Abstract
Aim
To evaluate the bond strength (BS) and analysis of the adhesive interface in root canals filled with bioceramic gutta percha sealers and cones.
Material and methods
Ninety-six maxillary canines were divided into eight groups according to the endodontic sealer (AH Plus, AH Plus Bioceramic, Bio-C Sealer or Bio-C Sealer Ion+ and gutta percha cones (conventional or bioceramic) tested. They were analyzed using the BS test, failure pattern, analysis of the adhesive interface by scanning electron microscopy and confocal laser scanning microscopy. The BS data were compared between groups using the analysis of variance test with the Turkey post-test. The chi-square test was used to assess the type of failure and the non-parametric Mann–Whitney and Kruse-Wallis tests (P < 0.05).
Results
Analysis of variance showed higher BS values for the groups of bioceramic gutta percha cones in Bio-C Sealer Ion+ (8.38 ± 4.27), AH Plus Bioceramic (6.19 ± 3.28), Bio-C Sealer (5.70 ± 3.18), AH Plus (4.61 ± 2.11) and for conventional gutta percha cones in AH Plus sealers (4.26 ± 2.35), Bio-C Sealer Ion + (3.63 ± 2.29), Bio-C Sealer (2.94 ± 2.32) and AH Plus Bioceramic (1.19 ± 0.89) (P < 0.05). Relative to the type of failure and adaptation of the types of filling material, a higher percentage of mixed failures was observed (gaps between 1 µm-10 µm) for the group with bioceramic gutta percha cones (P < 0.001).
Conclusion
The bond between sealers and bioceramic gutta percha cones showed higher bond strength values and greater penetration into the dentin tubules.
Clinical relevance
The filling the root canal system with bioceramic sealers should be associated with bioceramic gutta percha cones.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The primary endodontic treatment is considered the option for maintaining inflamed and/or infected teeth because it removes the cause (eg, biofilm and inflamed pulp tissue) and establishes adequate conditions to repair or maintain periapical tissues health [1, 2]. According to the study by Silva et al. [2], it was observed that patients diagnosed with apical periodontitis undergoing primary endodontic treatment had a success rate of 81.1% to 87.4%. Successful endodontic therapy is contingent on the proper disinfection and complete obturation of the root canal system [1].
The epoxy resin-based sealer AH Plus resin sealer is currently widely used due to its excellent physical and handling properties [3, 4]. As a result, it is often used as a benchmark to compare to other formulations. However, there are disadvantages such as mutagenicity, cytotoxicity and hydrophobicity, which specifically reduce the compatibility within hydrophilic root canals [3, 4].
The calcium silicate-based bioceramic sealers contain concentrations of calcium di and trisilicates, alumina, zirconia, bioactive glass, glass ceramics, hydroxyapatite and calcium phosphates in their composition [5,6,7] and setting occurs in the presence of moisture due to contamination by fluids such as blood or saliva [8]. Calcium silicates, hydroxyapatite and calcium phosphates have bioactive characteristics [8,9,10,11,12,13,14,15,16,17,18,19], and osteoinductive potential in bone healing [12] that allow the proliferation of fibroblasts, collagen formation and osteocalcin production [13, 14]. This interaction with biological tissues has been attributed mainly to dissociation in an aqueous medium, with the release of calcium ions [10, 11, 13, 17, 20, 21] and the formation of the apatite layer on the dentin surface [9, 16].
However, despite the bioactive characteristics, the high solubility of calcium silicate-based sealers can result in root canal fillings with gaps that allow the extravasation of fluids and by subproducts of microorganisms to reach the periradicular region [22, 23]. Furthermore, the first generation of calcium silicate-based materials demonstrated a long hardening time [21, 24], low radiopacity (less than 3 mm of aluminum thickness), difficulty in handling and grayish discoloration due to the presence of bismuth oxide, which restricted the use of these materials for filling the root canal system [24].
Thus, with the aim of improving the physicochemical properties of bioceramic sealers, the Bio-C Sealer Ion+ sealer was developed. According to the manufacturer this product is composed of calcium and magnesium silicate, calcium sulfate, potassium sulfate, zirconium (radiopacifying agent) and silicon dioxide. Moreover, considering the low solubility of the AH Plus resin sealer, the development of the AH Plus Bioceramic sealer was proposed, with the aim of conferring bioactive properties on it, and improving its physicochemical properties such as radiopacity and film thickness, with the purpose of reducing the formation of gaps [25] incorporating dimethyl sulfoxide as a filler particle into the traditional composition, tricalcium silicate (5 to 15%), and zirconium dioxide (50–70%) as a radiopacifier [7, 25].
Root canal system filling requires not only a filling sealer but it is also necessary to associate it with gutta percha—a solid material [26]. From the time when bioceramic compounds were introduced into Endodontics, gutta percha cones coated with bioceramic compounds were developed. By chemical affinity, these are able to form a layer of bioactive components that help with the bond between the filling sealer and the root dentin, generating a byproduct of the biomineralization reaction between the dentin wall and calcium and hydroxyl ions [27,28,29,30]. This bioactive layer is responsible for eliminating the voids and fluid penetration at the bond interface [31,32,33,34]. Recently, gutta percha cones were developed. According to their manufacturer, in addition to being coated in the manufacturing process, bioceramic particles were incorporated into them, with the aim of developing a chemical interaction between the gutta percha cones and bioceramic sealers, and leading to their integration with tissues and dentin structures. This formulation was based on previous studies of coated cones, which showed evidence of the formation of a thick biomineralized layer, with a regular and stable morphology, favoring a possible micromechanical anchorage of the bioceramic filling sealer between the gutta percha cone and the root dentin [27,28,29,30].
Therefore, the aim of the present study was to evaluate the bond strength and the adhesive interface of the filling of root canals with coated gutta percha cones, with bioceramic compounds incorporated into them, and associated with epoxy resin-based sealers (AH Plus) and bioceramic-based (Bio-C Sealer, Bio-C Sealer Ion+ and AH Plus Bioceramic). The null hypothesis tested was that there would be no significant difference in (1) bond strength and (2) bond interface quality of bioceramic gutta percha sealers and cones.
Material and methods
This study was approved by the Research Ethics Committee of the Ribeirão Preto School of Dentistry, University of São Paulo (CAAE: 61010022.8.0000.5419). The sample calculation was made by using the SigmaPlot v.12.00 program (Systat Software, San Jose, CA) based on two-tailed parameters, 5% significance level (α = 0.05), 95% confidence interval, 90% statistical power (β = 0.10), 1:1 ratio of specimen allocation in the experimental groups, standard deviation (based on previous results) [35,36,37,38], which indicated the need to include a minimum of 10 specimens in each group. Furthermore, for the confocal laser scanning fluorescence microscopy analysis, 2 teeth were used for each group, totaling 16 samples.
Ninety-six human maxillary canines with a buccolingual/mesiodistal dimension ≤ 1.5 mm and a root length of 16 mm were used, determined by scanning in a PreXion 3D® cone beam computed tomography scanner (Prexion Co. Ltd, Tokyo, Japan), with an endodontic acquisition protocol of 90 kV, 4 mA, 37 s, isotropic voxel of 0.10 mm and field of view of 5 × 5 mm. Two-dimensional morphometric data of circularity and major and minor diameters were obtained using the OnDemand 3D Project Viewer program (Cybermed Inc., Tustin, CA, USA) to determine the degree of flattening of root canals [36,37,38,39,40,41]. The inclusion criteria were established following meticulous observation and cone-beam computed tomography. Only well-developed single-rooted teeth with a Vertucci type I configuration and straight root canals (curvature < 5°), without any prior root canal preparation or obturation, and featuring similar canal size and cross-sectional shape were eligible for selection. Teeth exhibiting cracks, resorptions, caries, or prior root canal treatment were excluded from the study.
An operator who was unaware of the study objectives generated a list of random numbers using the Sealed Envelope website (https://www.sealedenvelope.com/) with a 1:1 allocation ratio and random block sizes of 10. To maintain confidentiality, this list was stored in a file that was kept secure. The list was only opened by a blinded assistant after the teeth were included in the study and prior to the endodontic treatment. Based on the random numbers from the list, each tooth was assigned an enrollment number and then randomly allocated to one of eight groups according to the filling protocol.
After conventional endodontic access to the cavity, the root canals were irrigated with 2.5 mL of 2,5% sodium hypochlorite (NaOCl), applied with a disposable plastic syringe (Ultradent Products Inc., South Jordan, UT, USA). The specimens were explored with a manual type of instrument (Dentsply Maillefer, Ballaigues, Switzerland) until the free extremity of the instrument appeared in the apical foramen. From this measurement, 1.0 mm was subtracted to establish the working length (WL) [36, 38, 41]. Biomechanical preparation was performed with the Wave One Gold Large instrument (45./05) (Wave One Gold, Dentsply Sirona, Ballaigues, Switzerland) in accordance with the manufacturer's recommendation. Subsequently, irrigation was performed with 2 mL of 17% ethylenediaminetetraacetic acid (EDTA) for 5 min, followed by final irrigation with 5 mL of 2.5% NaOCl. The canals were dried using a Capillary Tip suction cannula (Ultradent Products Inc., South Jordan, UT, USA) and with absorbent R50 paper cones (Reciproc, VDW, GmbH, Munich, Germany).
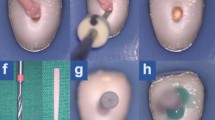
After biomechanical preparation, the specimens were randomly distributed, using the random.org program (http://www.random.org) to form eight experimental groups (n = 10), according to the conventional (Conform Fit, Dentsply Sirona, Ballaigues, Switzerland) or bioceramic gutta percha cones (Angelus, Londrina, Brazil) and epoxy resin-based endodontic sealers (AH Plus; Dentsply DeTrey GmbH, Konstanz, Germany) and calcium silicate-based AH Plus Bioceramic (Dentsply DeTrey GmbH, Konstanz, Germany), Bio-C Sealer (Angelus, Londrina, Brazil), Bio-C Sealer Ion+ (Angelus, Londrina, Brazil) (Fig. 1).
The canals were filled using the single cone technique, and adaptation of the gutta percha cones to the WL was verified by visual, tactile and radiographic means. The sealers were manipulated in accordance with the manufacturers' instructions and they were inserted into the canals with the aid of a manual K #45 file (Dentsply Sirona, Ballaigues, Switzerland) in movements of counterclockwise rotation. The gutta percha cones with endodontic sealer were introduced up to the WL and the excess filling material at the canal entrance was removed with Paiva-type tampers (Golgran, São Paulo, Brazil), followed by vertical condensation of the filling mass with light pressure in the apical direction for 5 s. The canal entrance was temporarily sealed with glass ionomer sealer (Ketac Molar EasyMix; 3M, Maplewood, MN, USA) [36, 38, 41].
Subsequently the teeth were sectioned, obtaining 3 slices of each root third (cervical, middle and apical), with a thickness of approximately 1.0 ± 0.3 mm. The first and second slices of each third were used for testing extrusion of the root-end filling (push-out test); the third slice was used to assess the quality of the bond interface by scanning electron microscopy (SEM) analysis [36, 38, 41].
The slices were placed on metal bases with holes measuring 1.2 mm, 1.5 mm and 2.5 mm in diameter in their central portion and metal rods with an active tip of 0.8 mm, 1 mm and 1.5 mm in diameter, for the cervical, middle and apical thirds, respectively. The specimens were positioned in the same direction as the hole in the metal base, with their cervical surface facing downwards, and the rods were fixed in the upper portion of the testing machine and positioned over the filling material. The testing machine was activated at a constant speed of 0.5 mm/min until the maximum stress required for displacing the filling material was reached [36, 38, 41, 42]. The force required for displacing the filling material was measured in Newtons (N). To calculate the bond strength (BS), the resulting force was converted into Megapascals (MPa) by dividing the extrusion force of the material by the lateral area of the material [36, 38, 41].
For analysis of the failure type, the slices were evaluated with the aid of a Leica M165C stereomicroscope, (Leica Mycrosystems, Mannheim, Germany) at 25 × magnification, and the LAS v4.4 software program (Leica Mycrosystems, Mannheim, Germany). The failures observed were determined in percentages and classified as follows: a) adhesive to dentin: if the filling material was displaced from the dentin; b) adhesive to filling sealer: if the gutta percha was displaced from the filling sealer; c) mixed: if the gutta percha was displaced from both the dentin and the filling sealer; d) cohesive in dentin: if fracture occurred in the dentin; e) cohesive in the filling sealer: if fracture occurred in the filling sealer [36, 38, 41].
Qualitative analysis of the bond interface and of sealer penetration—SEM
For the SEM analysis, the third slice of dentin of each root third (cervical, middle and apical) was used. The preparation for SEM was carried out by polishing the dentin specimens with abrasive paper of decreasing grain size up to 1200 grit. After this, the specimens were rinsed in distilled water and superficially decalcified in 6 M hydrochloric acid (HCl) for 30 s and deproteinized in 2% NaOCl for 10 min [36,37,38, 41]. Subsequently, the specimens were rinsed, dehydrated and fixed on cylindrical aluminum structures (10 × 10 mm) using double-sided adhesive tape according to the methodology described in previous studies [36,37,38], 41]. After vacuum sputter-coating, the specimens were analyzed by a scanning electron microscopy (JSM 5410, JEOL Ltd., Tokyo, Japan) operating at 20 kV.
The photomicrographs were captured at 100x, 250x, and 500 × magnification. In the images captured at 250 × magnification, 12 measurements were performed at equidistant points on the bond interface to identify empty spaces (voids or gaps). According to the methodology described in a previous study [43], adaptation of the filling sealer to the root canal wall was classified according to the following criteria: a) good: the majority of sections did not show any gaps between the sealer and the dentin; b) reasonable: the majority of sections showed some small flaws (< 1 µm) between sealer and dentin; c) poor: the majority of sections showed many gaps (between 1 and 10 µm) between sealer and dentin; d) no adaptation: the majority of sections showed no adaptation between sealer and dentin (gaps > 10 µm) [36,37,38], 41].
Qualitative analysis of the bond interface and of sealer penetration—Confocal laser scanning microscopy (CLSM)
For the confocal laser scanning fluorescence microscopy analysis, the following dyes, respectively, were used at the time of filling: Fluo-3 (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for sealers based on bioceramic compounds (AH Plus Bioceramic, Bio-C Sealer and Bio-C Sealer Ion+) and Rhodamine B (Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) for epoxy resin-based sealer (AH Plus). The 16 samples were used and distributed into eight groups according to the filling protocol.
Subsequently, the samples were sectioned into 1-mm-thick slices, and qualitatively evaluated using laser confocal scanning microscopy with inverted fluorescence Leica TCS-SPS (Leica, Mannheim, Germany). Images of the filled areas were acquired using the epifluorescence mode with absorption and emission wavelengths for Rhodamine B of 553/568 nm and for Fluo-3 of 360/449 nm, respectively, using the Leica Application Suite- Advanced Fluorescence (Leica Systems).
Samples were analyzed 10 m below the sampling surface using an objective lens
with 20 × magnification in a 5 × 5 mm field of view, with a resolution of 512 × 512 pixels. The slices were qualitatively analyzed for each group, subgroup and thirds in which sealer penetration and the density of tags formed were observed.
Statistical analysis
Parametric tests were used for statistical analysis of the bond strength values since they showed normal distribution (Shapiro–Wilk, P > 0.05) and homogeneity of variance (Levene test, P > 0.05). Three-way analysis of variance was used to evaluate the influence of endodontics sealers (AH Plus, AH Plus Bioceramic, Bio-C Sealer and Bio-C Sealer Ion+), root thirds (cervical, middle and apical) and gutta percha cones (conventional and bioceramic) on bond strength values. The Turkey test was used for multiple comparisons between groups (P > 0.05).
The chi-square test was used to assess the type of failure after the bond strength test (P > 0.05). The non-parametric Mann–Whitney and Kruse-Wallis tests (P < 0.05) were used to analyze the data relative to the adaptation of filling material to the dentin wall. All the statistical tests were performed using SPSS v.25 software (IBM, USA) with the significance level set at 95% (P > 0.05).
Results
Table 1 presents the mean and standard deviation values of the bond strength according to the gutta percha cones (conventional and bioceramic) and endodontic sealers (AH Plus, AH Plus Bioceramic, Bio-C Sealer, Bio-C Sealer Ion+).
The results of the analysis of variance showed that filling with bioceramic gutta percha cones associated with bioceramic sealers showed higher bond strength values (Bio-C Sealer Ion+: 8.38 ± 4.27; AH Plus Bioceramic: 6.19 ± 3.28 and Bio-C Sealer: 5.70 ± 3.18) (P < 0.05) (Table 1).
Whereas relative to conventional gutta percha cones, when associated with bioceramic sealers (Bio-C Sealer Ion + : 3.63 ± 2.29; Bio-C Sealer: 2.94 ± 2.32 E AH Plus Bioceramic: 1.19 ± 0.89) (P < 0.05), showed the lowest bond strength values (Table 1).
Whereas the bond strength values of conventional (4.26 ± 2.35) and bioceramic (4.61 ± 2.11) gutta-percha cones associated with AH Plus sealer showed no statistically significant difference between them (P > 0.05) (Table 1).
Relative to the different thirds evaluated, the cervical third showed the highest bond strength values, followed by the middle and apical thirds, irrespective of the gutta percha cone (conventional or bioceramic) or endodontics sealer (epoxy resin-based or bioceramic) evaluated (P < 0.05). When the middle and apical thirds of Bio-C Sealer sealer associated with conventional gutta percha cones were evaluated, no statistically significant differences were observed (P > 0.05) (Table 2).
Table 3 presents the failure pattern data for the different types of gutta percha cones (conventional and bioceramic) in the different endodontic sealers (AH Plus, AH Plus Bioceramic, Bio-C Sealer, Bio-C Sealer Ion+). The chi-square test showed a statistically significant difference with a higher percentage of adhesive failures to the filling material for conventional gutta percha cones associated with AH Plus, AH Plus Bioceramic, Bio-C Sealer, Bio-C Sealer Ion+ sealers, and for the bioceramic gutta percha cones associated with AH Plus sealer (P < 0.001). Whereas for the bioceramic gutta-percha cones associated with AH Plus Bioceramic, Bio-C Sealer and Bio-C Sealer Ion+ sealers, a higher percentage of mixed failures was observed (P < 0.001).
Table 4 presents the scores obtained according to Balguerie et al. [43] based on the SEM images. The non-parametric Kruse-Wallis test showed evidence of statistically significant difference between the different types of gutta percha cones (conventional and bioceramic) and endodontic sealers (AH Plus, AH Plus Bioceramic, Bio-C Sealer, Bio-C Sealer Ion+). The SEM analysis showed evidence of a higher percentage of reasonable failures with gaps smaller than 1 m for the bioceramic gutta percha cones associated with the AH Plus Bioceramic sealer; bad failures with gaps ranging between 1 and 10 m for the conventional gutta percha cones associated with AH Plus sealer, Bio-C Sealer Ion+, Bio-C Sealer and for the bioceramic gutta percha cones associated with the Bio-C Sealer Ion+ sealer. Furthermore, failures greater than 10 μm were observed, with maladaptation at the adhesive interface, for conventional gutta percha cones associated with AH Plus Bioceramic and Bio-C Sealer sealers.
The statistical analysis observed in the evaluation of the experimental groups could be confirmed by the qualitative analysis of the SEM images (Figs. 2, 3 and 4), which allowed the observation of areas of maladaptation (yellow arrows) and adaptation (yellow asterisks) at the bond interface between the gutta percha cone, endodontic sealer and root dentin. In the apical third, irrespective of the Experimental Group, it was possible to observe a predominance of endodontic sealer in the polar areas (Figs. 2C, F, I, L and 3C, F, L). For bioceramic gutta percha cones associated with Bio-C Sealer and Bio-C Sealer Ion+ sealers (3G, 3H, 3I, 3 J, 3 K, 3L) it was possible to observe perfect adaptation at the bond interface, with integrity of the filling margins and the lowest percentage of gaps. SEM analysis at 250 × magnification allowed a more uniform and thinner layer of endodontic sealer to be verified in the specimens filled with gutta percha cones and bioceramic sealers (Fig. 4D, F, H).
Photomicrographs of the bond interfaces of filling material to root dentin in conventional gutta percha cone in different endodontic sealers (100x). A, B, C Bond interface between AH Plus sealer, conventional gutta percha cone and root dentin in the cervical, middle and apical thirds, respectively. D, E, F Bond interface between AH Plus Bioceramic sealer, conventional gutta percha cone and root dentin in the cervical, middle and apical thirds, respectively. G, H, I Bond interface between Bio-C Sealer sealer, conventional gutta percha cone and root dentin in the cervical, middle and apical thirds, respectively. J, K, L Bond interface between Bio-C Sealer Ion+ sealer, conventional gutta percha cone and root dentin in the cervical, middle and apical thirds, respectively. d: dentin; c: endodontic sealer; g: gutta percha; yellow arrows: gaps at the bond interface; yellow asterisks: regions of juxtaposition at the bond interface
Photomicrographs of the bond interfaces of filling material to root dentin in bioceramic gutta percha cone in different endodontic sealers (100x). A, B, C Bond interface between AH Plus sealer, bioceramic gutta percha cone and root dentin in the cervical, middle and apical thirds, respectively. D, E, F Bond interface between AH Plus Bioceramic sealer, bioceramic gutta percha cone and root dentin in the cervical, middle and apical thirds, respectively. G, H, I Bond interface between Bio-C Sealer sealer, bioceramic gutta percha cone and root dentin in the cervical, middle and apical thirds, respectively. J, K, L Bond interface between Bio-C Sealer Ion+ sealer, bioceramic gutta percha cone and root dentin in the cervical, middle and apical thirds, respectively. d: dentin; c: endodontic sealer; g: gutta percha; yellow arrows: gaps at the bond interface; yellow asterisks: regions of juxtaposition at the bond interface
Photomicrographs of the bond interfaces of filling material to root dentin in conventional and bioceramic gutta percha cones in different endodontic sealers (250x). A, B Bond interface between AH Plus sealer, conventional gutta percha cone and root dentin in the cervical, middle and apical thirds, respectively. C, D Bond interface between AH Plus Bioceramic sealer, conventional and bioceramic gutta percha cones, respectively. E, F Bond interface between Bio-C Sealer sealer, conventional and bioceramic gutta percha cones, respectively. G, H Bond interface between Bio-C Sealer Ion+ sealer, conventional and bioceramic gutta percha cones, respectively. d: dentin; c: endodontic sealer; g: gutta percha; yellow arrows: gaps at the bond interface; yellow asterisks: regions of juxtaposition at the bond interface
For the conventional gutta percha cones associated with the AH Plus sealer, the analysis by confocal laser scanning fluorescence microscopy showed greater penetration into the interior of the dentin tubules in a regular and homogeneous manner, with the formation of longer tags (Fig. 5A, B, C). When associated with bioceramic sealers, however, there was no uniform penetration of the sealer into the adhesive interface and there were more empty spaces with the presence of shorter, irregularly shaped tags (Fig. 5D, E, F, G, H, I, J, K, L).
Representative images of confocal laser scanning fluorescence microscopy of the different experimental groups of the conventional gutta percha cone. A, B, C Penetration of the AH Plus sealer into the dentin tubules in the cervical, middle and apical thirds, respectively. D, E, F Penetration of the AH Plus Bioceramic sealer into the dentin tubules in the cervical, middle and apical thirds, respectively. G, H, I Penetration of the Bio-C Sealer sealer into the dentin tubules in the cervical, middle and apical thirds, respectively. J, K, L Penetration of the Bio-C Sealer Ion+ sealer into the dentin tubules into the cervical, middle and apical thirds, respectively
For the bioceramic gutta percha cones associated with the AH Plus sealer, penetration into the interior of the dentin tubules in a regular and homogeneous manner was observed, with the formation of longer tags (Fig. 6A, B, C). Whereas for the bioceramic sealers, regular penetration of the filling sealer into the dentin tubules was observed, with the formation of shorter and less numerous tags, however with the formation of a homogeneous layer between cone/sealer/dentin (Fig. 6D, E, F, G, H, I, J, K, L).
Representative confocal laser scanning fluorescence microscopy images of the different experimental groups of the bioceramic gutta percha cone. A, B, C Penetration of the AH Plus sealer into the dentin tubules in the cervical, middle and apical thirds, respectively. D, E, F Penetration of the AH Plus Bioceramic sealer into the dentin tubules in the cervical, middle and apical thirds, respectively. G, H, I Penetration of the Bio-C Sealer sealer into the dentin tubules in the cervical, middle and apical thirds, respectively. J, K, L
Discussion
In this study an evaluation of the bond strength and analysis of the bond interface was performed in root canals filled with bioceramic gutta percha sealers and cones. The null hypothesis tested was not accepted since the teeth endodontically treated with bioceramic gutta-perch sealers and cones showed higher bond strength values. This was due to interparticle bonds between the bioceramic sealer, bioceramic gutta percha cones and dentin calcium hydroxyapatite during the solidification and hydration reaction, which was able to eliminate empty spaces and fluid penetration at the bond interface.
Relative to the methodology, a push-out test was performed. This allowed evaluation of the mechanical performance of the bond between gutta percha and filling sealer in the cervical, middle and apical thirds. This was followed by complementary analysis of the failure pattern in a stereomicroscope and analysis of the adhesive interface by laser scanning confocal microscopy and scanning electron microscopy, thereby enabling visualization of the endodontic filler sealer layer and evaluation of empty spaces present at the adhesive interface [36, 38, 40, 41, 44,45,46]. Although this test shows limitations regarding the variations to sample thickness, tip and root canal diameter [47, 48], in the present study these variables were standardized, as recommended by Brichko, Burrow & Parashos [49], so that the risk of bias was minimized.
For the analysis under confocal laser scanning microscopy, Fluo-3 was used. This emits fluorescence in the presence of calcium ions corresponding to the specific waveband of the argon laser (488 nm) that detects the presence of calcium silicate-based sealers [28, 50,51,52]. Thus, the calcium present in calcium silicate-based sealers binds to Fluo-3 and its fluorescence, in a green tone observed in the confocal images, and increases according to the stability of the bonds formed. Whereas Rhodamine B is the red fluorescent marker used for the epoxy resin-based filling sealer (AH Plus), since this marker has affinity for humidity and less affinity for calcium ions, due to the leaching phenomenon [28].
Relative to the results obtained, when the bioceramic gutta percha cones associated with bioceramic sealers (AH Plus Bioceramic, Bio-C Sealer and Bio-C Sealer Ion+) were evaluated, higher bond strength values were observed, due to the occurrence of ionic exchanges between the silicates of gutta percha cones and bioceramic sealers during the hydration process. During this process, the hydrates formed by covalent bonds are less soluble and precipitate forming calcium and hydroxyl ions, favoring the formation of hydroxyapatite, which provides the material with greater resistance [53], and probably increases the bond strength to the root canal walls. Moreover, calcium silicate nanoparticles expand inside the dentin tubules after the hydration reaction, thereby allowing physical–mechanical imbrication to occur [46, 54, 55].
According to Roussel [52], based on the hydration reaction, the adhesive interaction of hydroxyapatite to root dentin occurs by integrated bonds between the sealer and bioceramic gutta percha cones, forming a unique network with covalent bonds between the calcium silicates and water, thereby allowing a three-dimensional filling, and consequently, increasing the bond strength [56]. This interaction of the bioceramic material is responsible for the elimination of empty spaces and consequent reduction of fluid penetration into the adhesive interface [31, 34, 46] as observed in the present study in the SEM photomicrographs. This made it possible to verify the more uniform and thinner endodontic sealer layer in the bioceramic gutta percha cones, with a prevalence of mixed failures and the presence of gaps ranging between 1 µm and 10 µm at the adhesive interface. This was also shown in the confocal laser scanning fluorescence microscopy, in which regular penetration of the filling sealer into the dentin tubules was observed since the homogeneous mass of the filling sealer allowed distribution of forces and stresses throughout the root. The mixed failure pattern could be an indicator of bond strength, due to the similarity between the chemical properties of the root dentin, sealers, calcium phosphate and apatite coating of the bioceramic cones [31], suggesting that the endodontic sealer adheres to the bioceramic gutta percha cone and root dentin by means of covalent bonds [53].
Furthermore, more extensive tubular penetration was observed in the cervical third compared to the middle and apical thirds. This corroborates the findings of studies in the literature, in which they reveal that the depth of penetration of the sealer varies in the cervical, middle and apical thirds [34, 57,58,59], since the apical region has the lowest number and smallest diameter of dentin tubules per square millimeter [60, 61]. Whereas Osiri et al. [32] observed that filling root canals with sealer and bioceramic gutta percha cones provided higher bond strength values in the apical area and better sealer penetration into the cervical, middle, and apical thirds than canals filled with AH Plus and conventional gutta percha cones.
Penetration of the bioceramic sealer is influenced by the physicochemical properties of the sealers, such as solubility and dimensional alteration, the filling technique, anatomy of the root canal system, number and diameter of dentin tubules [28]. Penetration of bioceramic sealers into the dentin tubules occurs by means of mechanical and chemical imbrication, thereby reducing microleakage and favoring the chemical formation of hydroxyapatite [62]. Differences in the chemical composition of bioceramic sealers may effect its interaction with root dentin [46, 63], as observed in the present study, in which the magnesium and potassium ions present in the chemical composition of the Bio-C Sealer Ion+ sealer, allowed greater compatibility and chemical bonding with the phosphate ions of root dentin, favoring bond strength and less formation of gaps, in agreement with the study by Janini et al. [19].
Whereas the group of conventional gutta percha cones with bioceramic sealers (AH Plus Bioceramic, Bio-C Sealer and Bio-C Sealer Ion+) showed lower bond strength values and a thicker filling line. These factors probably caused an increase in stress, thereby generating gaps greater than 10 µm observed in the qualitative-quantitative evaluation of the specimens by SEM. Thus, there was no occurrence of uniform penetration of sealer into the adhesive interface leading to more empty spaces with the presence of shorter, irregular-shaped tags. In this case, the bonding process between the calcium silicate particles of the bioceramic sealer to the conventional gutta percha cones occurred only by means of interparticle adhesion of the Bronsted-Lorry acid–base type, with low chemical interaction and physical anchorage by roughness between the surfaces of materials [64]. These results corroborate with Cimpean et al. [46], which also observed in their study, that the most prevalent failure pattern was adhesive to filling material, suggesting limited chemical bonding between endodontic filling sealer and root dentin.
For the groups filled with epoxy resin-based sealer, irrespective of the type of gutta percha cone (conventional or bioceramic), similar results were observed in terms of bond strength, failure pattern, analysis of the bond interface and intratubular penetration. Interaction between the latex of conventional gutta percha cones and the organic solvents present in the chemical composition of epoxy resin-based sealers, resulted in an interaction with greater solubilization of the filling material, mainly when there was a thicker layer of sealer [65], as observed in the single cone filling technique performed in the present study. Moreover, the interaction between the bioceramic gutta percha cone (inorganic compound) and the epoxy resin-based sealer (low polarity organic compound) was weak due to the incompatibility between the materials, and thus, the polymerization reaction of the sealer would occur differently in an aqueous media [64], weakening the filling material bond to root dentin. This was contrary to the situation observed when using bioceramic gutta percha sealers and cones, in which there was greater interaction and chemical compatibility.
It is pointed out that the degree of moisture in the root canal system [46, 66, 67], suggested by the manufacturers when using bioceramic materials, may interfere with the penetration of the sealer into complex anatomies of the root canal system, such as polar areas, isthmuses, dentin tubules. This allowed less anchorage of the filling material to the dentin walls, and consequently, led to lower bond strength values [15, 67]. SEM photomicrographs and on focal laser scanning fluorescence microscopy made it possible to verify a uniform, thin layer of AH Plus sealer on conventional and bioceramic cones, with a prevalence of adhesive failures in the filling material and the presence of gaps ranging between 1 µm and 10 µm at the adhesive interface. Thus, more extensive penetration into the dentin tubules occurred in a regular and homogeneous way with the formation of longer tags.
Thus, based on the results of the present study, it can be concluded that filling the root canal system with bioceramic sealers should be associated with bioceramic gutta percha cones, with the aim of obtaining higher bond strength values of the filling material to the root dentin, and greater adaptation of at the adhesive interface formed between the sealer, gutta percha cone and dentin wall. Furthermore, it is essential to highlight that, although these results provide valuable insights into the materials and their bonding properties, clinical studies should be conducted to establish the direct clinical significance of these findings.
Conclusion
In conclusion, the adhesive interface formed between sealers and bioceramic gutta percha cones showed higher bond strength values and greater penetration into the dentin tubules. The bond between the Bio-C Sealer Ion+ endodontic sealer and the bioceramic gutta percha cone was the most effective.
Data Availability
Data may be provided upon request.
References
Alves Dos Santos GN, Faria-E-Silva AL, Ribeiro VL et al (2022) Is the quality of root canal filling obtained by cone-beam computed tomography associated with periapical lesions? A systematic review and meta-analysis. Clin Oral Investig 26:5105–5116. https://doi.org/10.1007/s00784-022-04558-y
da Silva TA, de Araújo LP, Gobbo LB et al (2023) Outcome of root canal treatment of teeth with asymptomatic apical periodontitis treated with foraminal enlargement and 2% chlorhexidine gel: a retrospective cohort study. J Endod 49:972–979. https://doi.org/10.1016/j.joen.2023.06.005
Shieh K, Yang J, Zhu EH, Peters OA, Hosseinpour S (2023) Dentinal tubule penetrability and bond strength of two novel calcium silicate-based root canal sealers. Materials (Basel) 16:3309. https://doi.org/10.3390/ma16093309
Chang SW, Lee YK, Zhu Q et al (2015) Comparison of the rheological properties of four root canal sealers. Int J Oral Sci 7:56–61. https://doi.org/10.1038/ijos.2014.33
Loushine BA, Bryan TE, Looney SW et al (2011) Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J Endod 37:673–677. https://doi.org/10.1016/j.joen.2011.01.003
Singh G, Gupta I, Elshamy FMM, Boreak N, Homeida HE (2016) In vitro comparison of antibacterial properties of bioceramic-based sealer, resin-based sealer and zinc oxide eugenol based sealer and two mineral trioxide aggregates. Eur J Dent 10:366–369. https://doi.org/10.4103/1305-7456.184145
Donnermeyer D, Schemkämper P, Bürklein S, Schäfer E (2022) Short and long-term solubility, alkalizing effect, and thermal persistence of premixed calcium silicate-based sealers: AH plus bioceramic sealer vs total fill BC sealer. Materials (Basel) 15:7320. https://doi.org/10.3390/ma15207320
Bilvinaite G, Drukteinis S, Brukiene V, Rajasekharan S (2022) Immediate and long-term radiopacity and surface morphology of hydraulic calcium silicate-based materials. Materials (Basel) 15:6635. https://doi.org/10.3390/ma15196635
Gandolfi MG, Ciapetti G, Taddei P et al (2010) Apatite formation on bioactive calcium-silicate cements for dentistry affects surface topography and human marrow stromal cells proliferation. Dent Mater 26:974–992. https://doi.org/10.1016/j.dental.2010.06.002
Gandolfi MG, Spagnuolo G, Siboni F et al (2015) Calcium silicate/calcium phosphate biphasic cements for vital pulp therapy: chemical-physical properties and human pulp cells response. Clin Oral Investig 19:2075–2089. https://doi.org/10.1007/s00784-015-1443-2
Pedano MS, Li X, Yoshihara K, Landuyt KV, Van Meerbeek B (2020) Cytotoxicity and bioactivity of dental pulp-capping agents towards human tooth-pulp cells: a systematic review of in-vitro studies and meta-analysis of randomized and controlled clinical trials. Materials (Basel) 13:2670. https://doi.org/10.3390/ma13122670
Gandolfi MG, Iezzi G, Piattelli A, Prati C, Scarano A (2017) Osteoinductive potential and bone-bonding ability of ProRoot MTA, MTA plus and biodentine in rabbit intramedullary model: Microchemical characterization and histological analysis. Dent Mater 33:e221–e238. https://doi.org/10.1016/j.dental.2017.01.017
Sanz JL, López-García S, Lozano A et al (2021) Microstructural composition, ion release, and bioactive potential of new premixed calcium silicate-based endodontic sealers indicated for warm vertical compaction technique. Clin Oral Investig 25:1451–1462. https://doi.org/10.1007/s00784-020-03453-8
Huang CY, Huang TH, Kao CT, Wu YH, Chen WC, Shie MY (2017) Mesoporous calcium silicate nanoparticles with drug delivery and odontogenesis properties. J Endod 43:69–76. https://doi.org/10.1016/j.joen.2016.09.012
Dewi A, Upara C, Sastraruji T, Louwakul P (2022) Effect of a heat-based root canal obturation technique on push-out bond strength of the classical bioceramic and new HiFlow sealer. Aust Endod J 48:116–120. https://doi.org/10.1111/aej.12602
Sanz JL, López-García S, Rodríguez-Lozano FJ et al (2022) Cytocompatibility and bioactive potential of AH plus bioceramic sealer: an in vitro study. Int Endod J 55:1066–1080. https://doi.org/10.1111/iej.13805
Queiroz MB, Inada RNH, Jampani JLA et al (2023) Biocompatibility and bioactive potential of an experimental tricalcium silicate-based cement in comparison with Bio-C repair and MTA Repair HP materials. Int Endod J 56:259–277. https://doi.org/10.1111/iej.13863
Souza LC, Neves GST, Kirkpatrick T, Letra A, Silva R (2023) Physicochemical and biological properties of AH plus bioceramic. J Endod 49:69–76. https://doi.org/10.1016/j.joen.2022.10.009
Janini ACP, Pelepenko LE, Boldieri JM et al (2023) Biocompatibility analysis in subcutaneous tissue and physico-chemical analysis of pre-mixed calcium silicate-based sealers. Clin Oral Investig 27:2221–2234. https://doi.org/10.1007/s00784-023-04957-9
Abu Zeid ST, Alnoury A (2023) Characterisation of the bioactivity and the solubility of a new root canal sealer. Int Dent J S0020–6539:00070–00079. https://doi.org/10.1016/j.identj.2023.04.003
Rabello CZ, Kopper PMP, Ferri LJM et al (2022) Physicochemical properties of three bioceramic cements. Braz Oral Res 36:e069. https://doi.org/10.1590/1807-3107bor-2022.vol36.0069
Aminoshariae A, Kulild JC (2020) The impact of sealer extrusion on endodontic outcome: a systematic review with meta-analysis. Aust Endod J 46:123–129. https://doi.org/10.1111/aej.12370
Aminoshariae A, Primus C, Kulild JC (2022) Tricalcium silicate cement sealers: do the potential benefits of bioactivity justify the drawbacks? J Am Dent Assoc 153:750–760. https://doi.org/10.1016/j.adaj.2022.01.004
Camilleri J, Gandolfi MG (2010) Evaluation of the radiopacity of calcium silicate cements containing different radiopacifiers. Int Endod J 43:21–30. https://doi.org/10.1111/j.1365-2591.2009.01621.x
Zamparini F, Prati C, Taddei P, Spinelli A, Di Foggia M, Gandolfi MG (2022) Chemical-physical properties and bioactivity of new premixed calcium silicate-bioceramic root canal sealers. Int J Mol Sci 23:13914. https://doi.org/10.3390/ijms232213914
Bhandi S, Mashyakhy M, Abumelha AS et al (2021) Complete obturation-cold lateral condensation vs. thermoplastic techniques: a systematic review of micro-CT studies. Materials (Basel) 14:4013. https://doi.org/10.3390/ma14144013
Khalil I, Naaman A, Camilleri J (2016) Properties of tricalcium silicate sealers. J Endod 42:1529–1535. https://doi.org/10.1016/j.joen.2016.06.002
Jeong JW, DeGraft-Johnson A, Dorn SO, Di Fiore PM (2017) Dentinal tubule penetration of a calcium silicate-based root canal sealer with different obturation methods. J Endod 43:633–637. https://doi.org/10.1016/j.joen.2016.11.023
Villa N, Santos VVD, Costa UMD et al (2020) A new calcium silicate-based root canal dressing: physical and chemical properties, cytotoxicity and dentinal tubule penetration. Braz Dent J 31:598–604. https://doi.org/10.1590/0103-6440202003376
Guerreiro JCM, Ochoa-Rodrígez VM, Rodrigues EM et al (2021) Antibacterial activity, cytocompatibility and effect of Bio-C Temp bioceramic intracanal medicament on osteoblast biology. Int Endod J 54:1155–1165. https://doi.org/10.1111/iej.13502
Al-Haddad AY, Kutty MG, Che Ab Aziz ZA (2018) Push-out bond strength of experimental apatite calcium phosphate based coated gutta-percha. Int J Biomater 1:1731857. https://doi.org/10.1155/2018/1731857
Osiri S, Banomyong D, Sattabanasuk V, Yanpiset K (2018) Root Reinforcement after obturation with calcium silicate-based sealer and modified gutta-percha cone. J Endod 44:1843–1848. https://doi.org/10.1016/j.joen.2018.08.011
Rajda M, Miletić I, Baršić G, Krmek SJ, Šnjarić D, Baraba A (2021) Efficacy of reciprocating instruments in the removal of bioceramic and epoxy resin-based sealers: micro-CT analysis. Materials (Basel) 14:6670. https://doi.org/10.3390/ma14216670
Casino Alegre A, Aranda Verdú S, Zarzosa López JI, Plasencia Alcina E, Rubio Climent J, PallarésSabater A (2022) Intratubular penetration capacity of HiFlow bioceramic sealer used with warm obturation techniques and single cone: a confocal laser scanning microscopic study. Heliyon 8:e10388. https://doi.org/10.1016/j.heliyon.2022.e10388
Pereira RD, Brito-Júnior M, Leoni GB, Estrela C, de Sousa-Neto MD (2017) Evaluation of bond strength in single-cone fillings of canals with different cross-sections. Int Endod J 50:177–183. https://doi.org/10.1111/iej.12607
Bertolini GR, Alves Dos Santos GN, Paula-Silva FWG et al (2022) Impact of the removal of filling material from the post space with ultrasonic insert and magnification with a surgical microscope on the bond strength and adhesive interface of multifilament fiberglass posts onto flat-oval root canals. J Mech Behav Biomed Mater 132:105264. https://doi.org/10.1016/j.jmbbm.2022.105264
Rosa E, Silva VL, Silva FASD, Alves Dos Santos GN et al (2022) The impact of provisional intraradicular retainers cementation with temporary methacrylate-based resin in the bond strength of glass fiber posts to root dentin. J Mech Behav Biomed Mater 135:105486. https://doi.org/10.1016/j.jmbbm.2022.105486
Alves Dos Santos GN, Silva-Sousa YTC, Alonso ALL et al (2023) Evaluation of the push-out bond strength of an adjustable fiberglass post system to an endodontically treated oval root canal [published online ahead of print] Dent Mater J. https://doi.org/10.4012/dmj.2022-248
Versiani MA, Pécora JD, de Sousa-Neto MD (2011) Flat-oval root canal preparation with self-adjusting file instrument: a micro-computed tomography study. J Endod 37:1002–1007. https://doi.org/10.1016/j.joen.2011.03.017
Assis RS, Lopes FC, Roperto R et al (2020) Bond strength and quality of bond interface of multifilament fiberglass posts luted onto flat-oval root canals without additional dentin wear after biomechanical preparation. J Prosthet Dent 124:738.e1-738.e8. https://doi.org/10.1016/j.prosdent.2020.04.021
Lopes FC, Roperto R, Akkus A, de Queiroz AM, Francisco de Oliveira H, Sousa-Neto MD (2020) Effect of carbodiimide and chlorhexidine on the bond strength longevity of resin cement to root dentine after radiation therapy. Int Endod J 53:539–552. https://doi.org/10.1111/iej.13252
Zanatta RF, Barreto Bde C, Xavier TA, Versluis A, Soares CJ (2015) Effect of punch and orifice base sizes in different push-out test setups: stress distribution analysis. J Adhes Dent 17:45–50. https://doi.org/10.3290/j.jad.a33516
Balguerie E, van der Sluis L, Vallaeys K, Gurgel-Georgelin M, Diemer F (2011) Sealer penetration and adaptation in the dentinal tubules: a scanning electron microscopic study. J Endod 37:1576–1579. https://doi.org/10.1016/j.joen.2011.07.005
Paiola FG, Lopes FC, Mazzi-Chaves JF et al (2018) How to improve root canal filling in teeth subjected to radiation therapy for cancer. Braz Oral Res 32:e121. https://doi.org/10.1590/1807-3107bor-2018.vol32.0121
Yamin PA, Pereira RD, Lopes FC et al (2018) Longevity of bond strength of resin cements to root dentine after radiation therapy. Int Endod J 2018;51(11):1301–1312. https://doi.org/10.1111/iej.12945
Cimpean SI, Burtea ALC, Chiorean RS et al (2022) Evaluation of bond strength of four different root canal sealers. Materials (Basel) 15:4966. https://doi.org/10.1016/j.heliyon.2022.e10388
Reddy P, Neelakantan P, Sanjeev K, Matinlinna J (2018) Effect of irrigant neutralizing reducing agents on the compromised dislocation resistance of an epoxy resin and a methacrylate resin-based root canal sealer in vitro. Int J Adhes Adhes 82:206–210. https://doi.org/10.1016/j.ijadhadh.2018.01.018
Marques Ferreira M, Martinho JP, Duarte I et al (2022) Evaluation of the sealing ability and bond strength of two endodontic root canal sealers: an in vitro study. Dent J (Basel) 10:201. https://doi.org/10.3390/dj10110201
Brichko J, Burrow MF, Parashos P (2018) Design variability of the push-out bond test in endodontic research: a systematic review. J Endod 44:1237–1245. https://doi.org/10.1016/j.joen.2018.05.003
Paredes RM, Etzler JC, Watts LT, Zheng W, Lechleiter JD (2008) Chemical calcium indicators. Methods 46:143–151. https://doi.org/10.1016/j.ymeth.2008.09.025
Candeiro GT, Correia FC, Duarte MA, Ribeiro-Siqueira DC, Gavini G (2012) Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J Endod 38:842–845. https://doi.org/10.1016/j.joen.2012.02.029
Martins MP, de Andrade FB, Bramante CM et al (2022) Effect of obturation technique on penetration of calcium silicate-based sealer into dentinal tubules after endodontic retreatment of mandibular premolars. Clin Oral Investig 26:7143–7148. https://doi.org/10.1007/s00784-022-04675-8
Roussel N (2011) Understanding the rheology of concrete. Woodhead Publishing; 1ª eddition.
Zhou HM, Shen Y, Zheng W, Li L, Zheng YF, Haapasalo M (2013) Physical properties of 5 root canal sealers. J Endod 39:1281–1286. https://doi.org/10.1016/j.joen.2013.06.012
McMichael GE, Primus CM, Opperman LA (2016) Dentinal tubule penetration of tricalcium silicate sealers. J Endod 42:632–636. https://doi.org/10.1016/j.joen.2015.12.012
Huang G, Liu SY, Qiu D, Dong YM (2023) Effect of a bioactive glass-based root canal sealer on root fracture resistance ability. J Dent Sci 18:27–33. https://doi.org/10.1016/j.jds.2022.08.004
Gharib SR, Tordik PA, Imamura GM, Baginski TA, Goodell GG (2007) A confocal laser scanning microscope investigation of the epiphany obturation system. J Endod 33:957–961. https://doi.org/10.1016/j.joen.2007.03.011
Kara Tuncer A, Tuncer S (2012) Effect of different final irrigation solutions on dentinal tubule penetration depth and percentage of root canal sealer. J Endod 38:860–863. https://doi.org/10.1016/j.joen.2012.03.008
Akcay M, Arslan H, Durmus N, Mese M, Capar ID (2016) Dentinal tubule penetration of AH Plus, iRoot SP, MTA fillapex, and guttaflow bioseal root canal sealers after different final irrigation procedures: a confocal microscopic study. Lasers Surg Med 48:70–6. https://doi.org/10.1002/lsm.22446
Bolles JA, He J, Svoboda KK, Schneiderman E, Glickman GN (2013) Comparison of vibringe, EndoActivator, and needle irrigation on sealer penetration in extracted human teeth. J Endod 39:708–711. https://doi.org/10.1016/j.joen.2013.01.006
Mobaraki B, Yeşildal Yeter K (2020) Quantitative analysis of SmearOFF and different irrigation activation techniques on removal of smear layer: a scanning electron microscope study. Microsc Res Tech 83:1480–1486. https://doi.org/10.1002/jemt.23541
Escobar PM, Silva-Sousa AC, Camargo RV et al (2023) Influence of bioceramic intracanal medication on the bond strength of bioceramic root canal sealer. Braz Oral Res 37:e056. https://doi.org/10.1590/1807-3107bor-2023.vol37.0056
Kebudi Benezra M, Schembri Wismayer P, Camilleri J (2018) Interfacial characteristics and cytocompatibility of hydraulic sealer cements. J Endod 44:1007–1017. https://doi.org/10.1016/j.joen.2017.11.011
Granier V, Sartre A (1995) Ordering and adhesion of latex particles on model inorganic surfaces. Langmuir 11:2179–2186
Ha JY, Son JS, Kim KH, Kwon TY (2015) Simple heat treatment of zirconia ceramic pre-treated with silane primer to improve resin bonding. J Nanosci Nanotechnol 15:587–590. https://doi.org/10.1166/jnn.2015.8359
Hammad M, Qualtrough A, Silikas N (2008) Extended setting shrinkage behavior of endodontic sealers. J Endod 34:90–93. https://doi.org/10.1166/jnn.2015.8359
Atmeh AR, AlShwaimi E (2017) The effect of heating time and temperature on epoxy resin and calcium silicate-based endodontic sealers. J Endod 43:2112–2118. https://doi.org/10.1016/j.joen.2017.08.008
Acknowledgements
The authors gratefully acknowledge financial support from Coordination for the Improvement of Higher Education Personnel, CAPES Brazil, nº 33002029032P4 and São Paulo Research Foundation, FAPESP, nº 2018/14450-1.
Funding
The work was supported by Coordination for the Improvement of Higher Education Personnel, CAPES Brazil, nº 33002029032P4 and by São Paulo Research Foundation, FAPESP, nº 2018/14450–1.
Author information
Authors and Affiliations
Contributions
Sérgio A.L. Quaresma, Guilherme N. Alves dos Santos and Alice C. Silva-Sousa: Conceptualization, methodology, data curation, formal analysis, investigation, writing – original draft, project administration. Rafael V. Camargo: Data curation, formal analysis, investigation. Jardel F. Mazzi-Chaves, Yara T. Silva-Sousa, and Fabiane C. Lopes-Olhê: Visualization, investigation, writing-review & editing. Manoel D. Sousa-Neto: Conceptualization, methodology, visualization, investigation, writing-review & editing, supervision, project administration.
Corresponding author
Ethics declarations
Ethical approval
This study was approved by the Research Ethics Committee of the Ribeirão Preto School of Dentistry, University of São Paulo (CAAE: 61010022.8.0000.5419).
Informed consent
For this type of study, formal consent is not required. Informed consent was obtained from all individual participants included in the study.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Quaresma, S.A.L., Alves dos Santos, G.N., Silva-Sousa, A.C. et al. Influence of bioceramic cones on the quality of root canal filling relative to bond strength and adaptation of the adhesive interface. Clin Oral Invest 27, 7919–7933 (2023). https://doi.org/10.1007/s00784-023-05385-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05385-5