pediatric CNS tumor patients from serum, plasma and CSF (García-Romero et al. 2019). The results showed that ctDNA derived from serum rather than plasma yielded the best performance for genetic characteristics such as BRAF V600E mutation of the original tumor by dPCR detection. Kang et al. tested the feasibility of detecting circulating BRAF V600E in a cohort of nine patients with known BRAF mutant gliomas or metastatic disease, showing a sensitivity up to 80% and a specificity up to 100% (Kang et al. 2021).
The US NCCN guidelines recommend epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitors (TKIs) like osimertinib for the first-line treatment of NSCLC patients with T790M‐positive mutations in the EGFR gene, but acquired resistance of TKIs is prevalent in clinical management. BMs are frequently found in NSCLCs that possess at least one oncogenic driver mutation, which can be a potential therapy target. Wei et al. assessed 375 patients harboring either an EGFR 19del or L858R mutation that sensitizes TKIs ascertained by initial biopsy and implemented ddPCR and ARMS-PCR assay on evaluating T790M mutation, which approximately explains for 50% of the acquired TKI resistance in Caucasian and Asian populations, through plasma extracted ctDNA validated by NGS (Wei et al. 2019). Their results indicated that acquired T790M mutation is positively correlated with primary EGFR 19 del and brain metastasis as revealed by univariate analyses. Kaplan–Meier survival curves suggested that progression‐free survival of initial EGFR-TKI treatment was significantly longer in T790M-positive patients than negative ones.
Another study involving 71 GBMs was reported by Figueroa et al. The CSF of patients was collected, and the EVs were isolated and assessed for EGFR-vIII mutation and EGFR amplification status, with comparison of cells from primary tumor biopsies (Figueroa et al. 2017). The results elucidated that CSF-derived EVs contain RNA signatures reflecting wild-type EGFR expression and EGFR-vIII status in GBMs, with a sensitivity up to 61% and a specificity up to 98%.
Monitoring
Evaluating early treatment response
For DMGs which are impossible for surgical resection, radiotherapy remains the standard of care at diagnosis. In a recent study aiming at differentiating pseudoprogression (PsP) and DMG progression carried out by Panditharatna et al., 88% of patients with pediatric DMGs were H3K27M positive using CSF and matched plasma, with the highest enrichment of ctDNA in CSF (Panditharatna et al. 2018). Following radiotherapy, a significant decrease in H3K27M plasma ctDNA (at least 50% decrease) consistent with MRI assessment (at least 10% decrease) of tumor volume alterations was found in 83% of patients, which indicates that liquid biopsy could function as a strong supplement.
Compared with genomic alterations, the specificity of detecting epigenic changes is often lower, but ctDNA methylation often yields good sensitivity, since methylated DNA often occurs in early carcinogenesis. The detection of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation status through cfDNA helps predict the treatment response of temozolomide in adults (Maire 2021). Sabedot et al. identified a cfDNA-derived methylation signature through genome-wide DNA methylation profiling of gliomas (Sabedot 2021). Based on the signature, they further verified a score metric (the "glioma epigenetic liquid biopsy score" or GeLB) that optimally distinguished patients with or without glioma (sensitivity: 100%, specificity: 97.78%). Following up patient’s GeLB score can also reflect their tumor progression and treatment response.
Accessing minimal residual disease
A traditional way of monitoring the dynamics of tumor is to access the sugar, cholesterol and/or protein circulating biomarkers, such as prostate-specific antigen (PSA) for prostate cancer and cancer antigen (CA) 19-9 for pancreatic cancer. However, these biomarkers are found only in a small portion of tumor and lack specificity for an individual. The analysis of cfDNA deals with the above problems, since the genetic alteration happens in every tumor. To discriminate ctDNA from normal cfDNA, genetic alterations in cfDNA must be detected and compared with DNA in normal cells in the same individual. Also, circulating biomarkers are not accurate for onset time prediction, since the protein biomarkers are persistent in circulation for several weeks. The half-life of cfDNA is comparatively short (approximately 2 h), so changes in cfDNA can be identified before beingseen on regular imaging or employing protein biomarkers with weeks to months ahead (Diehl et al. 2008; Dawson et al. 2013). This characteristic allows liquid biopsy to be utilized in early detection and monitoring of tumor progression during or after treatment.
During routine cancer surveillance, MRI and CTCs found in CSF cytology and flow cytometry analysis are highly specific of CNS relapse, but harboring low sensitivity. Ignatiadis et al. introduced a term ‘ctDNA relapse’ for those with detectable ctDNA, but without obvious imaging evidence after completion of primary cancer surgery and neoadjuvant and/or adjuvant chemotherapy (Ignatiadis et al. 2021). EscuderoIn et al. reported that CSF ctDNA analysis not only facilitated MB subgroup and risk stratification, but also helped to identify minimal residual disease in two cases, as ctDNA obtained in the follow-up CSF samples had similar mutations and/or loss of chromosome arms (Escudero et al. 2020). Liu enrolled 123 MB patients and 476 CSF samples, and applied low-coverage whole-genome sequencing for cfDNA detection of tumor-associated CNVs, which were used as a biomarker for measurable residual disease (MRD) and showed good detectability (85% in patients with metastatic MB, 54% for those with non-metastatic disease and 64% for those with negative CSF cytology findings) (SHH, 32%; WNT 63%; Group 3, 77%; Group 4, 75%). Persistent MRD positivity at the end of treatment in patients, regardless of initial risk grouping, is correlated with shorter PFS, which is detectable months ahead of radiographic progression (Liu et al. 2021).
Bobilo et al. evaluated CSF and plasma from 19 patients with CNS B-cell lymphomas and observed that CSF ctDNA levels increased earlier than CTCs by flow cytometry, which confirmed CNS relapse after months (Bobillo et al. 2021). Besides, ctDNA also showed better performance in monitoring CNS tumor burden and response to treatment. In another trial exploiting the effect of ibrutinib with HD-MTX–based chemotherapy in CNS lymphomas, Grommes applied MSK-HemePACT targeted panel to sequence CSF ctDNA and observed a considerable fraction of patients with subsequent recurrence or refractory harbor tumor DNA in CSF (Grommes et al. 2019).
Tracking evolution of resistance in real time
GBM is characterized by a high infiltration rate and treatment resistance, remaining the most fatal brain tumor. While GBMs may be primary, or secondary ones that evolve from LGGs, it makes tracking gliomas evolution of great importance. Zeng et al. looked into the mechanism of TMZ resistance by characterizing exosomes from two TMZ-resistant cell lines, patients’ serum and matched CSF using qPCR, and demonstrated that miR-151a in exosomes not only plays as a positive predictor to TMZ response, but also potentially serves as a target for treatment of refractory GBMs. The results suggested that recurrent GBM samples and TMZ-resistant cells usually present lower levels of miR-151a, while high levels of miR-151a can strengthen the response to TMZ in vitro via inhibiting XRCC4-mediated DNA repair (Zeng et al. 2018).
Brain tumor may directly release ctDNA into CSF and its level is related to tumor burden and adverse outcome. In extensive research carried out by Miller et al., 85 patients with WHO II-IV gliomas were evaluated using CSF-derived ctDNA (Miller et al. 2019). The data suggested that the alterations detected in CSF ctDNA from diffuse gliomas represent the genome landscape found in tumor biopsies. They exploited this technique to track the pattern of genomic evolution within the same patient and found out that truncal genetic alterations are characterized by IDH1 and 1p/19q codeletion in early tumorigenesis and subsequently in TP53, TERT and ATRX, which mostly correlate with the core pathways of gliomas, especially growth factor receptor pathways.
To investigate liquid biopsy techniques and mediums in leptomeningeal metastases (LM) frequently reported in NSCLC with EGFR mutations, Jiang et al. detected the driver and resistance mutations in 21 NSCLC patients diagnosed with LMs and investigated the performance of CellSearch Assay™, the Thinprep cytologic test (TCT), and brain MRI (Jiang et al. 2017a). The results supported CellSearch as it yielded the highest sensitivity (95.2%) and specificity in capturing CSF CTCs among other single methods. By CellSearch, they also found significantly higher concentration of CTCs in CSF than peripheral blood samples.
Predicting survival time
To noninvasively detect MBs and discriminate the subtypes, Li et al. performed whole-genome bisulfite sequencing (WGBS) and CMS-IP–seq analysis that elucidated 6598 differentially methylated CpGs in the CSF-derived ctDNA paralleling the mutation direction of MB tumors. By associating DNA methylation level at this CpG site with survival rates, they found that high DNA methylation may function as a poor prognostic marker for pediatric MB patients (Li et al. 2020).
Since IDH mutations are common in LGGs and the mutated enzymes result in increasing abundance of tissue total 2-HG, including R-2-hydroxyglutarate (R-2-HG) and S-2-hydroxyglutarate (S-2-HG), Sim et al. quantitated R-2-HG and S-2-HG and calculated the individual ratio of R-2-HG/S-2-HG (rRS) in tissues and blood samples from glioma patients intraoperatively, based on HPLC–mass spectrometry (Sim et al. 2019). The tissue rRS proved sensitive and specific for IDH mutations, while blood rRS did not; it is rapid and can be implemented during surgery. Higher rRS is associated with poorer prognosis, which may be an additional target of CSF liquid biopsy.
Future development
Tissue-based multi-panel gene analysis has been contributing to the clinical application of liquid biopsy. As more and more novel genes are discovered, gene panels in panel sequencing are rapidly developing, for instance, TAM-Seq (tagged-amplicon deep sequencing) and CAPP-Seq (cancer personalized profiling by deep sequencing) both harbor high sensitivity and coverage depth with balanced cost and coverage mutation number (Thierry et al. 2010). The earliest application of liquid biopsy in brain tumor may take place in most prevalent mutation points or panels of different miRNA, such as IDH mutation, TERT mutation, a marker correlated with ATRX loss on total blood cells, and miR-21.
For example, GliomaDx assay, an LNA-based allele-specific qPCR method developed by Diplas et al., has yielded much sensitivity and rapidity in diffuse glioma classification (Diplas et al. 2019). Based on the sensitivity analysis, GliomaDx targets recurrent mutations in the TERT promoter and codon 132 of IDH1 in samples, which is over 200 times more sensitive than Sanger sequencing and can be performed within an hour. Besides exploiting CTCs or ctDNA in plasma, voided urine, or CSF, GliomaDx also showed ability in genotyping surgically resected tissue or frozen specimens of variable tumor purity, making intraoperative diagnosis more practical. Other researches like the circTeloDIAG, a liquid biopsy tool combining detection of three markers for glioma, are ongoing (NCT04931732).
Recently, urine is emerging as a CNS tumor liquid biopsy source. With the development of a nanowire device that needs only 1 mL of urine from mainly glioma patients and noncancer individuals, an artificial intelligence (AI) diagnostic model detecting miRNAs of such tumors in urine samples was established at a sensitivity and specificity of 100 and 97% (Kitano et al. 2021). Mouliere et al. detected tumor-derived DNA in vast samples (in CSF, 7/8; in plasma, 10/12; and in urine, 10/16) (Mouliere 2021). Based on cfDNA fragmentation features in urine samples, they further built a random forest (RF) to classify gliomas and non-cancer patients that exhibited a median AUC = 0.91.
Combining liquid biopsy with machine learning is promising to improve the accuracy and efficiency in recognizing mutational patterns and identifying key features predictive of progression, which has been proven by several studies (Zhang et al. 2021; Nassiri et al. 2020; Sol 2020; Li et al. 2020). With the development of machine learning and the prevalence of big data from multiple centers, it is also feasible to uncover the unknown driver genes by sequencing multiple mutational patterns and further utilize these patterns for the construction of predictive models. However, aging, chemo and popular germline mutations may affect the analysis of patterns and they have been not extensively catalogued. Like other noninvasive diagnosis tools, artificial intelligence is promising to be involved in future liquid biopsy workflow and accelerate liquid biopsy into clinical practice. Multiple studies have utilized machine learning for sequencing analysis. However, deep learning requires a large quantity of data. While deep learning in radiomics can easily gain images for analysis, liquid biopsy lacks extensive clinical application which limits the source of learning material. A public database with standard criteria of reporting is crucial for future development of liquid biopsy.
Based on multiple noninvasive diagnostic tools, somatic mutation accumulation and driver genes can be evaluated, the characteristics of tumor can be assessed, and progression of tumor can be recorded and visualized. For example, Cucchiara et al. integrated liquid biopsy and radiomics to monitor clonal heterogeneity of EGFR-positive NSCLC (Cucchiara 2020). As a result, more individualized treatment plans can be tailored and patients with imperceptible premalignant lesion or those who undergo surgery can also benefit. Future studies should focus on improving the sensitivity and specificity.
Conclusion
The emergence of noninvasive detection techniques such as liquid biopsy has yielded much progress in recent years. The liquid biopsy, analyzing molecules or macrostructures in low concentration from body liquid, shows minimal invasiveness toward patients who are susceptible to tumor or cannot withstand biopsy. The blossoming of sequencing technique, such as ddPCR and NGS, ensures more accurate molecular profile, higher specificity, and more clinical practicability. The plasma is the most preferable source for brain tumor liquid biopsy due to convenient sampling. For patients with primary brain tumor, the CSF may be a better source of liquid biopsy, since the BBB blocks the spreading of the target molecule or macrostructure, which may be safely and transiently opened by techniques like MRgFUS. For patients with metastases, the comparison among CSF, blood and normal tissue may provide more information regarding intratumoral and intermetastatic heterogeneity. Though cfDNA is more convenient, EV-derived DNA may hold inherent stability due to its lipid bilayer enclosed structures, yet isolation and purification of EVs are associated with more technical difficulties. Due to the limitations in the low concentration, discrimination and quantification of the target molecules, the overall profiling is still challenging, thus needing more reliable source and more sophisticated sequencing techniques. Another barrier hindering liquid biopsy’s extensive clinical application is its high cost. Shallow WGS or targeted extreme including ddPCR may be relatively cost-effective (Mouliere et al. 2018). Other noninvasive detection techniques like DSC, FTIR, nanoDSF or CancerSEEK (Tsvetkov et al. 2018; Tsvetkov et al. 2021; Engh 2011; Chagovetz et al. 2013; Sala et al. 2020; Cohen et al. 2018) may initially replace sequencing to reduce the cost and may prepare for further larger-scale sequencing, but require more studies to prove feasibility.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AUC:
-
Area under curve
- BBB:
-
Blood–brain barrier
- BEAMing:
-
Beads, emulsion, amplification, and magnetics
- BM:
-
Brain metastases
- cfDNA:
-
Circulating free DNA
- CNS:
-
Central nervous system
- CNV:
-
Copy number variations
- CSF:
-
Cerebrospinal fluid
- CTC:
-
Circulating tumor cells
- ctDNA:
-
Circulating tumor DNA
- ddPCR:
-
Droplet digital polymerase chain reaction
- DMG:
-
Diffuse midline glioma
- DSC:
-
Differential scanning calorimetry
- EGFR:
-
Epidermal growth factor receptor
- EV:
-
Extracellular vesicle
- FTIR spectroscopy:
-
Fourier transform infrared spectroscopy
- GBM:
-
Glioblastomas
- GSC:
-
Glioblastoma stem cell
- HGG:
-
High-grade glioma
- IDH:
-
Isocitrate dehydrogenase
- LC–MS:
-
Liquid chromatography-mass spectrometry
- LGG:
-
Low-grade glioma
- LM:
-
Leptomeningeal metastases
- MB:
-
Medulloblastoma
- MGMT:
-
O6-methylguanine-DNA methyltransferase
- MV:
-
Microvesicle
- nanoDSF:
-
Nano differential scanning fluorimetry
- NGS:
-
Next-generation sequencing
- NSCLC:
-
Non-smal cell lung cancer
- PAP:
-
Pyrophosphorolysis-activated polymerization
- SCNA:
-
Somatic copy number alteration
- SVM:
-
Supporting vector machine
- TAM-seq:
-
Tagged-amplicon deep sequencing
- TCA:
-
Tricarboxylic acid cycle
- TEP:
-
Tumor-educated platelet
- TERT:
-
Telomerase reverse transcriptase
- WES:
-
Whole-exosome sequencing
- WGS:
-
Whole-genome sequencing
References
Aceto N et al (2015) En route to metastasis: circulating tumor cell clusters and epithelial-to-mesenchymal transition. Trends Cancer 1(1):44–52
Acinas SG et al (2005) PCR-induced sequence artifacts and bias: insights from comparison of two 16S rRNA clone libraries constructed from the same sample. Appl Environ Microbiol 71(12):8966–8969
Aldea M et al (2020) Circulating tumor DNA analysis for patients with oncogene-addicted NSCLC with isolated central nervous system progression. J Thoracic Oncol 15(3):383–391
Ali N et al (2017) Current nucleic acid extraction methods and their implications to point-of-care diagnostics. Biomed Res Int 2017:9306564
Alix-Panabières C, Pantel K (2013) Circulating tumor cells: liquid biopsy of cancer. Clin Chem 59(1):110–118
Ballester LY et al (2018) Analysis of cerebrospinal fluid metabolites in patients with primary or metastatic central nervous system tumors. Acta Neuropathol Commun 6(1):85
Baraniskin A et al (2011) Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood 117(11):3140–3146
Baraniskin A et al (2012) Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro Oncol 14(1):29–33
Barthel FP, Wesseling P, Verhaak RGW (2018) Reconstructing the molecular life history of gliomas. Acta Neuropathol 135(5):649–670
Best MG et al (2015) RNA-seq of tumor-educated platelets enables blood-based pan-cancer, multiclass, and molecular pathway cancer diagnostics. Cancer Cell 28(5):666–676
Best MG et al (2017) Swarm intelligence-enhanced detection of non-small-cell lung cancer using tumor-educated platelets. Cancer Cell 32(2):238-252.e9
Best MG, Wesseling P, Wurdinger T (2018) Tumor-educated platelets as a noninvasive biomarker source for cancer detection and progression monitoring. Can Res 78(13):3407–3412
Best MG et al (2019) RNA sequencing and swarm intelligence-enhanced classification algorithm development for blood-based disease diagnostics using spliced blood platelet RNA. Nat Protoc 14(4):1206–1234
Bettegowda C et al (2014) Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med 6(224):224ra24
Billard P et al (2021) The TeloDIAG: how telomeric parameters can help in glioma rapid diagnosis and liquid biopsy approaches. Ann Oncol 32(12):1608–1617
Bobillo S et al (2021) Cell free circulating tumor DNA in cerebrospinal fluid detects and monitors central nervous system involvement of B-cell lymphomas. Haematologica 106(2):513–521
Butler TM, Spellman PT, Gray J (2017) Circulating-tumor DNA as an early detection and diagnostic tool. Curr Opin Genet Dev 42:14–21
Calabrese C et al (2020) Genomic basis for RNA alterations in cancer. Nature 578:129–136
Campanella R et al (2020) Tumor-educated platelets and angiogenesis in glioblastoma: another brick in the wall for novel prognostic and targetable biomarkers, changing the vision from a localized tumor to a systemic pathology. Cells 9(2):294
Capper D et al (2010) Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol (zurich, Switzerland) 20(1):245–254
Capper D et al (2011) Assessment of BRAF V600E mutation status by immunohistochemistry with a mutation-specific monoclonal antibody. Acta Neuropathol 122(1):11–19
Carlino MS et al (2015) Targeting oncogenic BRAF and aberrant MAPK activation in the treatment of cutaneous melanoma. Crit Rev Oncol Hematol 96(3):385–398
Chagovetz AA et al (2013) Differential scanning calorimetry of gliomas: a new tool in brain cancer diagnostics? Neurosurgery 73(2):289–295
Chan K et al (2005) Effects of preanalytical factors on the molecular size of cell-free DNA in blood. Clin Chem 51(4):781–784
Cohen JD et al (2018) Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 359(6378):926–930
Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289
Cucchiara F et al (2020) Integrating liquid biopsy and radiomics to monitor clonal heterogeneity of EGFR-positive non-small cell lung cancer. Front Oncol 10:593831
Dawson S et al (2013) Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med 368(13):1199–1209
Di Meo A et al (2017) Liquid biopsy: a step forward towards precision medicine in urologic malignancies. Mol Cancer 16(1):80
Di Vito C et al (2017) Platelets from glioblastoma patients promote angiogenesis of tumor endothelial cells and exhibit increased VEGF content and release. Platelets 28(6):585–594
Diaz LAJ, Bardelli A (2014) Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol 32(6):579–586
Diehl F et al (2008) Circulating mutant DNA to assess tumor dynamics. Nat Med 14(9):985–990
Diplas BH et al (2019) Sensitive and rapid detection of TERT promoter and IDH mutations in diffuse gliomas. Neuro Oncol 21(4):440–450
El Messaoudi S et al (2013) Circulating cell free DNA: preanalytical considerations. Clin Chim Acta 424:222–230
Engh JA (2011) Differential scanning calorimetry applied to cerebrospinal fluid analysis in glioblastoma. Neurosurgery 69(4):N22–N23
Erpenbeck L, Schön MP (2010) Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood 115(17):3427–3436
Escudero L et al (2020) Circulating tumour DNA from the cerebrospinal fluid allows the characterisation and monitoring of medulloblastoma. Nat Commun 11(1):5376
Fanfani V et al (2021) The landscape of the heritable cancer genome. Cancer Res 81(10):2588–2599
Fernando MR et al (2017) New evidence that a large proportion of human blood plasma cell-free DNA is localized in exosomes. PLoS ONE 12(8):e0183915
Figueroa JM et al (2017) Detection of wild-type EGFR amplification and EGFRvIII mutation in CSF-derived extracellular vesicles of glioblastoma patients. Neuro Oncol 19(11):1494–1502
Fleischhacker M, Schmidt B (2007) Circulating nucleic acids (CNAs) and cancer–a survey. Biochem Biophys Acta 1775(1):181–232
Fonkem E, Lun M, Wong ET (2011) Rare phenomenon of extracranial metastasis of glioblastoma. J Clin Oncol 29(34):4594–4595
García-Romero N et al (2019) BRAF V600E detection in liquid biopsies from pediatric central nervous system tumors. Cancers 12(1):66
Gautschi O et al (2004) Circulating deoxyribonucleic acid as prognostic marker in non-small-cell lung cancer patients undergoing chemotherapy. J Clin Oncol 22(20):4157–4164
Gay LJ, Felding-Habermann B (2011) Contribution of platelets to tumour metastasis. Nat Rev Cancer 11(2):123–134
Geraldo LHM et al (2019) Glioblastoma therapy in the age of molecular medicine. Trends Cancer 5(1):46–65
Godlewski J et al (2010) MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell 37(5):620–632
Grommes C et al (2019) Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood 133(5):436–445
Hansen TB et al (2013) Natural RNA circles function as efficient microRNA sponges. Nature 495(7441):384–388
Haque IS, Elemento O (2017) Challenges in using ctDNA to achieve early detection of cancer. bioRxiv. https://doi.org/10.1101/237578
Hermoso-Durán S et al (2020) Thermal liquid biopsy (TLB) focused on benign and premalignant pancreatic cyst diagnosis. J Personalized Med 11(1):25
Huang TY et al (2017) Detection of Histone H3 mutations in cerebrospinal fluid-derived tumor DNA from children with diffuse midline glioma. Acta Neuropathol Commun 5(1):28
Ignatiadis M, Sledge GW, Jeffrey SS (2021) Liquid biopsy enters the clinic - implementation issues and future challenges. Nat Rev Clin Oncol 18(5):297–312
Indira Chandran V et al (2019) Ultrasensitive immunoprofiling of plasma extracellular vesicles identifies syndecan-1 as a potential tool for minimally invasive diagnosis of glioma. Clin Cancer Res 25(10):3115–3127
Jahr S et al (2001) DNA fragments in the blood plasma of cancer patients: quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res 61(4):1659–1665
Jalali M et al (2021) Plasmonic nanobowtiefluidic device for sensitive detection of glioma extracellular vesicles by Raman spectrometry. Lab Chip 21(5):855–866
Jiang P et al (2015) Lengthening and shortening of plasma DNA in hepatocellular carcinoma patients. Proc Natl Acad Sci USA 112(11):E1317–E1325
Jiang B et al (2017a) Detection of driver and resistance mutations in leptomeningeal metastases of NSCLC by next-generation sequencing of cerebrospinal fluid circulating tumor cells. Clin Cancer Res 23(18):5480–5488
Jiang X et al (2017b) Microfluidic isolation of platelet-covered circulating tumor cells. Lab Chip 17(20):3498–3503
Jung M et al (2003) Changes in concentration of DNA in serum and plasma during storage of blood samples. Clin Chem 49(1):1028–1029
Kahlert C, Kalluri R (2013) Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (berl) 91(4):431–437
Kalokhe G et al (2012) Metastatic glioblastoma: case presentations and a review of the literature. J Neuro Oncol 107(1):21–27
Kammesheidt A et al (2016) Mutation detection in cell free DNA from healthy donors. J Clinical Oncol 34(15):e23054
Kane JR (2019) The role of brain vasculature in glioblastoma. Mol Neurobiol 56(9):6645–6653
Kang KM et al (2021) Blood-based detection of BRAF V600E in gliomas and brain tumor metastasis. Cancers 13(6):1227
Khuong-Quang D et al (2012) K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol 124(3):439–447
Khwaja FW et al (2006) Attractin is elevated in the cerebrospinal fluid of patients with malignant astrocytoma and mediates glioma cell migration. Clin Cancer Res 12(21):6331–6336
Khwaja FW et al (2007) Proteomic identification of biomarkers in the cerebrospinal fluid (CSF) of astrocytoma patients. J Proteome Res 6(2):559–570
Kilgour E et al (2020) Liquid biopsy-based biomarkers of treatment response and resistance. Cancer Cell 37(4):485–495
Kitamura T, Qian B, Pollard JW (2015) Immune cell promotion of metastasis. Nat Rev Immunol 15(2):73–86
Kitano Y et al (2021) Urinary MicroRNA-based diagnostic model for central nervous system tumors using nanowire scaffolds. ACS Appl Mater Interfaces 13(15):17316–17329
Konstantopoulos K, Thomas SN (2009) Cancer cells in transit: the vascular interactions of tumor cells. Annu Rev Biomed Eng 11:177–202
Krol I et al (2018) Detection of circulating tumour cell clusters in human glioblastoma. Br J Cancer 119(4):487–491
Kros JM et al (2015) Circulating glioma biomarkers. Neuro Oncol 17(3):343–360
Kulasinghe A et al (2017) Enrichment of circulating head and neck tumour cells using spiral microfluidic technology. Sci Rep 7:42517
Labelle M, Begum S, Hynes RO (2014) Platelets guide the formation of early metastatic niches. Proc Natl Acad Sci USA 111(30):E3053–E3061
Lassaletta A et al (2017) Therapeutic and prognostic implications of BRAF V600E in pediatric low-grade gliomas. J Clin Oncol 35(25):2934
Lawrence MS et al (2014) Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505(7484):495–501
Lawson C et al (2016) Microvesicles and exosomes: new players in metabolic and cardiovascular disease. J Endocrinol 228(2):R57-71
Lee Y, El Andaloussi S, Wood MJA (2012) Exosomes and microvesicles: extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet 21(R1):R125–R134
Lennon AM et al (2020) Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science (new York, NY) 369(6499):eabb9601
Li CCY et al (2013) Glioma microvesicles carry selectively packaged coding and non-coding RNAs which alter gene expression in recipient cells. RNA Biol 10(8):1333–1344
Li Y et al (2015) Circular RNA is enriched and stable in exosomes: a promising biomarker for cancer diagnosis. Cell Res 25(8):981–984
Li YS et al (2018) Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann Oncol 29(4):945–952
Li J et al (2020) Reliable tumor detection by whole-genome methylation sequencing of cell-free DNA in cerebrospinal fluid of pediatric medulloblastoma. Sci Adv 6(42):5427
Li P et al (2021) Circular RNA sequencing reveals serum exosome circular RNA panel for high-grade astrocytoma diagnosis. Clin Chem 68(2):343
Lianidou ES et al (2010) What’s new on circulating tumor cells? A meeting report. Brest Cancer Res 12(4):307
Lin B et al (2021) Microfluidic-based exosome analysis for liquid biopsy. Small Methods 5(3):e2001131
Liu APY et al (2021) Serial assessment of measurable residual disease in medulloblastoma liquid biopsies. Cancer Cell 39(11):1519-1530.e4
Louis DN et al (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131(6):803–820
Lucero R et al (2020) Glioma-derived miRNA-containing extracellular vesicles induce angiogenesis by reprogramming brain endothelial cells. Cell Rep 30(7):2065-2074.e4
Lui Y et al (2002) Predominant hematopoietic origin of cell-free DNA in plasma and serum after sex-mismatched bone marrow transplantation. Clin Chem 48(3):421–427
Ma L et al (2021) Sensitive detection and conjoint analysis of promoter methylation by conjugated polymers for differential diagnosis and prognosis of glioma. ACS Appl Mater Interfaces 13(8):9291–9299
Maas SLN et al (2020) Orally administered 5-aminolevulinic acid for isolation and characterization of circulating tumor-derived extracellular vesicles in glioblastoma patients. Cancers 12(11):3297
Maire CL et al (2021) Genome-wide methylation profiling of glioblastoma cell-derived extracellular vesicle DNA allows tumor classification. Neuro Oncol 23(7):1087–1099
Martignano F (2019) Cell-Free DNA: an Overview of Sample Types and Isolation Procedures. Methods Mol Biol (clifton, N.J.) 1909:13–27
McDonald BR et al (2019) Personalized circulating tumor DNA analysis to detect residual disease after neoadjuvant therapy in breast cancer. Sci Transl Med 11(504):7392
Meng S et al (2017) CircRNA: functions and properties of a novel potential biomarker for cancer. Mol Cancer 16(1):94
Meng Y et al (2021) MR-guided focused ultrasound liquid biopsy enriches circulating biomarkers in patients with brain tumors. Neuro Oncol 23(10):1789–1797
Miller AM et al (2019) Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature 565(7741):654–658
Miller KD et al (2021) Brain and other central nervous system tumor statistics, 2021. CA 71(5):381–406
Mouliere F et al (2011) High fragmentation characterizes tumour-derived circulating DNA. PLoS ONE 6(9):e23418
Mouliere F et al (2018) Detection of cell-free DNA fragmentation and copy number alterations in cerebrospinal fluid from glioma patients. EMBO Mol Med 10(12):e9323
Mouliere F et al (2021) Fragmentation patterns and personalized sequencing of cell-free DNA in urine and plasma of glioma patients. EMBO Mol Med 13(8):e12881
Müller C et al (2014) Hematogenous dissemination of glioblastoma multiforme. Sci Transl Med 6(247):247ra101
Muralidharan K et al (2021) TERT Promoter mutation analysis for blood-based diagnosis and monitoring of gliomas. Clin Cancer Res 27(1):169–178
Nash GF et al (2002) Platelets and cancer. Lancet Oncol 3(7):425–430
Nassiri F et al (2020) Detection and discrimination of intracranial tumors using plasma cell-free DNA methylomes. Nat Med 26(7):1044–1047
Nieswandt B et al (1999) Lysis of tumor cells by natural killer cells in mice is impeded by platelets. Can Res 59(6):1295–1300
Nilsson RJA et al (2011) Blood platelets contain tumor-derived RNA biomarkers. Blood 118(13):3680–3683
Nishizaki T et al (2001) Detection of craniospinal dissemination of intracranial germ cell tumours based on serum and cerebrospinal fluid levels of tumour markers. J Clin Neurosci 8(1):27–30
Northcott PA et al (2017) The whole-genome landscape of medulloblastoma subtypes. Nature 547(7663):311–317
Palumbo JS et al (2005) Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 105(1):178–185
Pan C et al (2019) Molecular profiling of tumors of the brainstem by sequencing of CSF-derived circulating tumor DNA. Acta Neuropathol 137(2):297–306
Panditharatna E et al (2018) Clinically Relevant and minimally invasive tumor surveillance of pediatric diffuse midline gliomas using patient-derived liquid biopsy. Clin Cancer Res 24(23):5850–5859
Pantel K, Alix-Panabières C (2010) Circulating tumour cells in cancer patients: challenges and perspectives. Trends Mol Med 16(9):398–406
Pasquier B et al (1980) Extraneural metastases of astrocytomas and glioblastomas: clinicopathological study of two cases and review of literature. Cancer 45(1):112–125
Patil DP et al (2016) m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537(7620):369–373
Pittella-Silva F et al (2020) Plasma or serum: which is preferable for mutation detection in liquid biopsy? Clin Chem 66(7):946–957
Placke T et al (2012) Platelet-derived MHC class I confers a pseudonormal phenotype to cancer cells that subverts the antitumor reactivity of natural killer immune cells. Cancer Res 72(2):440–448
Qaddoumi I et al (2012) Diagnostic utility and correlation of tumor markers in the serum and cerebrospinal fluid of children with intracranial germ cell tumors. Child’s Nervous Syst 28(7):1017–1024
Raz T et al (2011) Protocol dependence of sequencing-based gene expression measurements. PLoS ONE 6(5):e19287
Ricklefs FL et al (2018) Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv 4(3):2766
Rodrigo A et al (2019) Thermal liquid biopsy (TLB): a predictive score derived from serum thermograms as a clinical tool for screening lung cancer patients. Cancers 11(7):1012
Romero A et al (2021) Comprehensive cross-platform comparison of methods for non-invasive EGFR mutation testing: results of the RING observational trial. Mol Oncol 15(1):43–56
Sabedot T et al (2021) A serum-based DNA methylation assay provides accurate detection of glioma. Neuro Oncol 23(9):1494–1508
Sabrkhany S, Griffioen AW, Egbrink MGOA (2011) The role of blood platelets in tumor angiogenesis. Biochem Biophys Acta 1815(2):189–196
Sala A et al (2020) Biofluid diagnostics by FTIR spectroscopy: a platform technology for cancer detection. Cancer Lett 477:122–130
Sanchez JN, Wang T, Cohen MS (2018) BRAF and MEK inhibitors: use and resistance in BRAF-mutated cancers. Drugs 78(5):549–566
Santangelo A et al (2018) A microRNA signature from serum exosomes of patients with glioma as complementary diagnostic biomarker. J Neurooncol 136(1):51–62
Scher HI et al (2009) Circulating tumour cells as prognostic markers in progressive, castration-resistant prostate cancer: a reanalysis of IMMC38 trial data. Lancet Oncol 10(3):233–239
Seoane J et al (2019) Cerebrospinal fluid cell-free tumour DNA as a liquid biopsy for primary brain tumours and central nervous system metastases. Ann Oncol 30(2):211–218
Serrano MJ et al (2020) Precision prevention and cancer interception: the new challenges of liquid biopsy. Cancer Discov 10(11):1635–1644
Shankar GM et al (2017) Liquid biopsy for brain tumors. Expert Rev Mol Diagn 17(10):943–947
Shah N, Lankerovich M, Lee H, Yoon JG, Schroeder B, Foltz G (2013) Exploration of the gene fusion landscape of glioblastoma using transcriptome sequencing and copy number data. BMC genomics 14(1):818. https://doi.org/10.1186/1471-2164-14-818
Shen F et al (2014) Proteomic analysis of cerebrospinal fluid: toward the identification of biomarkers for gliomas. Neurosurg Rev 37(3):367–380
Siegel RL et al (2021) Cancer Statistics, 2021. CA 71(1):7–33
Sim H et al (2019) Tissue 2-hydroxyglutarate as a biomarker for isocitrate dehydrogenase mutations in gliomas. Clin Cancer Res 25(11):3366–3373
Sol N, Wurdinger T (2017) Platelet RNA signatures for the detection of cancer. Cancer Metastasis Rev 36(2):263–272
Sol N et al (2020) Tumor-educated platelet RNA for the detection and (pseudo)progression monitoring of glioblastoma. Cell Rep Med 1(7):100101
Sprouse ML et al (2019) PMN-MDSCs enhance CTC metastatic properties through reciprocal interactions via ROS/Notch/Nodal signaling. Int J Mol Sci 20(8):1916
Srinivasan VM et al (2016) Modern management of medulloblastoma: molecular classification, outcomes, and the role of surgery. Surg Neurol Int 7(Suppl 44):S1135–S1141
Srivastava S et al (2019) Cancer overdiagnosis: a biological challenge and clinical dilemma. Nat Rev Cancer 19(6):349–358
Sullivan JP et al (2014) Brain tumor cells in circulation are enriched for mesenchymal gene expression. Cancer Discov 4(11):1299–1309
Teplyuk NM et al (2012) MicroRNAs in cerebrospinal fluid identify glioblastoma and metastatic brain cancers and reflect disease activity. Neuro Oncol 14(6):689–700
Thakur BK et al (2014) Double-stranded DNA in exosomes: a novel biomarker in cancer detection. Cell Res 244(6):766–769
Thakur A et al (2017) Direct detection of two different tumor-derived extracellular vesicles by SAM-AuNIs LSPR biosensor. Biosens Bioelectron 94:400–407
Thakur A et al (2021) In vivo liquid biopsy for glioblastoma malignancy by the AFM and LSPR based sensing of exosomal CD44 and CD133 in a mouse model. Biosens Bioelectron 191:113476
Théry C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev 2(8):569–579
Thierry A et al (2010) Origin and quantification of circulating DNA in mice with human colorectal cancer xenografts. Nucleic Acids Res 38(18):6159–6175
Tsvetkov PO et al (2018) Differential scanning calorimetry of plasma in glioblastoma: toward a new prognostic / monitoring tool. Oncotarget 9(10):9391–9399
Tsvetkov PO et al (2021) An AI-powered blood test to detect cancer using NanoDSF. Cancers 13(6):1294
Umetani N, Hiramatsu S, Hoon DSB (2006) Higher amount of free circulating DNA in serum than in plasma is not mainly caused by contaminated extraneous DNA during separation. Ann N Y Acad Sci 1075:299–307
Underhill HR et al (2016) Fragment length of circulating tumor DNA. PLoS Genet 12(7):e1006162
van Bodegraven EJ et al (2019) Importance of GFAP isoform-specific analyses in astrocytoma. Glia 67(8):1417–1433
Velazquez-Campoy A et al (2018) Thermal liquid biopsy for monitoring melanoma patients under surveillance during treatment: a pilot study. Biochim Biophys Acta 1862(8):1701–1710
Wang Y et al (2015a) Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci USA 112(31):9704–9709
Wang E et al (2015b) Predictive genomics: a cancer hallmark network framework for predicting tumor clinical phenotypes using genome sequencing data. Semin Cancer Biol 30:4–12
Wang H et al (2019) Evaluation of serum extracellular vesicles as noninvasive diagnostic markers of glioma. Theranostics 9(18):5347–5358
Wang F et al (2020) Cerebrospinal fluid-based metabolomics to characterize different types of brain tumors. J Neurol 267(4):984–993
Wang L, Yekula A, Muralidharan K, Small JL, Rosh ZS, Kang KM, Carter BS, Balaj L (2020) Novel Gene Fusions in Glioblastoma Tumor Tissue and Matched Patient Plasma. Cancers 12(5):1219. https://doi.org/10.3390/cancers12051219
Watanabe A et al (2006) Expression of syndecans, a heparan sulfate proteoglycan, in malignant gliomas: participation of nuclear factor-kappaB in upregulation of syndecan-1 expression. J Neurooncol 77(1):25–32
Watanabe S et al (2012) A highly sensitive and specific chemiluminescent enzyme immunoassay for placental alkaline phosphatase in the cerebrospinal fluid of patients with intracranial germinomas. Pediatr Neurosurg 48(3):141–145
Weber C, Springer TA (1997) Neutrophil accumulation on activated, surface-adherent platelets in flow is mediated by interaction of Mac-1 with fibrinogen bound to alphaIIbbeta3 and stimulated by platelet-activating factor. J Clin Investig 100(8):2085–2093
Wei B et al (2019) Combined plasma and tissue genotyping of EGFR T790M benefits NSCLC patients: a real-world clinical example. Mol Oncol 13(5):1226–1234
Wong F et al (2016) Cell-free DNA in maternal plasma and serum: a comparison of quantity, quality and tissue origin using genomic and epigenomic approaches. Clin Biochem 49(18):1379–1386
Wu X et al (2020) Circulating tumor DNA as an emerging liquid biopsy biomarker for early diagnosis and therapeutic monitoring in hepatocellular carcinoma. Int J Biol Sci 16(9):1551–1562
Xu H et al (2018) NFIX circular RNA promotes glioma progression by regulating miR-34a-5p via notch signaling pathway. Front Mol Neurosci 11:225
Yin K, Liu X (2020) CircMMP1 promotes the progression of glioma through miR-433/HMGB3 axis in vitro and in vivo. IUBMB Life 72(11):2508–2524
Zeng A et al (2018) Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma. Cancer Lett 436:10–21
Zhang M et al (2018) A peptide encoded by circular form of LINC-PINT suppresses oncogenic transcriptional elongation in glioblastoma. Nat Commun 9(1):4475
Zhang B et al (2020) m(6)A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol Cancer 19(1):53
Zhang B et al (2021) m(6)A target microRNAs in serum for cancer detection. Mol Cancer 20(1):170
Zhao Y et al (2020) Cytoplasm protein GFAP magnetic beads construction and application as cell separation target for brain tumors. J Nanobiotechnol 18(1):169
Acknowledgements
We are grateful to all of those with whom we have had the pleasure to work during this and other related projects.
Funding
The research was funded by China Postdoctoral Science Foundation 2018M643006.
Author information
Authors and Affiliations
Contributions
ZY and YZ reviewed the literature and wrote the manuscript. CQ reviewed the literature and generated Table 2. YZ and ZL designed the research. All authors contributed to writing and critically revising the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no competing interests.
Ethical approval
The study was approved by the ethics committee of the Xiangya Hosptial, Central South University.
Consent for publication
All listed authors have actively participated in the study and have read and approved the submitted manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yi, Z., Qu, C., Zeng, Y. et al. Liquid biopsy: early and accurate diagnosis of brain tumor. J Cancer Res Clin Oncol 148, 2347–2373 (2022). https://doi.org/10.1007/s00432-022-04011-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-022-04011-3