Abstract
Objectives
To examine the effect of lipopolysaccharide (LPS) on cellular senescence induction of human apical papilla cells (hAPCs) and evaluate the potential use of 50 μg/ml ascorbic acid to recover cellular senescence and regenerative functions.
Materials and methods
hAPCs were treated with LPS at 1 and 10 μg/ml either with or without 50 μg/ml ascorbic acid for 48 h. The cellular senescence biomarkers were analyzed by senescence-associated β-galactosidase (SA-β-gal) staining and senescence-related gene expression, p16 and p21. Cell migration, at 12 h and 24 h, was evaluated using a scratch wound assay. Mineralization potential was assessed at 21 days using Alizarin red S staining and dentine sialophosphoprotein (DSPP) and bone sialoprotein (BSP) gene expression.
Results
1 μg/ml and 10 μg/ml LPS stimulation for 48 h induced cellular senescence, as shown by remarkable SA-β-gal staining and p16 and p21 gene expression. The percentage of wound closure and mineralized formation was reduced. The co-incubation with ascorbic acid significantly down-regulated the level of SA-β-gal staining. The reduction of senescence-associated gene expressions was observed. Ascorbic acid improved cell migration, mineralized nodule formation, and the expression of DSPP and BSP genes in LPS-treated hAPCs.
Conclusions
LPS significantly promoted cellular senescence on hAPCs and diminished the cell function capacity. Co-presence of ascorbic acid could impede cellular senescence and possibly improve the regenerative capacity of LPS-induced senescent hAPCs in vitro.
Clinical relevance
The data support the in vitro potential benefit of ascorbic acid on cellular senescence recovery of apical papilla cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Regenerative endodontic procedures (REPs) are one of the treatment options for infected immature teeth with pulp necrosis. Although current clinical studies demonstrated promising successful outcomes [1], some unfavorable outcomes have also been consistently reported [2,3,4,5,6]. The stem cell of the dental papilla (SCAP) is mentioned to play roles in REPs. However, the information regarding the fate and potential of these stem cells after an infection is limited [7]. Lipopolysaccharide (LPS), the gram-negative bacterial endotoxin, stimulates the production of the inflammatory cytokines and intracellular reactive oxygen species (ROS) production and also has different effects on cell proliferation, differentiation, ROS production, and mitochondrial dynamic alteration [8,9,10,11,12,13,14,15,16,17,18,19]. Various studies reported the correlation between ROS alteration and the induction of cellular senescence [20].
Cellular senescence is an irreversible arrest of cell division. The arrested cells remain viable and can secrete a variety of substances into the environment. Diverse stress inducers can promote cellular senescence, especially oxidative stress, causing irreparable DNA damage to cells. The accumulated DNA damage can trigger the signaling pathways and activate the cell cycle arrest [21, 22]. Previous studies revealed the alteration of stem cell proliferation or differentiation capacity under a senescent environment [23, 24]. While there are various models for cellular senescence induction, LPS is a model that has been confirmed to induce cellular senescence in a variety of cells including mesenchymal cells [25,26,27], dental pulp stem cells [28], and alveolar osteocytes [29]. However, there are no studies on the role of LPS in triggering cellular senescence in cells associated with REPs, including apical papilla cells (APCs). One of the senescent biomarkers commonly used to detect cellular senescence is the detection of senescence-associated beta-galactosidase (SA-β-gal), a lysosomal enzyme associated with cells with senescence conditions. In addition, the expression of p16 and p21, which are inhibitors of cyclin-dependent kinases (CDK) involved in the progression of the cell cycle, also shows great accuracy in detecting senescence.
Ascorbic acid (AA) has been known as a cofactor for various enzyme activities. It is a critical antioxidant acting as a free radical scavenger by eliminating ROS production, resulting in attenuating oxidative stress [30]. Studies in dental fields confirm the positive effects of AA under an inflammatory-induced environment by reducing ROS production [31,32,33]. Moreover, it has other valuable benefits, including enhancing cellular stemness, proliferation, and differentiation in various dental stem cells [33,34,35,36,37]. It also appears to have the ability to counteract inflammatory cytokines, which are considered one of the senescence-associated secretory phenotypes (SASPs) [22, 32], potentially contributing to the subsiding of the senescent condition. Recent studies demonstrated that AA could retard cellular senescence in stem cells [38,39,40,41,42], suggesting that it can be a possible intervention to attenuate cellular senescence.
Therefore, this study examined the effect of LPS on cellular senescence induction and evaluated the potential use of AA to recover the cellular senescence and regenerative potential of hAPCs by detecting cellular senescence biomarkers, cell migration, and mineralization potential.
Materials and methods
This study was approved by the Human Experimental Committee, Faculty of Dentistry, Chiang Mai University (No.5/2021).
Apical papilla cells obtention
With written informed consent, unsorted hAPCs were harvested from the apical papilla tissues of non-carious immature mandibular third molars from 16- to 20-year-old patients (N = 3). The apical papilla tissues were gently separated from the root apex, minced, and digested with 3 mg/ml Collagenase I (Gibco/Invitrogen, Gaithersburg, MD, USA) and 4 mg/ml Dispase II (Sigma-Aldrich, St Louis, MO, USA) for 45 min under 37 °C. Cells were cultured in complete ⍺-MEM (Sigma-Aldrich) comprised of 10% fetal bovine serum (Sigma-Aldrich), 1% penicillin-streptomycin (Sigma-Aldrich), and 100 mol/L L-ascorbic acid (Sigma-Aldrich) under a 37 °C humidified atmosphere containing 95% air and 5% CO2 with the medium change every three days. Cells at the second to third passages were used. Cells were designed into 6 groups as follows (Fig. 1):
-
Control: hAPCs in regular complete media
-
D-gal 20 mg/ml: hAPCs in media containing 10 mg/ml of D-galactose (D-gal) for 48 h (as a positive control)
-
LPS 1 μg/ml: hAPCs in complete media containing 1 μg/ml of LPS for 48 h
-
LPS 1 μg/ml + AA: hAPCs in complete media containing 1 μg/ml of LPS and 50 μg/ml of ascorbic acid for 48 h
-
LPS 10 μg/ml: hAPCs in complete media containing 10 μg/ml of LPS for 48 h
-
LPS 10 μg/ml + AA: hAPCs in complete media containing 1 μg/ml of LPS and 50 μg/ml of ascorbic acid for 48 h
hAPCs cultured in regular complete media served as the negative control group, while the hAPCs in the positive control group were treated with 20 mg/ml D-galactose (G5388, Sigma-Aldrich). D-gal was selected because it is a common model for cellular senescence induction [41, 43, 44]. In the experimental groups, LPS from Escherichia coli (O111:B4; Sigma-Aldrich), either at 1 μg/ml or 10 μg/ml was added to hAPCs in order to mimic the inflammatory state of endodontic infection [12]. In groups containing AA, freshly prepared L-ascorbic acid (A4544; Sigma-Aldrich) at 50 μg/ml was coincubated until the end of the experiment; 50 μg/ml of AA was selected because it did not adversely impact the hAPCs viability (from our pilot study) while having anti-inflammation and ROS reduction effects [32]. For each experiment, all groups were performed in triplicate.
Senescence-associated-β-galactosidase (SA-β-gal) staining
To evaluate the cellular senescence, an SA-β-gal staining kit (Cell signaling Technology, USA) was used following the manufacturer’s protocol. Cells at 2×104 cells/well in 24-well plates were assigned into experimental groups, as previously mentioned. After 48 h of incubation, cells were fixed and incubated overnight with 1 ml of the staining solution in a dry incubator without CO2. Five random fields of each well were captured at ×200 magnification using a light microscope (DMi8 Microscope, Leica Microsystem CMS, Germany). The quantification was developed at the same standard command in ImageJ software (RRID:SCR_003070, National Institutes of Health, Bethesda, MD, USA) and measured the percent area of blue-stained SA-β-gal-positive cells [45].
The expression of senescence-associated genes: p16 and p21
The expressions of senescence-associated genes (p16 and p21) were evaluated using a quantitative reverse-transcription polymerase chain reaction (qRT-PCR). hAPCs at 3×105 cells/well in 6-well plates treated as previously mentioned for 48 h were analyzed. Gene expression analysis per sample was performed in triplicate. Total RNAs were extracted using TRIzolTM Reagent (Invitrogen, Burlington, ON, Canada) and evaluated using Nanodrop equipment (NanoDrop Technologies Inc., Wilmington, DE). Isolated RNA was then converted to cDNA with ReverTra Ace® qPCR RT Master Mix (TOYOBO CO., LTD., Japan). The qRT-PCR was performed using SensiFASTTM SYBR® no-ROX kit (Bioline, London, UK) with a LightCycler 480 Real-Time PRC system (Roche Applied Science, Rotkreuz, Switzerland). The denaturing, annealing, and extension conditions for each PCR cycle were at 95 °C for 5 s, 60 °C for 10 s, and 72 °C for 20 s, respectively. Relative differences in amplified products were calculated using the comparative cycle threshold (CT) method. The housekeeping enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize with CT values. The 2−ΔΔCT was calculated to analyze the relative changes in gene expression obtained from the control group. Primer sequences used for the analysis are described in Table 1.
Cell migration by wound healing assay
Similarly assigned to the previous experiment, a direct wound was carefully created in each well using a sterile plastic pipette tip generating a similar size of cell-free zone in the middle of each well. Then, the media was refreshed to remove the floating cells and debris. The plates were incubated for 24 h under automated live-cell imaging platforms (DMi8 Microscope, Leica Microsystems CMS, Germany) for live monitoring and imaging of cell behavior. A random field from each well of the scratched area was captured at 0 h, 12 h, and 24 h at ×5 magnification. The experiments were repeated three times, and migration distance from the wound edge was analyzed using ImageJ software (Software 1.48q, Rayne Rasband, National Institutes of Health, USA). The percentage of cell migration was quantified by calculating the %Wound Closure by using the %Area of the cell-free zone measured at the initial time (0 h) compared to 12 h and 24 h [46]. (Wound closure (%) = ((At=0−At=∆h)/A0) × 100, where At=0 is the area of the wound measured immediately after scratching (t = 0h) and At=∆h is the area of the wound measured h hours after the scratch is performed.)
Mineralization formation and the expression of osteo-/odontogenic differentiation genes
After 48 h of incubation as per the previous experimental design, the medium was replaced with a differentiation medium containing complete α-MEM, 50 mg/ml of ascorbic acid (Sigma-Aldrich), 10 nmol/ml of dexamethasone (Sigma-Aldrich), and 10 mmol/ml of ß-glycerophosphate (Sigma-Aldrich) and renewed every 3 days. On day 21, Alizarin Red S staining was used to qualify mineralization formation. After fixation with 4% paraformaldehyde, cells were incubated with 0.5 ml Alizarin red S (Sigma-Aldrich) for 15 min, washed, and left air-dry for 1 week. To quantitatively measure the amount of calcium deposit, 10% cetylpyridinium chloride monohydrate (Sigma-Aldrich) in 10 mmol/L of sodium phosphate was used to destain, while absorbance was measured using a spectrophotometer (Bio-Rad Laboratories Inc., Hercules, CA, USA) at the wavelength of 550 nm.
Osteo-/odontogenic gene expression was evaluated using qRT-PCR, hAPCs at 3×105 cells/well in 6-well plates cultured in differentiation media for 21 days. Similar standard protocols for gene expression were conducted, as previously mentioned. The relative changes of BSP and DSPP expressions were calculated using 2−ΔΔCT. Primer sequences of BSP and DSPP genes are described in Table 1.
Statistical analysis
The experiment was conducted in triplicates and presented in mean ± SD (standard deviation). Data were statistically analyzed with either one-way analysis of variance (ANOVA) or Tukey’s or Dunnett’s T3 test using SPSS 22.0 software (SPSS Inc, Chicago, IL, USA). A statistically significant was set at P < 0.05.
Results
Ascorbic acid impedes the cellular senescence of hAPCs
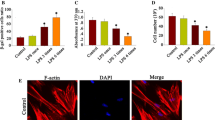
LPS stimulation-induced cellular senescence in hAPCs was confirmed by the expressions of cellular senescent biomarkers. APCs treated with LPS at both concentrations exhibited a significantly high percentage of stained SA-β-gal-positive cells when compared to the negative control group (P < 0.001) (Fig. 2a,b). The percentage area of SA-β-gal staining was observed at 16.35 ± 2.78% and 12.05 ± 2.18% in cells treated with 10 μg/ml LPS and 1 μg/ml LPS, respectively. The level of p16 (P = 0.001) and p21 (P < 0.001) expression was significantly upregulated in both LPS groups (Fig. 2c,d) in a dose-dependent manner. APCs stimulated with LPS at 10 μg/ml showed the highest level of p16 and p21 expression compared to the untreated control group.
The effect of LPS on cellular senescence. The percentage area of SA-β-gal-positive cells was determined by SA-β-gal staining (a). SA-β-gal-positive cells represented in blue under the light microscope (×200 magnification, bar 138 μm) (b). The expression of senescence-associated gene: p16 (c), and the expression of senescence-associated gene: p21 (d). *P < 0.05 compared with the control group. #P < 0.05 compared between two different groups. Data are presented as mean ± SD
Compared to LPS groups without AA treatment, co-incubation of AA was found to reduce the percentage area of stained SA-β-gal cells both in 1 μg/ml and 10 μg/ml LPS-treated groups (P < 0.001). The levels of p16 and p21 expressions were also reduced in groups with AA with significance was only observed in the 1 μg/ml LPS-treated group (P = 0.001 and P = 0.004, respectively) (Fig. 2c,d).
Potential of ascorbic acid to improve regenerative functions of LPS-induced cellular senescence of hAPCs
Cell migration capacity
To explore the migration capacity of LPS-treated apical cells, the percentage of wound closure was analyzed using the scratch wound assay. The level of %wound closure significantly decreased in all groups containing D-gal and 1 μg/ml and 10 μg/ml LPS when compared to the control group at both 12 h (P = 0.027, P < 0.001, and P < 0.001, respectively) or 24 h (P = 0.013, P = 0.002, and P < 0.001, respectively) (Fig. 3a,b). Among the groups, cells treated with 10 μg/ml LPS showed the least migration. AA tended to elevate the migration potential of 1 μg/ml and 10 μg/ml LPS-treated APCs (at 12 h P = 0.408 and P = 0.979, respectively, and at 24 h P = 0.996 and P = 1.000, respectively) compared to those without AA.
The effect of cellular senescence on the regenerative potential of hAPCs. The results of migration potential showed in the percentage area of %Wound closure at 12- and 24-h time points (a) *P < 0.05 compared with the control group; and representative images of the migrated cells from scratch wound assay at 0 h, 12 h, and 24 h, scale bar = 100 μm (b). The quantitative analysis of mineralized matrix formation at 21 days (c); and the captures from cell culture plates and light microscope, scale bar = 500 μm (d). Data are presented as mean ± SD. Statistical analyses were performed using one-way ANOVA and Tukey’s test. The effect of cellular senescence on osteo-/odontogenic differentiation as determined by DSPP and BSP gene expression (e, f). *P < 0.05 compared with the control group. #P < 0.05 compared between two different groups. Data are presented as mean ± SD. Statistical analyses were performed using one-way ANOVA and either Tukey’s or Dunnett’s T3 test
Mineralization capacity
All samples showed positive Alizarin red S staining, as shown by red nodules in the samples (Fig. 3d). The quantitative analysis exhibited reduction trends of calcium deposition in all groups containing D-gal and LPS when compared to the control group. A significant reduction of mineralization production was observed in both APCs treated with 10 μg/ml LPS and D-gal (P = 0.008 and P = 0.042, respectively) (Fig. 3c). Co-incubation of AA tended to elevate the ratio of mineralization in APCs treated with 1 μg/ml (P = 0.791) and 10 μg/ml (P = 0.895) LPS compared to those without AA.
Osteo-/odontogenic differentiation capacity
The expression of the DSPP gene was significantly down-regulated in APCs treated with 10 μg/ml LPS when compared to the control groups (P = 0.045). Co-incubation of AA was likely to upregulate the DSPP gene expression in both APCs treated with 1 μg/ml and 10 μg/ml LPS (P = 0.730 and 0.182, respectively) compared to those without AA.
A similar trend of BSP gene expression was observed as the reductions of gene expressions were observed in APCs treated with 10 μg/ml LPS when compared to the control group (P = 0.457). Moreover, AA was found to upregulate the BSP expression in both APCs treated with 1 μg/ml and 10 μg/ml LPS (P = 0.214 and 0.848) compared to those without AA (Fig. 3e,f).
Discussion
REPs are currently a treatment option for infected immature teeth with pulp necrosis. Cells located at the apical papilla play an essential role in the regenerative phase of REPs. A concern has been raised regarding the impairment of stem cells under the inflammatory condition which would affect the treatment [2, 47,48,49,50]. Cellular senescence, characterized as a permanent cell cycle arrest, is one of those conditions that should be considered since it would impair the regeneration process [51]. To date, there are no studies investigating the consequences of cellular senescence in cells associated with REPs, including APCs.
LPS is one possible stimulant that generates stress-induced senescence in various cell types [25,26,27]. Generally, it is proven to mediate inflammatory responses and plays roles in reactive oxygen species (ROS) production, creating oxidative stress conditions in many kinds of cells including dental pulp stem cells [11,12,13,14, 28]. Previous studies have reported that the repetitive stimulation of LPS could induce chronic inflammation, and the term “inflammaging” is generally used to define a connection between aging and chronic inflammation [52, 53]. However, information regarding the effects of LPS on the induction of cellular senescence is limited to certain areas, such as mesenchymal stem cells, dental pulp stem cells, and alveolar osteocytes [25,26,27,28,29]. Therefore, it is valuable for the endodontic field to investigate these effects in APCs, cells that play major roles in REPs. The present findings would fill the gap in knowledge associated with the current unpredictable clinical outcome after REPs.
This present study demonstrated the application of 1 μg/ml and 10 μg/ml of LPS-induced cellular senescence in APCs. Later, it impacted the regenerative function of the cells, as shown by the reduction of cell migration, mineralization, and differentiation capacities. These findings are in accordance with other studies examining the effects of LPS in other cell types. For instance, previous studies using repeated stimulation of 10 ng/ml E.coli LPS for 36 h and P. gingivalis for 144 h reported cellular senescence induction in dental pulp stem cells [28] and alveolar osteocytes [29], respectively. The term “inflammaging” is used to explain this situation since a connection between aging and chronic inflammation is reported. Oxidative stress, generated after inflammation, is a key factor that induces DNA damage by disturbing the cell cycle progression via the activation of p16 and p21.
Various studies confirmed that LPS stimulation could induce cellular senescence via the p16 and p21 pathways [25, 27, 54]. The remarkable expressions of p16 and p21 were recently reported in DPSCs and alveolar osteocytes after receiving repeated LPS stimulation [28, 29, 55]. Similar findings were observed in our study, showing the significant upregulation of p16 and p21 in APCs after LPS stimulation for 48 h. To compare the effect of LPS-induced cellular senescence, D-galactose (D-gal), a common model used to study cellular senescence [41, 43, 44], was used in this study. The results showed that LPS could induce more potent of senescence markers than the D-gal group, suspecting that LPS might has other cooperative pathways. LPS could also directly initiate cellular senescence by the release of pro-inflammatory cytokines, but D-gal normally relies only on the pathway of ROS production. Further investigations are required to support this hypothesis.
Considering the function of the cells, age-related cellular senescence or replicative senescence is usually associated with the loss of cell migration and differentiation capacity [56,57,58,59,60,61]. This present study demonstrated that LPS-induced APCs showed the declination of cell migration, and also mineralization. However, at the gene level, the reduction of DSPP gene expression was significantly observed only in the 10 μg/ml LPS group. This implicates that the high concentration of LPS is a key point generating negative effects on both gene level and mineralization potential. Interestingly, it was found that the expression of the BSP gene, associated with osteogenic differentiation, did not significantly alter, meaning that LPS-induced senescent cells might produce bone rather than dentin [15, 62].
To control cellular senescence, this study attempted to explore an antioxidant intervention which is able to prevent or reverse senescent phenotypes. Ascorbic acid (AA), an essential vitamin, is one of the well-known antioxidants that participates in redox oxidative pathways [30]. Previous research has shown that it has a positive impact on dental stem cells by promoting stemness marker expression, proliferation, and differentiation [34,35,36,37]. Also, it has been reported to effectively delay cellular senescence in various kinds of cells [38,39,40,41,42]. In this present study, the co-incubation of 50 μg/ml AA could impede LPS-induced cellular senescence, as shown by the reduction of SA-β-gal staining and the expression of p16 and p21. Also, it was shown to improve cell migration, mineralization, and osteo-/odontogenic differentiation capacity, especially in APCs treated with LPS 1 μg/ml. There are various reasons that can be hypothesized to support the positive role of AA under inflammatory conditions. Firstly, AA has the potential to neutralize pro-inflammatory cytokines, which are recognized as senescence-associated secretory phenotypes (SASPs) [22, 32]. Secondly, as a common ROS scavenger, AA should reduce the ROS level, which may increase during LPS induction. Lastly, AA may act on the AKT/mTOR signaling pathway, as it has been shown to inhibit this pathway and subsequently improve mineralization potential via dentinogenic gene expression [41, 63, 64]. While this study does not directly observe inflammatory cytokines, ROS levels, and specific signaling pathways, it is important to note some inherent limitations. Therefore, further studies are required to confirm the proposed hypothesis, focusing on the exact mechanism and pathways of AA in cellular senescence.
In relation to clinical endodontics, this present study revealed that infection induced by LPS not only affects pulpal status but also induces APCs into a cellular senescence condition, which has an additional negative effect on their regenerative function. This may implicate many unpredictable histological outcomes reported in teeth treated with REPs. Therefore, in this study, we attempted to reverse the cellular senescence condition using an antioxidant, AA, which is readily available. The results support the use of AA, which could potentially be employed as medication or in other forms of supplements, to improve cellular conditions. Nevertheless, further studies are required to examine various concentrations of AA and explore other aspects of this vitamin. Certainly, there are some limitations in this study as it was limited to in vitro experiments that may not completely replicate the cells’ conditions in a clinical situation. Therefore, the results should be interpreted with caution. Moreover, another concern in this study is the incubation period of LPS which was only one specific time. We speculate that the effects might vary if longer or shorter incubation times are applied. Therefore, further investigations focusing on senescent cells should be prioritized to enhance therapeutic benefits in REPs. In-depth research into these inflammatory conditions and cellular senescence is necessary to bridge the knowledge gap.
Conclusion
Based on the results of this study, 1 ug/ml and 10 ug/ml of LPS could induce cellular senescence in human APCs which negatively impaired the regenerative function. Co-incubation with 50 μg/ml AA could recover the cellular senescence while promoting the regenerative capacity of the cells. The findings imply that LPS-induced senescence causes negative effects on the regenerative capacity of APCs. Consequently, this senescence might disrupt pulp-dentine regeneration in REPs. However, AA could potentially be used to attenuate cellular senescence in APCs.
References
Torabinejad M, Nosrat A, Verma P, Udochukwu O (2017) Regenerative endodontic treatment or mineral trioxide aggregate apical plug in teeth with necrotic pulps and open apices: a systematic review and meta-analysis. J Endod 43:1806–1820. https://doi.org/10.1016/j.joen.2017.06.029
Chen MH, Chen KL, Chen CA, Tayebaty F, Rosenberg P, Lin L (2012) Responses of immature permanent teeth with infected necrotic pulp tissue and apical periodontitis/abscess to revascularization procedures. Int Endod J 45:294–305. https://doi.org/10.1111/j.1365-2591.2011.01978.x
Nosrat A, Homayounfar N, Oloomi K (2012) Drawbacks and unfavorable outcomes of regenerative endodontic treatments of necrotic immature teeth: a literature review and report of a case. J Endod 38:1428–1434. https://doi.org/10.1016/j.joen.2012.06.025
Becerra P, Ricucci D, Loghin S, Gibbs JL, Lin LM (2014) Histologic study of a human immature permanent premolar with chronic apical abscess after revascularization/revitalization. J Endod 40:133–139. https://doi.org/10.1016/j.joen.2013.07.017
Lin LM, Shimizu E, Gibbs JL, Loghin S, Ricucci D (2014) Histologic and histobacteriologic observations of failed revascularization/revitalization therapy: a case report. J Endod 40:291–295. https://doi.org/10.1016/j.joen.2013.08.024
Verma P, Nosrat A, Kim J, Price J, Wang P, Bair E, Xu H, Fouad A (2017) Effect of residual bacteria on the outcome of pulp regeneration in vivo. J Dent Res 96:100–106. https://doi.org/10.1177/0022034516671499
Huang GTJ, Sonoyama W, Liu Y, Liu H, Wang S, Shi S (2008) The hidden treasure in apical papilla: the potential role in pulp/dentin regeneration and bioroot engineering. J Endod 34:645–651. https://doi.org/10.1016/j.joen.2008.03.001
Martin FE, Nadkarni MA, Jacques NA, Hunter N (2002) Quantitative microbiological study of human carious dentine by culture and real-time PCR: association of anaerobes with histopathological changes in chronic pulpitis. J Clin Microbiol 40:1698–1704. https://doi.org/10.1128/jcm.40.5.1698-1704.2002
Narayanan LL, Vaishnavi C (2010) Endodontic microbiology. J Conserv Dent 13:233. https://doi.org/10.4103/2F0972-0707.73386
Cooper PR, Holder MJ, Smith AJ (2014) Inflammation and regeneration in the dentin-pulp complex: a double-edged sword. J Endod 40:S46–S51. https://doi.org/10.1016/j.joen.2014.01.021
Kim JC, Lee YH, Yu MK, Lee NH, Park JD, Bhattarai G, Yi HK (2012) Anti-inflammatory mechanism of PPARγ on LPS-induced pulp cells: role of the ROS removal activity. Arch Oral Biol 57:392–400. https://doi.org/10.1016/j.archoralbio.2011.09.009
Zhang J, Zhang Y, Lv H, Yu Q, Zhou Z, Zhu Q, Wang Z, Cooper PR, Smith AJ, Niu Z (2013) Human stem cells from the apical papilla response to bacterial lipopolysaccharide exposure and anti-inflammatory effects of nuclear factor IC. J Endod 39:1416–1422. https://doi.org/10.1016/j.joen.2013.07.018
Gölz L, Memmert S, Rath-Deschner B, Jäger A, Appel T, Baumgarten G, Götz W, Frede S (2014) LPS from P. gingivalis and hypoxia increases oxidative stress in periodontal ligament fibroblasts and contributes to periodontitis. Mediators Inflamm 2014:1–13. https://doi.org/10.1155/2014/986264
Cheng R, Choudhury D, Liu C, Billet S, Hu T, Bhowmick N (2015) Gingival fibroblasts resist apoptosis in response to oxidative stress in a model of periodontal diseases. Cell Death Discov 1:1–8. https://doi.org/10.1038/cddiscovery.2015.46
Lertchirakarn V, Aguilar P (2017) Effects of lipopolysaccharide on the proliferation and osteogenic differentiation of stem cells from the apical papilla. J Endod 43:1835–1840. https://doi.org/10.1016/j.joen.2017.06.024
Liu J, Du J, Chen X, Yang L, Zhao W, Song M, Wang Z, Wang Y (2019) The effects of mitogen-activated protein kinase signaling pathways on lipopolysaccharide-mediated osteo/odontogenic differentiation of stem cells from the apical papilla. J Endod 45:161–167. https://doi.org/10.1016/j.joen.2018.10.009
Lei S, Liu XM, Liu Y, Bi J, Zhu S, Chen X (2020) Lipopolysaccharide downregulates the osteo-/odontogenic differentiation of stem cells from apical papilla by inducing autophagy. J Endod. https://doi.org/10.1016/j.joen.2020.01.009
Jariyamana N, Chuveera P, Dewi A, Leelapornpisid W, Ittichaicharoen J, Chattipakorn S, Srisuwan T (2021) Effects of N-acetyl cysteine on mitochondrial ROS, mitochondrial dynamics, and inflammation on lipopolysaccharide-treated human apical papilla cells. Clin Oral Investig 25:3919–3928. https://doi.org/10.1007/s00784-020-03721-7
Weekate K, Chuenjitkuntaworn B, Chuveera P, Vaseenon S, Chompu-inwai P, Ittichaicharoen J, Chattipakorn S, Srisuwan T (2021) Alterations of mitochondrial dynamics, inflammation and mineralization potential of LPS-induced human dental pulp cells after exposure to N-acetyl cysteine, Biodentine or ProRoot MTA. Int Endod J 54:951–965. https://doi.org/10.1111/iej.13484
Tan DQ, Suda T (2018) Reactive oxygen species and mitochondrial homeostasis as regulators of stem cell fate and function. Antioxid Redox Signal 29:149–168. https://doi.org/10.1089/ars.2017.7273
Fridlyanskaya I, Alekseenko L, Nikolsky N (2015) Senescence as a general cellular response to stress: a mini-review. Exp Gerontol 72:124–128. https://doi.org/10.1016/j.exger.2015.09.021
Amaya-Montoya M, Pérez-Londoño A, Guatibonza-García V, Vargas-Villanueva A, Mendivil CO (2020) Cellular senescence as a therapeutic target for age-related diseases: a review. Adv Ther 37:1407–1424. https://doi.org/10.1007/s12325-020-01287-0
Campisi J (2005) Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell 120:513–522. https://doi.org/10.1016/j.cell.2005.02.003
Turinetto V, Vitale E, Giachino C (2016) Senescence in human mesenchymal stem cells: functional changes and implications in stem cell-based therapy. Int J Mol Sci 17:1164. https://doi.org/10.3390/ijms17071164
Yu H, Zhao Y, Luo X, Feng Y, Ren Y, Shang H, He Z, Luo X, Chen S, Wang X (2012) Repeated lipopolysaccharide stimulation induces cellular senescence in BV2 cells. Neuroimmunomodulation 19:131–136. https://doi.org/10.1159/000330254
Kim CO, Huh AJ, Han SH, Kim JM (2012) Analysis of cellular senescence induced by lipopolysaccharide in pulmonary alveolar epithelial cells. Arch Gerontol Geriatr 54:e35–e41. https://doi.org/10.1016/j.archger.2011.07.016
Zhao M, Chen X (2015) Effect of lipopolysaccharides on adipogenic potential and premature senescence of adipocyte progenitors. Am J Physiol - Endocrinol Metab 309:E334–E344. https://doi.org/10.1152/ajpendo.00601.2014
Feng X, Feng G, Xing J, Shen B, Tan W, Huang D, Lu X, Tao T, Zhang J, Li L (2014) Repeated lipopolysaccharide stimulation promotes cellular senescence in human dental pulp stem cells (DPSCs). Cell Tissue Res 356:369–380. https://doi.org/10.1007/s00441-014-1799-7
Aquino-Martinez R, Rowsey JL, Fraser DG, Eckhardt BA, Khosla S, Farr JN, Monroe DG (2020) LPS-induced premature osteocyte senescence: implications in inflammatory alveolar bone loss and periodontal disease pathogenesis. Bone 132:115220. https://doi.org/10.1016/j.bone.2019.115220
Monacelli F, Acquarone E, Giannotti C, Borghi R, Nencioni A (2017) Vitamin C, aging and Alzheimer’s disease. Nutrients 9:670. https://doi.org/10.3390/nu9070670
Staudte H, Güntsch A, Völpel A, Sigusch B (2010) Vitamin C attenuates the cytotoxic effects of Porphyromonas gingivalis on human gingival fibroblasts. Arch Oral Biol 55:40–45. https://doi.org/10.1016/j.archoralbio.2009.11.009
Diomede F, Marconi GD, Guarnieri S, D’Attilio M, Cavalcanti MF, Mariggiò MA, Pizzicannella J, Trubiani O (2019) A novel role of ascorbic acid in anti-inflammatory pathway and ROS generation in HEMA treated dental pulp stem cells. Materials 13:130. https://doi.org/10.3390/ma13010130
Fawzy El-Sayed KM, Nguyen N, Dörfer CE (2020) Ascorbic acid, inflammatory cytokines (IL-1β/TNF-α/IFN-γ), or their combination’s effect on stemness, proliferation, and differentiation of gingival mesenchymal stem/progenitor cells. Stem Cells Int 2020. https://doi.org/10.1155/2020/8897138
Bhandi S, Alkahtani A, Mashyakhy M, Abumelha AS, Albar NHM, Renugalakshmi A, Alkahtany MF, Robaian A, Almeslet AS, Patil VR (2021) Effect of ascorbic acid on differentiation, secretome and stemness of stem cells from human exfoliated deciduous tooth (SHEDs). J Pers Med 11:589. https://doi.org/10.3390/jpm11070589
Ishikawa S, Iwasaki K, Komaki M, Ishikawa I (2004) Role of ascorbic acid in periodontal ligament cell differentiation. J Periodontol 75:709–716. https://doi.org/10.1902/jop.2004.75.5.709
Van Pham P, Tran NY, Phan NL-C, Vu NB, Phan NK (2016) Vitamin C stimulates human gingival stem cell proliferation and expression of pluripotent markers. In Vitro Cell Dev Biol 52:218–227. https://doi.org/10.1007/s11626-015-9963-2
Diederich A, Fründ HJ, Trojanowicz B, Navarrete Santos A, Nguyen AD, Hoang-Vu C, Gernhardt CR (2023) Influence of ascorbic acid as a growth and differentiation factor on dental stem cells used in regenerative endodontic therapies. J Clin Med 12:1196. https://doi.org/10.3390/jcm12031196
Kashino G, Kodama S, Nakayama Y, Suzuki K, Fukase K, Goto M, Watanabe M (2003) Relief of oxidative stress by ascorbic acid delays cellular senescence of normal human and Werner syndrome fibroblast cells. Free Radic Biol Med 35:438–443. https://doi.org/10.1016/S0891-5849(03)00326-5
Kim JE, Jin DH, Lee SD, Hong SW, Shin JS, Lee SK, Jung DJ, Kang JS, Lee WJ (2008) Vitamin C inhibits p53-induced replicative senescence through suppression of ROS production and p38 MAPK activity. Int J Mol Sci 22:651–655. https://doi.org/10.3892/ijmm_00000068
Burger MG, Steinitz A, Geurts J, Pippenger BE, Schaefer DJ, Martin I, Barbero A, Pelttari K (2017) Ascorbic acid attenuates senescence of human osteoarthritic osteoblasts. Int J Mol Sci 18:2517. https://doi.org/10.3390/ijms18122517
Yang M, Teng S, Ma C, Yu Y, Wang P, Yi C (2018) Ascorbic acid inhibits senescence in mesenchymal stem cells through ROS and AKT/mTOR signaling. Cytotechnology 70:1301–1313. https://doi.org/10.1007/s10616-018-0220-x
Yang Y, Wang T, Zhang S, Jia S, Chen H, Duan Y, Wang S, Chen G, Tian W (2021) Vitamin C alleviates the senescence of periodontal ligament stem cells through inhibition of Notch3 during long-term culture. J Cell Physiol 236:1237–1251. https://doi.org/10.1002/jcp.29930
Azman KF, Zakaria R (2019) D-Galactose-induced accelerated aging model: an overview. Biogerontology 20:763–782. https://doi.org/10.1007/s10522-019-09837-y
Zhang D, Yan B, Yu S, Zhang C, Wang B, Wang Y, Wang J, Yuan Z, Zhang L, Pan J (2015) Coenzyme Q10 inhibits the aging of mesenchymal stem cells induced by D-galactose through Akt/mTOR signaling. Oxid Med Cell Longev 2015. https://doi.org/10.1155/2015/867293
Tominaga T, Shimada R, Okada Y, Kawamata T, Kibayashi K (2019) Senescence-associated-β-galactosidase staining following traumatic brain injury in the mouse cerebrum. PLoS One 14:e0213673. https://doi.org/10.1371/journal.pone.0213673
Grada A, Otero-Vinas M, Prieto-Castrillo F, Obagi Z, Falanga V (2017) Research techniques made simple: analysis of collective cell migration using the wound healing assay. J Invest Dermatol 137:e11–e16. https://doi.org/10.1016/j.jid.2016.11.020
Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB (2010) Challenges in regenerative endodontics: a case series. J Endod 36:536–541. https://doi.org/10.1016/j.joen.2009.10.006
Lenzi R, Trope M (2012) Revitalization procedures in two traumatized incisors with different biological outcomes. J Endod 38:411–414. https://doi.org/10.1016/j.joen.2011.12.003
Liu C, Xiong H, Chen K, Huang Y, Huang Y, Yin X (2016) Long-term exposure to pro-inflammatory cytokines inhibits the osteogenic/dentinogenic differentiation of stem cells from the apical papilla. Int Endod J 49:950–959. https://doi.org/10.1111/iej.12551
Yoo YJ, Oh JH, Lee WC, Woo KM (2016) Regenerative characteristics of apical papilla–derived cells from immature teeth with pulpal and periapical pathosis. J Endod 42:1626–1632. https://doi.org/10.1016/j.joen.2016.08.004
Herranz N, Gil J (2018) Mechanisms and functions of cellular senescence. J Clin Investig 128:1238–1246. https://doi.org/10.1172/JCI95148
Franceschi C, Campisi J (2014) Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J Gerontol A Biol Sci Med Sci 69:S4–S9. https://doi.org/10.1093/gerona/glu057
Sanada F, Taniyama Y, Muratsu J, Otsu R, Shimizu H, Rakugi H, Morishita R (2018) Source of chronic inflammation in aging. Front Cardiovasc Med 5:12. https://doi.org/10.3389/fcvm.2018.00012
Mytych J, Romerowicz-Misielak M, Koziorowski M (2017) Long-term culture with lipopolysaccharide induces dose-dependent cytostatic and cytotoxic effects in THP-1 monocytes. Toxicol in Vitro 42:1–9. https://doi.org/10.1016/j.tiv.2017.03.009
Feng G, Zheng K, Cao T, Zhang J, Lian M, Huang D, Wei C, Gu Z, Feng X (2018) Repeated stimulation by LPS promotes the senescence of DPSCs via TLR4/MyD88-NF-κB-p53/p21 signaling. Cytotechnology 70:1023–1035. https://doi.org/10.1007/s10616-017-0180-6
Mehrazarin S, Oh JE, Chung CL, Chen W, Kim RH, Shi S, Park N-H, Kang MK (2011) Impaired odontogenic differentiation of senescent dental mesenchymal stem cells is associated with loss of Bmi-1 expression. J Endod 37:662–666. https://doi.org/10.1016/j.joen.2011.02.009
Zhang J, An Y, Gao L-N, Zhang Y-J, Jin Y, Chen F-M (2012) The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials 33:6974–6986. https://doi.org/10.1016/j.biomaterials.2012.06.032
Feng X, Xing J, Feng G, Huang D, Lu X, Liu S, Tan W, Li L, Gu Z (2014) p16INK4A mediates age-related changes in mesenchymal stem cells derived from human dental pulp through the DNA damage and stress response. Mech Ageing Dev 141:46–55. https://doi.org/10.1016/j.mad.2014.09.004
Wu RX, Bi CS, Yu Y, Zhang LL, Chen FM (2015) Age-related decline in the matrix contents and functional properties of human periodontal ligament stem cell sheets. Acta Biomater 22:70–82. https://doi.org/10.1016/j.actbio.2015.04.024
Yi Q, Liu O, Yan F, Lin X, Diao S, Wang L, Jin L, Wang S, Lu Y, Fan Z (2017) Analysis of senescence-related differentiation potentials and gene expression profiles in human dental pulp stem cells. Cells Tissues Organs 203:1–11. https://doi.org/10.1159/000448026
Iezzi I, Cerqueni G, Licini C, Lucarini G, Mattioli Belmonte M (2019) Dental pulp stem cells senescence and regenerative potential relationship. J Cell Physiol 234:7186–7197. https://doi.org/10.1002/jcp.27472
Aguilar P, Mahanonda R, Sa-Ard-Iam N, Lertchirakarn V (2021) Effects of lipopolysaccharide on proliferation, migration and osteogenic differentiation of apical papilla cells from early and late stage of root development. Aust Endod J 47:281–289. https://doi.org/10.1111/aej.12475
Tanaka Y, Sonoda S, Yamaza H, Murata S, Nishida K, Hama S, Kyumoto-Nakamura Y, Uehara N, Nonaka K, Kukita T (2018) Suppression of AKT-mTOR signal pathway enhances osteogenic/dentinogenic capacity of stem cells from apical papilla. Stem Cell Res Ther 9:1–12. https://doi.org/10.1186/s13287-018-1077-9
Tanaka Y, Sonoda S, Yamaza H, Murata S, Nishida K, Kyumoto-Nakamura Y, Uehara N, Nonaka K, Kukita T, Yamaza T (2019) Acetylsalicylic acid treatment and suppressive regulation of AKT accelerate odontogenic differentiation of stem cells from the apical papilla. J Endod 45(591-598):e6. https://doi.org/10.1016/j.joen.2019.01.016
Acknowledgements
The authors would like to thank Adjunct Professor Richard L. Wilson, Faculty Consultant at the Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand, for his assistance in the English language editing of the manuscript. The research grant for this project was partially supported by the CMU Mid-career Research Fellowship program and research grants from the Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
Funding
The research grant for this project was partially supported by the CMU Mid-career Research Fellowship program and research grants from the Faculty of Dentistry, Chiang Mai University, Chiang Mai, Thailand.
Author information
Authors and Affiliations
Contributions
Chananporn Teawcharoensopa: data curation, formal analysis, investigation, software, visualization, writing – original draft preparation. Tanida Srisuwan: conceptualization, funding acquisition, methodology, project administration, resource, supervision, validation, writing – review and editing
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the Human Experimental Committee, Faculty of Dentistry, Chiang Mai University (No.5/2021), and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Teawcharoensopa, C., Srisuwan, T. The potential use of ascorbic acid to recover the cellular senescence of lipopolysaccharide-induced human apical papilla cells: an in vitro study. Clin Oral Invest 28, 49 (2024). https://doi.org/10.1007/s00784-023-05455-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00784-023-05455-8