Abstract
Dental pulp stem cells (DPSCs), one type of mesenchymal stem cells, are considered to be a type of tool cells for regenerative medicine and tissue engineering. Our previous studies found that the stimulation with lipopolysaccharide (LPS) might introduce senescence of DPSCs, and this senescence would have a positive correlation with the concentration of LPS. The β-galactosidase (SA-β-gal) staining was used to evaluate the senescence of DPSCs and immunofluorescence to show the morphology of DPSCs. Our findings suggested that the activity of SA-β-gal has increased after repeated stimulation with LPS and the morphology of DPSCs has changed with the stimulation with LPS. We also found that LPS bound to the Toll-like receptor 4 (TLR4)/myeloid differentiation factor (MyD) 88 signaling pathway. Protein and mRNA expression of TLR4, MyD88 were enhanced in DPSCs with LPS stimulation, resulting in the activation of nuclear factor-κB (NF-κB) signaling, which exhibited the expression of p65 improved in the nucleus while the decreasing of IκB-α. Simultaneously, the expression of p53 and p21, the downstream proteins of the NF-κB signaling, has increased. In summary, DPSCs tend to undergo senescence after repeated stimulation in an inflammatory microenvironment. Ultimately, these findings may lead to a new direction for cell-based therapy in oral diseases and other regenerative medicines.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dental pulp stem cells (DPSCs), one type of mesenchymal stem cells (MSCs), are multipotent cells which originate from dental pulp tissue, have been becoming tool cells for regenerative medicine and tissue engineering (Kolf et al. 2015; Meng et al. 2015). DPSCs can differentiate into distinct cell types, such as osteoblasts, chondroblasts, neuroblast, adipocytes, myoblasts, etc. (D’Alimonte et al. 2013; Hilkens et al. 2013). However, the differentiating capability of MSCs or DPSCs may be inhibited once those stem cells show senecsence (Sui et al. 2016; Trial et al. 2016). When those stem cells are transplanted into recipient region, they would be surrounded by the unclear microenvironment which includes stress state or cytokines released from ambient cells of the recipient tissue (Yang et al. 2012). Lipopolysaccharide (LPS), a major component of the cell wall of gram-negative bacteria, plays an important role in infectious diseases such as periodontitis and pulpitis (Huang et al. 2015; Rutherford and Gu 2000). LPS can induce the expression of proinflammatory cytokines, such as interleukin-6 (IL-6) and IL-8 and tumour necrosis factor-α (TNF-α) (Kalaiyarasu et al. 2016; Marques et al. 2016) and it exerts its biological effects by promoting the production of various inflammatory mediators. It has been proven that inflammatory mediators can stimulate MSCs to differentiation or senescence or some other physiological and pathological processes in different conditions (Brennen et al. 2013; Mantovani et al. 2014; Zhu et al. 2013). In our previous researches, we used LPS to imitate an inflammatory microenvironment, and we have proven that repeated LPS stimulation promotes senescence of DPSCs (Feng et al. 2014). The β-galactosidase (SA-β-gal) activity was stronly associated with senescent cells, since it was not detectable in quiescent cells or terminally differentiated cells, although there are exceptions. SA-β-gal activity is now a widely used biomarker in studies of cellular senescence in culture and in vivo (Debacq-Chainiaux et al. 2009; Dimri et al. 1995). SA-β-gal staining would be used to evaluate the senescence of DPSCs. Several signaling pathways, including MAPK, AP-1, NF-κB, have been proven to participate in LPS mediated senescence of DPSCs (Jung et al. 2016). However, the mechanism that LPS targets DPSCs on senescence is still unclear.
Increasing evidence suggested that LPS is a specific ligand of toll-like receptor 4 (TLR4) (Andonegui et al. 2009), and TLR4 plays a critical role in the recognition of G- bacterial components (LPS). Previous studies have shown that LPS can induce inflammatory liver injury via TLR4/myeloid differentiation factor (MyD) 88 signal pathway (Yao et al. 2016). TLR4 will activate NF-κB protein once stimulated by LPS via two major signaling pathways: a MyD88-dependent pathway that acts via NF-κB to induce pro-inflammatory cytokines such as TNF-α, and a MyD88-independent pathway that acts via type I interferons to enhance the expression of interferon-inducible genes (Broad et al. 2007; Hernandez et al. 2016). On the other hand, NF-κB signaling also plays an important role in the response to inflammation, and its activation leads to the expression of various proinflammatory cytokines including IL-1β, IL-6 and TNF-α, and the expression of p65 in nucleus will be increased while inhibitor of nuclear factor κB alpha (IκB-α) will be decreased in this process (Imam et al. 2015; Zhao et al. 2014, 2016). At the same time, the expression of p53 and p21, the downstream signal of NF-κB which had been reported in other inflammatory diseases, will be increased (Debelec-Butuner et al. 2014; O’Prey et al. 2010).
Senescence acts as a physiological or pathological phenomenon, which is characterized by a functional disorder, a decline of homoeostasis, and reduced capacity to respond properly to impair (Faner et al. 2012; Sui et al. 2016). Accumulating evidence has proven that the activation of p53 and p21 plays a key role in the senescence of the cells (Kim et al. 2016a; Oren 2003; Wang et al. 2016b). It has been proven that microenvironmental pathologic factors, such as inflammation factors LPS, TNF-α, IL, impair the biological functions of MSCs such as osteogenesis (Sui et al. 2016). Senescence will be evaluated by senescence-associated β-galactosidase (SA-β-gal) staining, which has been proven that the SA-β-gal-positive cells would increase along with the number of the aging cells increasing (Wang et al. 2016a). However, the mechanisms at the molecular level which act in inflammation microenvironment signals induce the senescence of DPSCs are not fully understood.
Materials and methods
Cell cultures
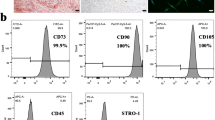
Normal human impacted third molars were collected from patients 13–23 years of age (n = 9) after giving the informed consents which had been approved by the Ethics Committee of the Affiliated Hospital of Nantong University. The ethical committee approval number was 2016–077. All subjects were free of carious lesions and oral infection. We isolated DPSCs by cleaning the tooth surface, cutting around the cementoenamel junction using sterilized dental fissure burs and then opening to reveal the pulp chamber. The pulp was then digested in a solution of 3 mg/ml collagenase type I for 1 h at 37 °C. Single-cell suspensions were obtained by passing the digested tissues through a 70-µm cell strainer (BD Falcon, Bedford, MA, USA). Cell suspensions of dental pulp were seeded into 25 cm2 culture dishes and cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 µg/ml streptomycin at 37 °C in 5% CO2. The medium was changed every 3 days. Approximately 7–10 days after seeding, the cells became nearly confluent. Cells were passaged at the ratio of 1:3 when they reached 85–90% confluence. The adherent cells were released from the dishes with 0.25% trypsin (Gibco, USA) and seeded into new fresh culture flasks. All the experiments described below were performed using DPSCs from the mixed population of cells at passage 3 (P3). In our previous expressions, our colleagues have analyzed the immunophenotype of DPSC (Feng et al. 2013), with both being highly positive for CD29 and CD105, but negative for CD31 and CD34. DPSCs were further identified by osteogenic, chondrogenic and adipogenic differentiation (Feng et al. 2013, 2014). The culture received either Escherichia coli LPS serotype 0111:B4 (Sigma, ST. Louis, MO, USA) stimulation (10 ng/ml) at the indicated times or normal saline as a control. Four groups were analyzed with the following stimuli added to the culture: (1) DPSCs stimulated with normal saline as a control; (2) DPSCs stimulated with LPS once for 6 h; (3) DPSCs stimulated with LPS 3 times, once every 48 h for 6 h each, and (4) DPSCs stimulated with LPS 6 times, once every 24 h for 6 h each (Yu et al. 2012). After stimulation, all groups were cultured in the SA-β-gal assay.
Cell proliferation assay
The LPS-treated DPSCs proliferation assay was performed using the BrdU assay kit according to the manufacturer’s protocol. Generally, cells were incubated with 100 μM BrdU labeling solution for 4 h at 37 °C. After removing the culture medium, the cells were fixed and the DNA was denatured by FixDenat solution. The anti-BrdU-POD working solution and substrate solution were then added, and the absorbances of the samples were measured by an ELISA plate reader at 370 nm wave length.
Determination of cell number
DPSCs were seeded at 0.7 × 104 cells/well into 6-well plates in triplicate for each experimental condition. DPSCs were collected after plating and dissociated. The total cell numbers were counted.
SA-β-gal assay
The SA-β-gal assay was used to detect cell senescence. The SA-β-gal activity was determined using a kit from the Chemical Company following the manufacturer’s instructions (Abcam, Cambridge, MA, USA). In brief, cells were cultured on slips in the 24-well plates overnight and fixed with paraformaldehyde. After incubation with SA-β-gal overnight, the slips were washed and analyzed under the microscope. The senescent cells were identified as blue-stained cells by standard light microscopy, and a total of 1000 cells were counted in 20 random fields on a slide to determine the percentage of SA-β-gal-positive cells.
siRNAs and Transfection
Double-stranded RNA nucleotides targeted to human p65 were obtained from Santa Cruz Biotechnology. Transfection of DPSCs with duplex synthetic siRNA was performed using Lipofectamine 2000 reagent (Invitrogen) according to the manufacturer’s instructions. Cells were assayed after 48 h of transfection. Cells were further stimulated by LPS.
Western blot
Cells were lysed in buffer consisting of 50 mM TRIS, 150 mM NaCl, 2% sodium dodecyl sulfate (SDS) and a protease inhibitor mixture. After centrifugation at 12,000 rpm for 12 min, protein concentrations were determined using the Bradford assay (Bio-Rad, Berkeley, CA, USA). The resulting supernatant (50 µg of protein) was subjected to SDS–polyacrylamide gel electrophoresis (PAGE). The separated proteins were transferred onto polyvinylidene fluoride (PVDF) membranes at 350 mA for 2.5 h in a blotting apparatus (Bio-RAD). Membranes were blocked with 5% nonfat milk and incubated with primary antibodies (1:400) at 4 °C overnight and subsequently with anti-rabbit horseradish peroxidase-conjugated secondary antibodies (1:1000) for 2 h at room temperature. Concomitantly, GAPDH was run as a reference protein. The following primary antibodies were used: GAPDH (anti-rabbit, Santa Cruz Biotechnology, Santa Cruz, CA, USA), p65 (anti-rabbit, Santa Cruz Biotechnology), β-actin (anti-rabbit, Santa Cruz Biotechnology), IκBα (anti-rabbit, Sigma), p53 (anti-rabbit, Sigma), p21 (anti-rabbit, Sigma).
Immunofluorescent staining
DPSCs were fixed with 4% PFA for 1 h, washed with phosphate buffer solution (PBS) containing 0.1% Triton X-100 (PBST), and blocked for 30 min in PBST supplemented with 10% FBS. Cells were then incubated with one of the following primary antibodies (1:100) in the same solution overnight at 4 °C: F-actin (anti-rabbit, Santa Cruz Biotechnology), p65 (anti-rabbit, Santa Cruz Biotechnology), p53 (anti-rabbit, Sigma), p21 (anti-rabbit, Sigma). Cells were then washed and incubated in secondary antibodies for 2 h at room temperature. Nuclei were stained with (4′6′-diamidino-2-phenylindole dihydrochloride) (DAPI) (1:800, Santa Cruz). The cells were examined using a Leica fluorescence microscope (Wetzlar, Germany). For immunofluorescence assay of the skeleton of DPSCs, DPSCs were washed once with PBS and fixed in 4% paraformaldehyde (PFA) for 15 min. After permeabilization and blocking, they were incubated with fluorescein isothiocyanate-conjugated phalloidin. The stained cells were then examined using a Leica fluorescence microscope. The total cell number of every field was estimated by counting DAPI stained nuclei.
Reverse transcription-polymerase chain reaction (RT–PCR) analysis
Total cellular RNA was isolated from cells and reverse transcribed using conventional protocols. PCR was performed to test the mRNA level of TLR4, MyD88, p53, p21, GAPDH and β-actin. Primers of mRNA for PCR analysis are listed in Table 1. All primer sequences were determined using established GenBank sequences. The primers were used to amplify the duplicate PCR reactions. Each sample was analyzed in triplicate and GAPDH or β-actin was used as a control.
Nucleocytoplasmic separation
Cultured DPSCs were collected, washed with PBS and suspended in hypotonic buffer to achieve nuclear extracts. The cultured DPSCs were homogenized, and the nuclei were pelleted. Then we removed the cytoplasmic extracts and re-suspended nuclei in a low-salt buffer. A high-salt buffer was added to release soluble proteins from the nuclei, and the nuclei were removed by centrifugation. The nuclear extracts were dialyzed into a moderate salt solution.
Statistical analysis
All data are presented as mean ± SEM from at least three independent experiments, each performed with triplicate samples. Differences between groups were tested for statistical significance using ANOVA. Student’s t test was used to determine significance with SPSS 16.0 software. A value for P < 0.05 was considered as statistically significant.
Results
Effects of LPS on DPSCs senescence
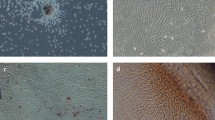
The number of SA-β-gal-positive DPSCs obviously increased after treatment with LPS, furthermore the strongest staining were seen in LPS stimulation 6 times (Fig. 1a, b). The proliferation of DPSCs was measured with a BrdU assay. The result suggested that the growth of DPSCs was slower after repeated stimulation with LPS for 3 or 6 times (Fig. 1c). After repeated stimulation with LPS for 3 or 6 times, the total number of DPSCs declined (Fig. 1d). The F-actin distribution was disordered and accumulated around the nuclear region in the LPS-treated DPSCs (Fig. 1e). These results indicated that repeated LPS stimulation promoted senescence of DPSCs.
Effects of LPS on the DPSCs senescence. a, b Senescence-associated-β-galactosidase (SA-β-gal) was induced by repeated stimulation with LPS in DPSCs. The senescent DPSCs were identified by blue staining using standard light microscopy and a total of 1000 cells was counted in 20 random fields on a slide to determine the percentage of SA-β-gal-positive cells. SA-β-gal-positive cells were identified in cells receiving repeated stimulation with LPS for 3 or 6 times much more easily, and the strongest staining being observed in cells receiving LPS stimulation 6 times (*P < 0.05, original magnification: ×200). Bar 50 μm (a). c The BrdU labeling assay was used to detect the cell proliferation ratio. The absorbance showed the proliferation rate. DPSCs from repeated stimulation with LPS for 3 or 6 times grew slowlier than those from the control group and only one single LPS stimulation (*P < 0.05). d The cell number was identified by counting after exposure to LPS. The population doubling time of DPSCs from the controls and only one single LPS stimulation were both faster than those from the repeated stimulation with LPS for 3 or 6 times (*P < 0.05). e DPSCs were stained by FITC-Phalloidin. Immuno-fluorescence showed that the F-actin distribution was abnormal in DPSCs from LPS treated 6 times (original magnification ×200). Scale bars: 50 µm (e)
LPS-induced expression of TLR4 and MyD88, and TLR4/MyD88 signaling promoted the nuclear translocation of p65
We examined the expression of TLR4 and MyD88 by RT–PCR. The results showed that expression level of TLR4 and MyD88 were up-regulated in a repeated-times dependent manner after stimulation with LPS (Fig. 2a, b). We also tested the expression of IκB-α and p65 by Western blot after LPS stimulation. The results suggested that the expression level of nuclear p65 had increased while the expression of IκB-α had been reduced dependent on the repeated-times of LPS stimulation (Fig. 2c, d). The immunofluorescence result also proved that LPS stimulation increased the nuclear translocation of p65 (Fig. 2e). These results indicated that repeated LPS stimulation enhanced the TLR4/MyD88 signaling, and the NF-κB pathway had been activated through the nuclear translocation of p65 and the inhibition of IκB-α.
Activation of TLR4/MyD88-NF-κB signaling pathway after LPS stimulation in DPSCs. a, b Total RNA was isolated after DPSCs have been stimulated with normal saline or with LPS only once, 3 times, or 6 times, followed by analysis with reverse transcription plus polymerase chain reaction (RT–PCR). D-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was determined as a control. c, d Western blot analysis of IκBα and cytoplasmic/nuclear p65 expression. The nuclear p65 expression was significantly increased in the LPS stimulation for once, 3 times and 6 times compared with that in the control group. The IκBα expression was significantly decreased in the LPS stimulation for once, 3 times and 6 times compared with that in the control group. GAPDH or β-tubulin or β-actin expression were determined as a control. e Immunofluorescent of p65. Blue, DAPI. In the LPS stimulation for 6 times group, there was a clear increase in the p65 expression in the nucleus. Bar 50 μm (e). (Color figure online)
The up-regulation of p53 and p21 expression was induced by LPS in DPSCs
We examined the expression of p53 and p21 by Western blot and RT–PCR. The results indicated after stimulation with LPS, that the expression of p53 and p21 was up-regulated in a repeated-times dependent mode both at a protein and messenger RNA level (Fig. 3a–d). The immunofluorescence staining showed the number of p53 and p21-positive cells significantly increased after treatment with LPS 6 times (Fig. 3e). These results indicated that repeated LPS stimulation enhanced the expression of p53 and p21.
The up-regulation of p53 and p21 expression was induced by LPS in DPSCs. a, b Western blot analysis of p53 and p21 expression. The p53 and p21 expression were significantly increased in the LPS stimulation for 3 times and 6 times compared with that in the control group or stimulation only for once. β-actin expression was determined as a control. c, d RT–PCR analysis of p53 and p21 expression had a similar result to western blot. β-actin expression was determined as a control. e Immunofluorescence of p53 and p21. Blue, DAPI. In the LPS stimulation for 6 times group, there was a clear increasing in p53 and p21 expression in nuclear. Bar 50 μm (e). (Color figure online)
Knockdown of p65 expression reversed the senescence characteristics of DPSCs treated with LPS
To further confirm the role of p65 in LPS induced senescence, p65 siRNA was transfected in DPSCs which stimulated with LPS. We found that the expression of p65 protein was remarkably decreased in the p65 siRNA-transfected DPSCs (Fig. 4a, b). Less SA-β-gal-positive cells were observed when p65 expression was knocked down in DPSCs treated with LPS (Control—34 ± 3.5%, LPS 6 times—81 ± 6.7%, p65 siRNA—44 ± 4.7%) (Fig. 4c, d). The proliferation was reversed in p65-knockdown DPSCs with LPS-treatment (Control—89 ± 8.2%, LPS 6 times—36 ± 3.2%, p65 siRNA—63 ± 5.6%) (Fig. 4e) and the total number of DPSCs had the same change in the above process (Fig. 4f). Further, the abnormal distribution of F-actin was effectively ameliorated in p65-knockdown LPS-treated DPSCs (Fig. 4g). The results indicated that p65 plays an antagonistic role in the process of senescence in DPSCs induced by LPS-stimulation.
Knockdown of p65 expression reversed the senescent characteristic of DPSCs treated with LPS. a The expression of p65 was analyzed by Western blot. b Quantification graph (relative optical density) of the intensity of staining of p65 to GAPDH at different numbers of stimulation (*P < 0.05). c, d Senescent DPSCs were identified in blue by standard light microscopy and a total of 1000 cells was counted in 20 random fields on a slide to determine the percentage of SA-β-gal-positive cells. The SA-β-gal-staining assay showed p65 knockdown could decrease SA-β-gal activity significantly in DPSCs after LPS stimulation for 6 times (*P < 0.05, original magnification: ×200). Bar 50 μm. e The BrdU labeling assay was used to detect the cell proliferation ratio. The absorbance showed the proliferation rate. DPSCs from the group of p65 knockdown could reverse the slow growth induced by repeated stimulation with LPS for 6 times (*P < 0.05). f The cell number was determined by counting after exposure to LPS. The population of DPSCs from the group of p65 knockdown reversed compared with the repeated stimulation with LPS for 6 times (*P < 0.05). g Immunofluorescence showing that the abnormal distribution of F-actin in DPSCs after LPS stimulation for 6 times was reversed after p65 knockdown (original magnification ×200). Scale bars: 50 µm (g). (Color figure online)
Discussion
In our experiments, we demonstrated for the first time that TLR4/MyD88-NF-κB-p53/p21 signaling pathway was activated in the senescence of DPSCs treated by LPS. As the expression of TLR4 and MyD88 in an inflammatory microenvironment increased, the expression of the senescence marker SA-β-gal was also increased while the proliferation and the total number of DPSCs were declined and the F-actin distribution was disordered. The above changes were reversed in the p65 siRNA-transfected DPSCs, which siRNA of p65 can weaken the NF-κB signal, and then influence the senescence of DPSCs.
An intimate relationship between inflammation and aging had been demonstrated in previous studies (Tran et al. 2016). Several signaling pathways, such as TLR4/p16INK4A, involved in this process have been reported (Feng et al. 2014). Previous experiments showed that LPS induced the expression of TLR-4 in DPSCs (Botero et al. 2010). DPSCs can be isolated from the inflammatory microenvironment, and the endodontist is the most common disease. DPSCs, from inflamed-pulps, would not lose the potential of tissue regeneration in vivo as well as the periodontal ligament stem cells isolated from the periodontitis tissue (Alongi et al. 2010; Liu et al. 2016). Replicative senescence and stress-induced premature senescence are two main types of senescence, and the latter is the situation at which stimulators such as inflammation or oxidative stress evoke a senescent cell phenotype (Dierick et al. 2002). Not only LPS but TNF-α or other inflammation factors may lead the senescence of stem cells (Qu et al. 2016; Yang et al. 2016). Our experiment tried to explain that the inflammation induced by LPS is a critical effector that leads to the premature senescence of DPSCs. The results also showed that the shape of DPSCs receiving repeated LPS stimulation were disordered observed through F-actin distribution compared to control cells or cells once stimulated. Additionally, as a maker of senescence, more SA-β-gal-positive cells were found after repeated LPS stimulation. Studies demonstrated that SA-β-gal activity was related to the increase of lysosomal enzymes which are probably linked to increased lysosomal biogenesis observed in senescent cells (Kurz et al. 2000; Lee et al. 2006). SA-β-gal was not detectable in quiescent cells or terminally differentiated cells in most situations (Debacq-Chainiaux et al. 2009; Price et al. 2002). Our findings are similar to previous evidence that an inflammatory microenvironment induces cell senescence, and so does it in DPSCs. Therefore, the way of fighting against inflammation will be searched continuously. A recent study found that the immunogenicity of human gingival mesenchymal stem cells would be reduced after treatment by Cannabidiol (Libro et al. 2016). More attention should be paid to promote the ability of multipotential differentiation or other biological function of DPSCs.
Comprehensive evidences suggest that the NF-κB signaling pathway plays an important role in the process of senescence (Rovillain et al. 2011). TLR4 is the pattern recognition receptor for the G− bacterial wall component LPS (Dunzendorfer et al. 2004). Two different pathways, MyD88-dependent and MyD88-independent or TRIF-dependent, were involved in the synthesis and secretion of a variety of inflammatory cytokines after activation of TLR4 signaling (Akira and Takeda 2004; Cinel and Opal 2009; Lu et al. 2008). MyD88 has been considered a kernel adaptor protein in signal transduction pathway of most TLRs and can interact with TLRs, leading to activation of the NF-κB or other pathways (Imai et al. 2008; Smyth et al. 2013). In addition, it has been proven that TLR4/NF-κB signaling seemed to play a role during osteogenic differentiation of adipose-derived mesenchymal stromal cells and proliferation in vitro stimulated by LPS in an appropriate condition (Kim et al. 2016b). More studies should be performed in this aspect to find the positive effect of LPS in clinical application of DPSCs. Recent data have also shown that the expression of the downstream molecules of TLR4/MyD88, such as IκBα which is an inhibitor of NF-κB signaling, would be significantly decreased, while the p65, an active subunit of NF-κB, would translocate into the nucleus (Yu et al. 2016; Zhang et al. 2016). Our results also showed similar changes that confirmed that NF-κB signaling pathway was activated (Fig. 2). After NF-κB signaling activation, p53 and p21, the downstream molecules, transmitted the stimulation of LPS sequentially, and led to correspondingly biological effect (Genov et al. 2016; Gu et al. 2013). Recent studies also suggested that NF-κB induces cell senescence by directly activating p53 and p21 (Ferrand et al. 2015; Rovillain et al. 2011). p53 activation is a typical response observed in cellular and replicative senescence (Beausejour et al. 2003). It had been reported that the p53/p21 pathway was found to play a more important role than the p38 MAPK/p16INK4a pathway in the senescence of CEP cells in vivo and in vitro (Zhou et al. 2016). Our experiment also concluded that p53/p21 pathway was involved in the senescence of DPSCs through TLR4/MyD88-NF-κB signaling stimulated by LPS (Fig. 3). Conversely, we acquired the knockdown p65 with siRNA so that we could test the influence of p65 in DPSCs senescence. Knockdown of p65 (siRNA-p65) could reverse the senescence of DPSCs acted as less SA-β-gal-positive cells, enhancing the proliferation and the increase of the total number of DPSCs with less abnormal distribution of F-actin (Fig. 4).
In summary, our data demonstrate that TLR4/MyD88-NF-κB-p53/p21 performed a link between inflammation led by LPS and senescence. Further studies should be undertaken to prevent senescence so that to extend the DPSCs strategies for the treatment of the diseases surrounding by inflammation.
References
Akira S, Takeda K (2004) Toll-like receptor signalling. Nat Rev Immunol 4:499–511
Alongi DJ, Yamaza T, Song Y, Fouad AF, Romberg EE, Shi S, Tuan RS, Huang GT (2010) Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med 5:617–631
Andonegui G, Zhou H, Bullard D, Kelly MM, Mullaly SC, McDonald B, Long EM, Robbins SM, Kubes P (2009) Mice that exclusively express TLR4 on endothelial cells can efficiently clear a lethal systemic Gram-negative bacterial infection. J Clin Investig 119:1921–1930
Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J (2003) Reversal of human cellular senescence: roles of the p53 and p16 pathways. EMBO J 22:4212–4222
Botero TM, Son JS, Vodopyanov D, Hasegawa M, Shelburne CE, Nor JE (2010) MAPK signaling is required for LPS-induced VEGF in pulp stem cells. J Dent Res 89:264–269
Brennen WN, Denmeade SR, Isaacs JT (2013) Mesenchymal stem cells as a vector for the inflammatory prostate microenvironment. Endocr Relat Cancer 20:R269–R290
Broad A, Kirby JA, Jones DE, Applied Immunology and Transplantation Research Group (2007) Toll-like receptor interactions: tolerance of MyD88-dependent cytokines but enhancement of MyD88-independent interferon-beta production. Immunology 120:103–111
Cinel I, Opal SM (2009) Molecular biology of inflammation and sepsis: a primer. Crit Care Med 37:291–304
D’Alimonte I, Nargi E, Lannutti A, Marchisio M, Pierdomenico L, Costanzo G, Iorio PD, Ballerini P, Giuliani P, Caciagli F, Ciccarelli R (2013) Adenosine A1 receptor stimulation enhances osteogenic differentiation of human dental pulp-derived mesenchymal stem cells via WNT signaling. Stem Cell Res 11:611–624
Debacq-Chainiaux F, Erusalimsky JD, Campisi J, Toussaint O (2009) Protocols to detect senescence-associated beta-galactosidase (SA-betagal) activity, a biomarker of senescent cells in culture and in vivo. Nat Protoc 4:1798–1806
Debelec-Butuner B, Alapinar C, Varisli L, Erbaykent-Tepedelen B, Hamid SM, Gonen-Korkmaz C, Korkmaz KS (2014) Inflammation-mediated abrogation of androgen signaling: an in vitro model of prostate cell inflammation. Mol Carcinog 53:85–97
Dierick JF, Eliaers F, Remacle J, Raes M, Fey SJ, Larsen PM, Toussaint O (2002) Stress-induced premature senescence and replicative senescence are different phenotypes, proteomic evidence. Biochem Pharmacol 64:1011–1017
Dimri GP, Lee X, Basile G, Acosta M, Scott G, Roskelley C, Medrano EE, Linskens M, Rubelj I, Pereira-Smith O et al (1995) A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci USA 92:9363–9367
Dunzendorfer S, Lee HK, Soldau K, Tobias PS (2004) TLR4 is the signaling but not the lipopolysaccharide uptake receptor. J Immunol 173:1166–1170
Faner R, Rojas M, Macnee W, Agusti A (2012) Abnormal lung aging in chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 186:306–313
Feng X, Xing J, Feng G, Sang A, Shen B, Xu Y, Jiang J, Liu S, Tan W, Gu Z, Li L (2013) Age-dependent impaired neurogenic differentiation capacity of dental stem cell is associated with Wnt/beta-catenin signaling. Cell Mol Neurobiol 33:1023–1031
Feng X, Feng G, Xing J, Shen B, Tan W, Huang D, Lu X, Tao T, Zhang J, Li L, Gu Z (2014) Repeated lipopolysaccharide stimulation promotes cellular senescence in human dental pulp stem cells (DPSCs). Cell Tissue Res 356:369–380
Ferrand M, Kirsh O, Griveau A, Vindrieux D, Martin N, Defossez PA, Bernard D (2015) Screening of a kinase library reveals novel pro-senescence kinases and their common NF-κB-dependent transcriptional program. Aging (Albany NY) 7:986–1003
Genov M, Kreiseder B, Nagl M, Drucker E, Wiederstein M, Muellauer B, Krebs J, Grohmann T, Pretsch D, Baumann K, Bacher M, Pretsch A, Wiesner C (2016) Tetrahydroanthraquinone derivative (±)-4-Deoxyaustrocortilutein induces cell cycle arrest and apoptosis in melanoma cells via upregulation of p21 and p53 and downregulation of NF-κB. J Cancer 7:555–568
Gu Z, Jiang J, Xia Y, Yue X, Yan M, Tao T, Cao X, Da Z, Liu H, Liu H, Miao Y, Li L, Wang Z (2013) p21 is associated with the proliferation and apoptosis of bone marrow-derived mesenchymal stem cells from non-obese diabetic mice. Exp Clin Endocrinol Diabetes 121:607–613
Hernandez A, Bohannon JK, Luan L, Fensterheim BA, Guo Y, Patil NK, McAdams C, Wang J, Sherwood ER (2016) The role of MyD88- and TRIF-dependent signaling in monophosphoryl lipid A-induced expansion and recruitment of innate immunocytes. J Leukoc Biol 100:1311–1322. https://doi.org/10.1189/jlb.1A0216-072R
Hilkens P, Gervois P, Fanton Y, Vanormelingen J, Martens W, Struys T, Politis C, Lambrichts I, Bronckaers A (2013) Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res 353:65–78
Huang Y, Jiang H, Gong Q, Li X, Ling J (2015) Lipopolysaccharide stimulation improves the odontoblastic differentiation of human dental pulp cells. Mol Med Rep 11:3547–3552
Imai Y, Kuba K, Neely GG, Yaghubian-Malhami R, Perkmann T, van Loo G, Ermolaeva M, Veldhuizen R, Leung YH, Wang H, Liu H, Sun Y, Pasparakis M, Kopf M, Mech C, Bavari S, Peiris JS, Slutsky AS, Akira S, Hultqvist M, Holmdahl R, Nicholls J, Jiang C, Binder CJ, Penninger JM (2008) Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 133:235–249
Imam F, Al-Harbi NO, Al-Harbi MM, Ansari MA, Zoheir KM, Iqbal M, Anwer MK, Al Hoshani AR, Attia SM, Ahmad SF (2015) Diosmin downregulates the expression of T cell receptors, pro-inflammatory cytokines and NF-κB activation against LPS-induced acute lung injury in mice. Pharmacol Res 102:1–11
Jung YS, Park JH, Kim H, Kim SY, Hwang JY, Hong KW, Bae SS, Choi BT, Lee SW, Shin HK (2016) Probucol inhibits LPS-induced microglia activation and ameliorates brain ischemic injury in normal and hyperlipidemic mice. Acta Pharmacol Sin 37:1031–1044
Kalaiyarasu S, Bhatia S, Mishra N, Sood R, Kumar M, SenthilKumar D, Bhat S, Dass Prakash M (2016) Elevated level of pro inflammatory cytokine and chemokine expression in chicken bone marrow and monocyte derived dendritic cells following LPS induced maturation. Cytokine 85:140–147
Kim JE, Shin JS, Moon JH, Hong SW, Jung DJ, Kim JH, Hwang IY, Shin YJ, Gong EY, Lee DH, Kim SM, Lee EY, Kim YS, Kim D, Hur D, Kim TW, Kim KP, Jin DH, Lee WJ (2016a) Foxp3 is a key downstream regulator of p53-mediated cellular senescence. Oncogene 36:219–230. https://doi.org/10.1038/onc.2016.193
Kim SH, Das A, Chai JC, Binas B, Choi MR, Park KS, Lee YS, Jung KH, Chai YG (2016b) Transcriptome sequencing wide functional analysis of human mesenchymal stem cells in response to TLR4 ligand. Sci Rep 6:30311
Kolf CM, Song L, Helm J, Tuan RS (2015) Nascent osteoblast matrix inhibits osteogenesis of human mesenchymal stem cells in vitro. Stem Cell Res Ther 6:258
Kurz DJ, Decary S, Hong Y, Erusalimsky JD (2000) Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci 113:3613–3622
Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, Kleijer WJ, DiMaio D, Hwang ES (2006) Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell 5:187–195
Libro R, Scionti D, Diomede F, Marchisio M, Grassi G, Pollastro F, Piattelli A, Bramanti P, Mazzon E, Trubiani O (2016) Cannabidiol modulates the immunophenotype and inhibits the activation of the inflammasome in human gingival mesenchymal stem cells. Front Physiol 7:559
Liu X, Tan GR, Yu M, Cai X, Zhou Y, Ding H, Xie H, Qu F, Zhang R, Lam CU, Cui P, Fu B (2016) The effect of tumour necrosis factor-alpha on periodontal ligament stem cell differentiation and the related signaling pathways. Curr Stem Cell Res Ther 11:593–602
Lu YC, Yeh WC, Ohashi PS (2008) LPS/TLR4 signal transduction pathway. Cytokine 42:145–151
Mantovani C, Terenghi G, Magnaghi V (2014) Senescence in adipose-derived stem cells and its implications in nerve regeneration. Neural Regen Res 9:10–15
Marques LC, Pinheiro AJ, Araujo JG, de Oliveira RA, Silva SN, Abreu IC, de Sousa EM, Fernandes ES, Luchessi AD, Silbiger VN, Nicolete R, Lima-Neto LG (2016) Anti-inflammatory effects of a pomegranate leaf extract in LPS-induced peritonitis. Planta Med 82:1463–1467. https://doi.org/10.1055/s-0042-108856
Meng Q, Man Z, Dai L, Huang H, Zhang X, Hu X, Shao Z, Zhu J, Zhang J, Fu X, Duan X, Ao Y (2015) A composite scaffold of MSC affinity peptide-modified demineralized bone matrix particles and chitosan hydrogel for cartilage regeneration. Sci Rep 5:17802
O’Prey J, Crighton D, Martin AG, Vousden KH, Fearnhead HO, Ryan KM (2010) p53-mediated induction of Noxa and p53AIP1 requires NFκB. Cell Cycle 9:947–952
Oren M (2003) Decision making by p53: life, death and cancer. Cell Death Differ 10:431–442
Price JS, Waters JG, Darrah C, Pennington C, Edwards DR, Donell ST, Clark IM (2002) The role of chondrocyte senescence in osteoarthritis. Aging Cell 1:57–65
Qu Y, Zhang Q, Ma S, Liu S, Chen Z, Mo Z, You Z (2016) Interleukin-17A differentially induces inflammatory and metabolic gene expression in the adipose tissues of lean and obese mice. Int J Mol Sci 17:522
Rovillain E, Mansfield L, Caetano C, Alvarez-Fernandez M, Caballero OL, Medema RH, Hummerich H, Jat PS (2011) Activation of nuclear factor-kappa B signalling promotes cellular senescence. Oncogene 30:2356–2366
Rutherford RB, Gu K (2000) Treatment of inflamed ferret dental pulps with recombinant bone morphogenetic protein-7. Eur J Oral Sci 108:202–206
Smyth K, Garcia K, Sun Z, Tuo W, Xiao Z (2013) TLR agonists are highly effective at eliciting functional memory CTLs of effector memory phenotype in peptide immunization. Int Immunopharmacol 15:67–72
Sui BD, Hu CH, Zheng CX, Jin Y (2016) Microenvironmental views on mesenchymal stem cell differentiation in aging. J Dent Res 95:1333-1340. https://doi.org/10.1177/0022034516653589
Tran JR, Chen H, Zheng X, Zheng Y (2016) Lamin in inflammation and aging. Curr Opin Cell Biol 40:124–130
Trial J, Entman ML, Cieslik KA (2016) Mesenchymal stem cell-derived inflammatory fibroblasts mediate interstitial fibrosis in the aging heart. J Mol Cell Cardiol 91:28–34
Wang M, Fu Y, Gao C, Jia Y, Huang Y, Liu L, Wang X, Wang W, Kong W (2016a) Cartilage oligomeric matrix protein prevents vascular aging and vascular smooth muscle cells senescence. Biochem Biophys Res Commun 478:1006–1013. https://doi.org/10.1016/j.bbrc.2016.08.004
Wang XM, Xiao H, Liu LL, Cheng D, Li XJ, Si LY (2016b) FGF21 represses cerebrovascular aging via improving mitochondrial biogenesis and inhibiting p53 signaling pathway in an AMPK-dependent manner. Exp Cell Res 346:147–156
Yang X, Zhang S, Pang X, Fan M (2012) Pro-inflammatory cytokines induce odontogenic differentiation of dental pulp-derived stem cells. J Cell Biochem 113:669–677
Yang R, Wu L, Chen J, Chen W, Zhang L, Zhang L, You R, Yin L, Li CH, Guan YQ (2016) Effects of differentiation and antisenescence from BMSCs to hepatocy-like cells of the PAAm-IGF-1/TNF-alpha biomaterial. ACS Appl Mater Interfaces 8:26638–26647. https://doi.org/10.1021/acsami.6b10377
Yao H, Hu C, Yin L, Tao X, Xu L, Qi Y, Han X, Xu Y, Zhao Y, Wang C, Peng J (2016) Dioscin reduces lipopolysaccharide-induced inflammatory liver injury via regulating TLR4/MyD88 signal pathway. Int Immunopharmacol 36:132–141
Yu HM, Zhao YM, Luo XG, Feng Y, Ren Y, Shang H, He ZY, Luo XM, Chen SD, Wang XY (2012) Repeated lipopolysaccharide stimulation induces cellular senescence in BV2 cells. NeuroImmunoModulation 19:131–136
Yu X, Jin D, Yu A, Sun J, Chen X, Yang Z (2016) p65 down-regulates DEPTOR expression in response to LPS stimulation in hepatocytes. Gene 589:12–19
Zhang W, Yin L, Tao X, Xu L, Zheng L, Han X, Xu Y, Wang C, Peng J (2016) Dioscin alleviates dimethylnitrosamine-induced acute liver injury through regulating apoptosis, oxidative stress and inflammation. Environ Toxicol Pharmacol 45:193–201
Zhao W, Ma G, Chen X (2014) Lipopolysaccharide induced LOX-1 expression via TLR4/MyD88/ROS activated p38MAPK-NF-κB pathway. Vascul Pharmacol 63:162–172
Zhao R, Liu Y, Wang H, Yang J, Niu W, Fan S, Xiong W, Ma J, Li X, Phillips JB, Tan M, Qiu Y, Li G, Zhou M (2016) BRD7 plays an anti-inflammatory role during early acute inflammation by inhibiting activation of the NF-κB signaling pathway. Cell Mol Immunol 14:830–841. https://doi.org/10.1038/cmi.2016.31
Zhou N, Lin X, Dong W, Huang W, Jiang W, Lin L, Qiu Q, Zhang X, Shen J, Song Z, Liang X, Hao J, Wang D, Hu Z (2016) SIRT1 alleviates senescence of degenerative human intervertebral disc cartilage endo-plate cells via the p53/p21 pathway. Sci Rep 6:22628
Zhu G, Chai J, Ma L, Duan H, Zhang H (2013) Downregulated microRNA-32 expression induced by high glucose inhibits cell cycle progression via PTEN upregulation and Akt inactivation in bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun 433:526–531
Funding
This work was supported by Natural Science Foundation of China Grant (Nos. 81500809, 81501076). Jiangsu Province Young Medical Key Talents Project of China (QNRC2016407). Nantong Health Bureau Youth Foundation of China (WQ2015016).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Feng, G., Zheng, K., Cao, T. et al. Repeated stimulation by LPS promotes the senescence of DPSCs via TLR4/MyD88-NF-κB-p53/p21 signaling. Cytotechnology 70, 1023–1035 (2018). https://doi.org/10.1007/s10616-017-0180-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10616-017-0180-6