Abstract
Objective
The aim of this study was to evaluate the microstructural composition, ion release, cytocompatibility, and mineralization potential of Bio-C Sealer ION+ (BCI) and EndoSequence BC Sealer HiFlow (BCHiF), compared with AH Plus (AHP), in contact with human periodontal ligament cells (hPDLCs).
Materials and methods
The sealers’ ionic composition and release were assessed using energy-dispersive spectroscopy (EDS) and inductively coupled plasma mass spectrometry (ICP-MS), respectively. For the biological assays, hPDLCs were isolated from third molars, and sealer extracts were prepared (undiluted, 1:2, and 1:4 ratios). An MTT assay, wound-healing assay, and cell morphology and adhesion analysis were performed. Activity-related gene expression was determined using RT-qPCR, and mineralization potential was assessed using Alizarin Red staining (ARS). Statistical analyses were performed using one-way ANOVA and Tukey’s post hoc test (α < 0.05).
Results
The three sealers exhibited variable levels of silicon, calcium, zirconium, and tungsten release and in their composition. Both BCI and BCHiF groups showed positive results in cytocompatibility assays, unlike AHP. The BCHiF group showed an upregulation of CAP (p < 0.01), CEMP1, ALP, and RUNX2 (p < 0.001) compared with the negative control, while the BCI group showed an upregulation of CEMP1 (p < 0.01), CAP, and RUNX2 (p < 0.001). Both groups also exhibited a greater mineralization potential than the negative and positive controls (p < 0.001).
Conclusions
The calcium silicate–based sealers considered in the present in vitro study exhibited a high calcium ion release, adequate cytocompatibility, upregulated osteo/cementogenic gene expression, and increased mineralized nodule formation in contact with hPDLCs.
Clinical relevance
From a biological perspective, BCI and BCHiF could be clinically suitable for root canal filling.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endodontic procedures require the use of materials with specific biological properties, in order to increase their predictability and improve the prognosis of the affected tooth [1]. Regarding root canal treatment, endodontic sealers will be placed in direct contact with both organic and inorganic tissue, with a substantial cellular component. Thus, these biomaterials should at least exhibit an absence of cytotoxicity towards surrounding tissues [2] and ideally promote an active biological response that favors cell survival, differentiation, and consequent tissue repair or neoformation [3].
Within endodontic sealers, those with calcium silicate–based compositions have shown high clinical success rates in root canal treatment [4, 5] and bioactive properties in vitro [6,7,8]. Their ion release and interchange with tissue fluids result in the formation of a hydroxyapatite-like layer on their surface, and a consequent mineral attachment to the inorganic component of dentine [9, 10].
The complex healing process of any pre-existent periapical lesion after root canal disinfection and sealing depends on the reparative potential of surrounding cells and tissues [11]. Current investigations with regard to endodontic sealers have centered on their interaction with dental stem cells (DSCs). Since the characterization of DSCs from different local sources [12,13,14] and the description of their active role in reparative dentinogenesis [15, 16], properties like cytocompatibility have become essential for endodontic sealers. Among DSCs, periodontal ligament stem cells (PDLSCs) have been shown to contribute to the repair and regeneration of the periodontium and other tissues [17].
Most recently, a new hydraulic calcium silicate–based endodontic sealer (hCSS), Bio-C Sealer ION+ (Angelus, Londrina, PR, Brazil), has been released. Along with other silicate-based endodontic sealers such as EndoSequence BC Sealer HiFlow (Brasseler, Savannah, GA, USA), Bio-C Sealer ION+ is presented in a pre-mixed ready-to-use format that can be used for both cold and warm vertical obturation techniques, according to the manufacturer. Recent evidence suggests that the traditional epoxy resin–based sealer AH Plus (Dentsply, Konstanz, Germany) could also be suitable for warm techniques [18]. Differences in biomaterial composition may lead to variable ionic interactions with surrounding tissues and cellular responses [19, 20]. To the authors’ knowledge, there is no evidence about the biological properties of the newly introduced Bio-C Sealer ION+, compared with traditional and well-established endodontic sealers which share its clinical indications.
Accordingly, the aim of this study was to determine the microstructural composition, ion release, and evaluate the cytocompatibility and mineralization potential of Bio-C Sealer ION+ and EndoSequence BC Sealer HiFlow, compared with AH Plus, in contact with human periodontal ligament cells (hPDLCs). The null hypothesis was that there is no difference between the tested materials in relation to their microstructural composition, ion release, cytocompatibility, and mineralization potential on hPDLCs.
Materials and methods
Cell isolation, culture, and characterization
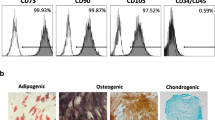
The present in vitro study was formerly approved by the Ethics Committee from the Universidad de Murcia (ID: 2199/2018), following the Helsinki Declaration guidelines. Human PDLCs were isolated from healthy extracted third molars (n = 10) and cells at passages 2–4 were used for consecutive experimentation, as described previously [6]. In brief, third molars were transported in Minimum Essential Medium with Alpha modifications (α-MEM; Gibco, Invitrogen, Waltham, MA, USA) supplemented with 1% penicillin/streptomycin (Sigma-Aldrich, St. Louis, MO, USA) and amphotericin B (Fungizone; Sigma-Aldrich, USA), at a temperature of 4 °C. The teeth were then rinsed thrice with phosphate-buffered saline (PBS) and their periodontal tissues were scraped from the middle and apical thirds of the root surface. Tissues were then sliced into smaller fragments and digested with collagenase type I solution (Gibco, USA) for 1 h at 37 °C. Finally, periodontal cells were seeded in α-MEM supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, USA) and 1% penicillin/streptomycin (Sigma-Aldrich, USA). Prior to their use, hPDLCs were characterized using flow cytometry (FACSCalibur Flow Cytometry System; BD Biosciences, San José, CA, USA) and the high expression of the mesenchymal stem cell (MSC)–specific surface markers CD73, CD90, and CD105, and low expression of the hematopoietic markers CD34, CD45, CD14, and CD20 were confirmed [6].
Preparation of BC-Sealer ION+, EndoSequence HiFlow, and AH Plus extracts
For the in vitro assays, the hCSSs Bio-C Sealer ION+ (BCI; Angelus, Londrina, PR, Brazil) and EndoSequence BC Sealer HiFlow (BCHiF; Brasseler, Savannah, GA, USA) were used, along with the epoxy resin–based sealer AH Plus (AHP; Dentsply, Konstanz, Germany). Their respective compositions, as stated by their manufacturers, are listed in Table 1.
The materials were prepared according to their manufacturers’ instructions under sterile conditions. To allow for a complete setting of the sealers, they were placed individually in 2-mm-depth and 5-mm-diameter casts with Hank’s balanced salt solution (HBSS; H6648; Sigma-Aldrich, Gillingham, UK), sterilized under UV radiation for 15 min, and stored in an incubator at 37 °C, 5% CO2, and 95% humidity for 48 h (n = 30). After the setting time, sample discs were incubated in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, USA) for 24 h at the previously mentioned conditions, achieving a sample surface area/medium ratio of 1.5 cm2/mL. Sample preparation and conditioning for in vitro experimentation were carried out in accordance with the current International Organization for Standardization (ISO) guidelines [21]. For the cell viability, migration, RT-qPCR, and mineralization assays, material extracts were filtered (0.22 μ pore size) and left undiluted, diluted to 1:2, and diluted to 1:4 ratios.
Scanning electronic microscopy and energy-dispersive spectroscopy
Two-millimeter-high and 5-mm-diameter sample discs for BCI, BCHiF, and AHP were prepared using the aforementioned process (n = 9), with a sample surface area/HBSS ratio of 6 cm2/mL. After 48 h of setting in the previously described conditions, i.e., 37 °C, 5% CO2, and 95% humidity, the discs underwent a carbon-coating process in a CC7650 SEM Carbon Coater unit (Quorum Technologies Ltd., East Sussex, UK). Then, the coated discs were examined individually under a SEM unit (Jeol 6100 EDAX; Jeol Inc., Peabody, MA, USA) attached to an EDS system (INCA 350 EDS; Oxford Instruments, Abingdon, UK) for element analysis.
Inductively coupled plasma mass spectrometry
Three sample discs with the previously described dimensions were immersed in 5-mL purified water (Milli-Q; Merck KGaA, Darmstadt, Germany) for 7 days and the proportion of calcium, iron, zirconium, silicon, and tungsten release from each sealer was determined by means of ICP-MS (Agilent 7900 ICP-MS; Agilent, Santa Clara, CA, USA).
Cytotoxicity assay (MTT)
Cell viability of hPDLCs in contact with sealer extracts was evaluated by means of the 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide (MTT) assay. Cells were seeded onto 96-well plates with 180 μL of DMEM and stored for 24 h at 37 °C, 5% CO2, and 95% humidity. 1 × 104 hPDLCs were then placed in contact with the undiluted, 1:2, and 1:4 material extracts (n = 3), and incubated for 24, 48, and 72 h in the same conditions. At each of the time intervals, 1 mg/mL of MTT solution was added to the hPDLC-sealer samples and incubated for a further 4 h. To solubilize the formazan crystals produced by viable cells through the reduction of MTT, 0.2 mL of dimethyl sulfoxide (DMSO) solution was added to each sample. Light absorption was then evaluated for each of the sample wells using a Synergy H1 multi-mode microplate reader (BioTek, Winooski, VT, USA) at 570 nm (Abs570), using the control group (cells cultured in unconditioned medium) as a reference, following previous evidence [22].
Cell migration assay (wound healing)
A wound healing assay was performed to assess hPDLC migration within the different material samples (undiluted, 1:2, and 1:4). Cells were seeded onto 6-well plates (2 × 105 hPDLCs per well, n = 3 for each dilution) and left to proliferate until confluent. Using a 200-μL pipette tip, a scratch was made on the surface of each cell monolayer, and each well was then rinsed three times with PBS to clear away any remaining cell debris. Wound healing (closure) was evaluated on an unconditioned sample (used as a control) and sealer-conditioned samples (non-diluted, 1:2, and 1:4) at 24, 48, and 72 h. Cell migration distances were assessed at three time-intervals: first 24-h period (0–24 h), second 24-h period (24–48 h), and third 24-h period (48–72 h). To account for width variations among the scratch wounds, migration rates were presented as percentage areas of relative wound closure or RWC and calculated as follows: RWC (%) = (wound closure area (pixels)/total number of pixels) × 100. Results were measured as the percentage of the total wound area at the different time points relative to the total wound area at 0 h for each respective well [23], using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Cell morphology and adhesion
Sample discs with the aforementioned standardized dimensions were obtained (n = 15) for each of the sealers and allocated into three groups (n = 5). 5 × 104 hPDLCs were seeded onto the surface of each disc and left to culture for 72 h. Cells were then fixed with 4% glutaraldehyde in PBS for 4 h, after which they were dehydrated via a gradual series of ethanol dilutions (30–90% v/v), air-dried, and coated with sputtered gold/palladium. Once coated, the analysis of cell morphology and adhesion was performed under SEM at × 100, × 300, and × 1500 magnifications.
Activity-related gene expression
hPDLC differentiation was evaluated by means of the expression of activity-related genes using real-time quantitative reverse-transcriptase polymerase chain reaction (RT-qPCR). The sequences of primers for the differentiation markers assessed are presented in Table 2. hPDLCs were seeded onto 12-well plates (2 × 104 cells/well, n = 3) with undiluted sealer-conditioned medium and incubated for 7 days, as previously reported [22]. The undiluted sealer-conditioned medium was previously prepared by immersing sealer discs in culture medium (DMEM; Gibco, USA) for 24 h. Total RNA of hPDLCs was extracted using the RNeasy Mini Kit (Qiagen, Hilden, Germany). RNA (1 μg) was reverse transcribed for first-strand complementary DNA (cDNA) synthesis using iScript™ Reverse Transcription Supermix for RT-qPCR (Bio-Rad Laboratories Inc., Hercules, CA, USA). Both the total RNA extraction and cDNA synthesis were carried out following their respective manufacturers’ instructions. The analysis of relative gene expression data was calculated using the 2−ΔΔCT method [24]. hPDLCs cultured in unconditioned medium (DMEM; Gibco, USA) acted as the negative control, and an osteo/cementogenic medium (OsteoDiff media; Miltenyi Biotec, Bergisch Gladbach, Germany) acted as the positive control.
Mineralization assay (Alizarin Red staining)
Alizarin Red staining (ARS) was used to assess the mineralization potential of hPDLCs in contact with BCI, BCHiF, and AHP. Cells were seeded onto 12-well plates (2 × 104 cells/well, n = 3) and left to proliferate until confluent. hPDLCs were then cultured in an undiluted sealer-conditioned medium for 21 days. After the culture period, the samples were washed (PBS) and fixed with 70% ethanol for 1 h, and then stained with 2% Alizarin Red solution (Sigma-Aldrich, USA) for 30 min in the dark at room temperature. Absorbance values of the samples were measured using Synergy H1 multi-mode microplate reader (BioTek, USA) at 405 nm. hPDLCs cultured in unconditioned medium (DMEM; Gibco, USA) acted as the negative control, and OsteoDiff media (Miltenyi Biotec, Germany) acted as the positive control.
Statistical analysis
All of the in vitro assays were performed in triplicate. Data are expressed as mean ± standard deviations (SD) and were analyzed using one-way ANOVA and Tukey’s post hoc test using GraphPad Prism v8.1.0 (GraphPad Software, San Diego, CA, USA). Statistical significance was considered at p < 0.05.
Results
SEM-EDS analysis
Regarding the chemical composition of the surface of each sealer, BCHiF presented the highest calcium (Ca) content. BCI, on the other hand, contained the highest percentage of zirconium (Zr) and silicon (Si). Interestingly, the percentage of Ca in BCHiF was higher than that of Zr, whereas the opposite was exhibited by BCI. With reference to AHP, the main difference in terms of composition was the presence of high amounts of tungsten (W). Both hCSSs exhibited an irregular prismatic crystalline structure on their surface, as opposed to the regular surface morphology shown by AHP (Fig. 1).
ICP-MS analysis
Results of the analysis of ion release from the different endodontic sealers are presented in Table 3. BCHiF exhibited a significantly higher concentration of Zr release when compared with BCI and AHP (p < 0.05). Both hCSSs showed a higher release of Ca than the epoxy resin–based sealer AHP (p < 0.05), which was greater in BCI than in BHiF (p < 0.05). BCI released a significantly higher amount of Si compared with the other sealers (p < 0.05), whereas the highest concentration of W release was detected in AHP (p < 0.05).
MTT assay
After the treatment of hPDLCs with endodontic sealer extracts, a cytotoxicity assay was performed using MTT. Cells which were cultured with BCHiF and BCI extracts (undiluted, 1:2, or 1:4) showed a similar formazan production in comparison with the control group, evidencing the cytocompatibility of both materials. At 48 h, the undiluted BCHiF group showed a higher production than the control (p < 0.05). Conversely, for all AHP extracts, the relative formazan formation was significantly lower than that of the control at all end time points (p < 0.001), indicating the cytotoxicity of the AHP extracts towards hPDLCs. The relative formazan formation by hPDLCs having been exposed to undiluted (1:1) and diluted (1:2 and 1:4) material extracts for 24, 48, and 72 h is summarized in Fig. 2.
Wound healing assay
The migration rates of hPDLCs exposed to undiluted (1:1) and diluted (1:2 and 1:4) sealer extracts are illustrated in Fig. 3 as changes in the open wound area at 24, 48, and 72 h. In the BCI group, significant differences were found in the 1:4 diluted group at 48 h when compared with the control group (p < 0.05), whereas no significant differences were observed at 24 nor 72 h in any dilution. At 24 h, significant differences were detected in all dilutions of BCHiF compared with that of the control group (undiluted, p < 0.01; 1:2 and 1:4, p < 0.05), while at 48 h, significant differences were only observed in the undiluted extracts (p < 0.05). Regarding the AHP group, at all time-periods and dilutions, the cell migration rate was significantly lower than that of the control group (p < 0.001), being unable to heal the wound.
Cell morphology and adhesion
The analysis of hPDLC morphology and adhesion onto the surfaces of the different sealers showed the presence of a low quantity of cells and debris in the AHP group, evidencing cell death, whereas BCI and BCHiF groups showed abundant cell adhesion with a greater spread. Additionally, cells adhered to the surface of BCHiF exhibited an intense growth and elongation (Fig. 4).
RT-qPCR assay
The relative expressions of CEMP1, CAP, RUNX2, and ALP from hPDLCs, cultured with undiluted sealers for 7 days, are summarized in Fig. 5. GAPDH was used to normalize the results. The BCHiF group showed an upregulation of CAP (p < 0.01), CEMP1, ALP, and RUNX2 (p < 0.001) compared with the negative control, while the BCI group showed an upregulation of CEMP1 (p < 0.01), CAP, and RUNX2 (p < 0.001). At the same time, cells exposed to BCHiF exhibited a higher expression of ALP (p < 0.01), CEMP1, and RUNX2 (p < 0.001) than those treated with BCI or Osteodiff (positive control). However, CAP expression was higher in the BCI and Osteodiff groups when compared with the BHiF group (p < 0.001). For the AHP group, RT-qPCR analysis was not performed, due to the evidenced cell death from the previous cytocompatibility assays.
Mineralization assay
The mineralization potential of the tested sealers is presented in Fig. 6. After 21 days of culture with undiluted sealer extracts, no mineralization was observed in the AHP group, showing similar rates to the untreated hPDLCs (control group). Both the BCI and BCHiF groups exhibited a greater mineralized nodule formation than the negative and positive control groups (p < 0.001). Likewise, cells treated with BCI showed a higher mineralization potential than those treated with BCHiF (p < 0.05).
Discussion
Traditionally, sealer-based obturations using hCSSs for root canal treatment were performed by means of cold compaction techniques, due to the lack of evidence on the effect of temperature and heating time on the properties of endodontic sealers [25, 26]. Recent evidence demonstrated that various sealers—with the well-established epoxy resin–based sealer AHP among them—can withstand a rise in temperature up until 100 °C and a subsequent cooldown, without suffering irreversible changes in their structure [18]. In view of this, AHP was used in the present study as a reference material to compare with two newly introduced hCSSs, as done by previous studies in the field [27, 28]. Both BCI and BCHiF are also indicated for warm compaction techniques according to their respective manufacturers, but their biological properties and, in particular, the biological response of hPDLCs towards them have not been described.
Accordingly, the present in vitro study aimed to outline the main clinically relevant chemical and biological properties of BCI and BCHiF, to anticipate their behavior in direct contact with the inorganic and organic tissues present within the root canal, and surrounding the root apex. The biological assays carried out comprise cell cytotoxicity, morphology, adhesion, proliferation, differentiation, and mineralization analyses, in accordance with similar studies within this framework [8, 29]. With regard to the chemical and ionic profiling of the assessed biomaterials, SEM-EDS and ICP-MS were used, respectively, as performed by various studies [6, 30].
The results from SEM-EDS and ICP-MS analyses revealed a high calcium content and ionic release in both BCI and BCHiF, as previously shown by other hCSSs: BioRoot RCS, MTA Fillapex [31]; Bio-C Sealer, TotalFill BC Sealer [32]; NeoMTA-Plus, Endocem-MTA [33]; EndoSequence BC Sealer, Ceraseal, Endoseal MTA [6]. The dissolution of calcium silicate–based biomaterials and release of their major cationic components, namely Ca2+, in contact with tissue fluids, results in an ionic interchange which resolves in the formation of a superficial mineral layer [34,35,36]. In the present study, calcium release was higher from BCI than from BCHiF samples after 48 h of setting (p < 0.05). The differences in ion release may influence the clinical sealing ability or, at least, the characteristics (i.e., thickness and composition) of the mineral attachment formed to the dentin substrate [36].
For the biological assays, hPDLCs were used as cell substrates, in an attempt to extrapolate their in vitro response to hCSSs, to their clinical behavior. These cells have shown long-term survival, self-renewal, and a capability to form bone/cementum-like mineralized tissue and ligament structures with associated vasculature [37, 38]. In root canal treatment, hPDLCs can be exposed directly to sealer extrusions from the root canals, and therefore these materials should at least ensure cell survival [32, 39]. Regarding the results from the present study, hPDLCs exposed to both BCI and BCHiF independently, exhibited adequate formazan production, high proliferation, and abundant adhesion when compared with AHP and the negative control. These results act as evidence of the cytocompatibility of hCSSs on hPDLCs, coinciding with previous studies in the field [6, 32, 39].
To assess the influence of sealer concentration on hPDLC viability and proliferation, various dilutions were prepared for each of the tested materials (undiluted, 1:2, and 1:4 ratios). With regard to cell viability, all BCI and BCHiF dilutions showed a similar formazan formation when compared with the control group after 48 h of incubation. In contrast, recent evidence reported that BCHiF eluates showed lower periodontal ligament fibroblast viability at higher concentrations (1:4 vs 1:8, 1:16, and 1:20 dilutions), significantly decreasing from 24 to 72 h of incubation [40]. Nevertheless, as highlighted in their study, it is expected that clinically, periapical tissues come into contact with considerably lower concentrations of the endodontic sealers and thus may exhibit a greater cytocompatibility.
Cytocompatibility assays, however, revealed negative results for the reference material used, AHP. hPDLCs incubated with the epoxy resin-based sealer extracts exhibited a significantly lower formazan production and migration than the control group in all of the sealer dilutions (undiluted, 1:2, 1:4). These results concur with those from previous in vitro studies [27, 41]. This, added to the evidenced hPDLC death on the surface of AHP sample discs observed under SEM and discouraged the inclusion of AHP on the posterior gene expression assays.
To evaluate the osteo/cementogenic differentiation of hPDLCs, the expression of a series of target genes was quantified using RT-qPCR, as follows: ALP, mediator in osteoblast activity and osteogenic potential [42, 43]; RUNX2, involved in the early stages osteogenesis as a transcriptional regulator factor [44]; CAP and CEMP1, cementoblast and cementocyte markers implicated in cementogenesis [45, 46]. hPDLCs cultured with both BCI or BCHiF exhibited a significant upregulation of osteogenic and cementogenic genes at 7 days of incubation compared with the negative control group. Likewise, both BCI and BCHiF groups showed a significant increase in mineralized nodule formation in comparison with the negative and positive control. Altogether, results indicate that both hCSSs are not only cytocompatible but also capable to induce the osteo/cementogenic differentiation and increase the mineralization potential of hPDLCs. These properties have also been reported for other hCSSs [6], supporting that they may be a shared characteristic among this group of biomaterials. In addition, recent evidence suggests that silicate-based endodontic materials may also induce the angiogenic stimulation of various DSCs [47].
Available evidence on the biological response of hPDLCs and other DSCs towards hCSSs is still limited and remains at an in vitro level, with the limitations that this may imply. The cellular behavior in controlled sealer-conditioned media shown in the present study and similar studies may provide an insight as to how these biomaterials may perform at a clinical level, but the presence of factors that may influence this behavior clinically [11] limits the extrapolation of the results. Nonetheless, to the authors’ knowledge, this is the first study to assess the biological properties of the recently introduced BCI. The release of calcium ions, the cytocompatibility and upregulation of osteo/cementogenic markers, and the increased mineralization in contact with hPDLCs shown in the present study act as evidence for its potential use in root canal treatment.
Conclusions
The calcium silicate–based sealers considered in the present in vitro study exhibited a high calcium ion release, adequate cytocompatibility, upregulated osteo/cementogenic gene expression, and increased mineralized nodule formation in contact with hPDLCs.
References
Primus CM, Tay FR, Niu LN (2019) Bioactive tri/dicalcium silicate cements for treatment of pulpal and periapical tissues. Acta Biomater 96:35–54. https://doi.org/10.1016/j.actbio.2019.05.050
Fonseca DA, Paula AB, Marto CM, Coelho A, Paulo S, Martinho JP, Carrilho E, Ferreira MM (2019) Biocompatibility of root canal sealers: a systematic review of in vitro and in vivo studies. Materials (Basel) 12. https://doi.org/10.3390/ma12244113
Sanz JL, Rodriguez-Lozano FJ, Llena C, Sauro S, Forner L (2019) Bioactivity of bioceramic materials used in the dentin-pulp complex therapy: a systematic review. Materials (Basel) 12. https://doi.org/10.3390/ma12071015
Chybowski EA, Glickman GN, Patel Y, Fleury A, Solomon E, He J (2018) Clinical outcome of non-surgical root canal treatment using a single-cone technique with endosequence bioceramic sealer: a retrospective analysis. J Endod 44:941–945. https://doi.org/10.1016/j.joen.2018.02.019
Zavattini A, Knight A, Foschi F, Mannocci F (2020) Outcome of root canal treatments using a new calcium silicate root canal sealer: a non-randomized clinical trial. J Clin Med 9. https://doi.org/10.3390/jcm9030782
Lopez-Garcia S, Myong-Hyun B, Lozano A, Garcia-Bernal D, Forner L, Llena C, Guerrero-Girones J, Murcia L, Rodriguez-Lozano FJ (2020) Cytocompatibility, bioactivity potential, and ion release of three premixed calcium silicate-based sealers. Clin Oral Investig 24:1749–1759. https://doi.org/10.1007/s00784-019-03036-2
Seo DG, Lee D, Kim YM, Song D, Kim SY (2019) Biocompatibility and mineralization activity of three calcium silicate-based root canal sealers compared to conventional resin-based sealer in human dental pulp stem cells. Materials (Basel):12. https://doi.org/10.3390/ma12152482
Zordan-Bronzel CL, Tanomaru-Filho M, Rodrigues EM, Chavez-Andrade GM, Faria G, Guerreiro-Tanomaru JM (2019) Cytocompatibility, bioactive potential and antimicrobial activity of an experimental calcium silicate-based endodontic sealer. Int Endod J 52:979–986. https://doi.org/10.1111/iej.13086
Vallittu PK, Boccaccini AR, Hupa L, Watts DC (2018) Bioactive dental materials-do they exist and what does bioactivity mean? Dent Mater 34:693–694. https://doi.org/10.1016/j.dental.2018.03.001
Donnermeyer D, Burklein S, Dammaschke T, Schafer E (2019) Endodontic sealers based on calcium silicates: a systematic review. Odontology 107:421–436. https://doi.org/10.1007/s10266-018-0400-3
Holland R, Gomes JEF, Cintra LTA, Queiroz IOA, Estrela C (2017) Factors affecting the periapical healing process of endodontically treated teeth. J Appl Oral Sci 25:465–476. https://doi.org/10.1590/1678-7757-2016-0464
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S (2002) Stem cell properties of human dental pulp stem cells. J Dent Res 81:531–535. https://doi.org/10.1177/154405910208100806
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT (2008) Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: a pilot study. J Endod 34:166–171. https://doi.org/10.1016/j.joen.2007.11.021
Wang L, Shen H, Zheng W, Tang L, Yang Z, Gao Y, Yang Q, Wang C, Duan Y, Jin Y (2011) Characterization of stem cells from alveolar periodontal ligament. Tissue Eng Part A 17:1015–1026. https://doi.org/10.1089/ten.tea.2010.0140
Smith AJ, Duncan HF, Diogenes A, Simon S, Cooper PR (2016) Exploiting the bioactive properties of the dentin-pulp complex in regenerative endodontics. J Endod 42:47–56. https://doi.org/10.1016/j.joen.2015.10.019
da Rosa WLO, Piva E, da Silva AF (2018) Disclosing the physiology of pulp tissue for vital pulp therapy. Int Endod J 51:829–846. https://doi.org/10.1111/iej.12906
Tomokiyo A, Wada N, Maeda H (2019) Periodontal ligament stem cells: regenerative potency in periodontium. Stem Cells Dev 28:974–985. https://doi.org/10.1089/scd.2019.0031
Atmeh AR, Hadis M, Camilleri J (2020) Real-time chemical analysis of root filling materials with heating: guidelines for safe temperature levels. Int Endod J 53:698–708. https://doi.org/10.1111/iej.13269
Tomson PL, Grover LM, Lumley PJ, Sloan AJ, Smith AJ, Cooper PR (2007) Dissolution of bio-active dentine matrix components by mineral trioxide aggregate. J Dent 35:636–642. https://doi.org/10.1016/j.jdent.2007.04.008
Tomson PL, Lumley PJ, Smith AJ, Cooper PR (2017) Growth factor release from dentine matrix by pulp-capping agents promotes pulp tissue repair-associated events. Int Endod J 50:281–292. https://doi.org/10.1111/iej.12624
ISO 10993-5. Biological evaluation of medical devices. Part 5. Test for in vitro cytotoxicity. 2009
Rodríguez-Lozano FJ, López-García S, García-Bernal D, Tomás-Catalá CJ, Santos JM, Llena C, Lozano A, Murcia L, Forner L (2020) Chemical composition and bioactivity potential of the new Endosequence BC Sealer formulation HiFlow. Int Endod J. https://doi.org/10.1111/iej.13327
Yue PY, Leung EP, Mak NK, Wong RN (2010) A simplified method for quantifying cell migration/wound healing in 96-well plates. J Biomol Screen 15:427–433. https://doi.org/10.1177/1087057110361772
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25:402–408
Viapiana R, Baluci CA, Tanomaru-Filho M, Camilleri J (2015) Investigation of chemical changes in sealers during application of the warm vertical compaction technique. Int Endod J 48:16–27. https://doi.org/10.1111/iej.12271
Atmeh AR, AlShwaimi E (2017) The effect of heating time and temperature on epoxy resin and calcium silicate-based endodontic sealers. J Endod 43:2112–2118. https://doi.org/10.1016/j.joen.2017.08.008
Lee JK, Kim S, Lee S, Kim HC, Kim E (2019) In vitro comparison of biocompatibility of calcium silicate-based root canal sealers. Materials (Basel):12. https://doi.org/10.3390/ma12152411
Giacomino CM, Wealleans JA, Kuhn N, Diogenes A (2019) Comparative biocompatibility and osteogenic potential of two bioceramic sealers. J Endod 45:51–56. https://doi.org/10.1016/j.joen.2018.08.007
Sultana N, Singh M, Nawal RR, Chaudhry S, Yadav S, Mohanty S, Talwar S (2018) Evaluation of biocompatibility and osteogenic potential of tricalcium silicate-based cements using human bone marrow-derived mesenchymal stem cells. J Endod 44:446–451. https://doi.org/10.1016/j.joen.2017.11.016
Tomas-Catala CJ, Collado-Gonzalez M, Garcia-Bernal D, Onate-Sanchez RE, Forner L, Llena C, Lozano A, Castelo-Baz P, Moraleda JM, Rodriguez-Lozano FJ (2017) Comparative analysis of the biological effects of the endodontic bioactive cements MTA-Angelus, MTA Repair HP and NeoMTA Plus on human dental pulp stem cells. Int Endod J 50:E63–E72. https://doi.org/10.1111/iej.12859
Siboni F, Taddei P, Zamparini F, Prati C, Gandolfi MG (2017) Properties of BioRoot RCS, a tricalcium silicate endodontic sealer modified with povidone and polycarboxylate. Int Endod J 50(Suppl 2):e120–e136. https://doi.org/10.1111/iej.12856
Lopez-Garcia S, Pecci-Lloret MR, Guerrero-Girones J, Pecci-Lloret MP, Lozano A, Llena C, Rodriguez-Lozano FJ, Forner L (2019) Comparative cytocompatibility and mineralization potential of Bio-C Sealer and TotalFill BC Sealer. Materials (Basel) 12. https://doi.org/10.3390/ma12193087
Rodriguez-Lozano FJ, Collado-Gonzalez M, Lopez-Garcia S, Garcia-Bernal D, Moraleda JM, Lozano A, Forner L, Murcia L, Onate-Sanchez RE (2019) Evaluation of changes in ion release and biological properties of NeoMTA-Plus and Endocem-MTA exposed to an acidic environment. Int Endod J 52:1196–1209. https://doi.org/10.1111/iej.13107
Sarkar NK, Caicedo R, Ritwik P, Moiseyeva R, Kawashima I (2005) Physicochemical basis of the biologic properties of mineral trioxide aggregate. J Endod 31:97–100. https://doi.org/10.1097/01.don.0000133155.04468.41
Watson TF, Atmeh AR, Sajini S, Cook RJ, Festy F (2014) Present and future of glass-ionomers and calcium-silicate cements as bioactive materials in dentistry: biophotonics-based interfacial analyses in health and disease. Dent Mater 30:50–61. https://doi.org/10.1016/j.dental.2013.08.202
Kim JR, Nosrat A, Fouad AF (2015) Interfacial characteristics of Biodentine and MTA with dentine in simulated body fluid. J Dent 43:241–247. https://doi.org/10.1016/j.jdent.2014.11.004
Han J, Menicanin D, Marino V, Ge S, Mrozik K, Gronthos S, Bartold PM (2014) Assessment of the regenerative potential of allogeneic periodontal ligament stem cells in a rodent periodontal defect model. J Periodontal Res 49:333–345. https://doi.org/10.1111/jre.12111
Menicanin D, Mrozik KM, Wada N, Marino V, Shi S, Bartold PM, Gronthos S (2014) Periodontal-ligament-derived stem cells exhibit the capacity for long-term survival, self-renewal, and regeneration of multiple tissue types in vivo. Stem Cells Dev 23:1001–1011. https://doi.org/10.1089/scd.2013.0490
Collado-Gonzalez M, Garcia-Bernal D, Onate-Sanchez RE, Ortolani-Seltenerich PS, Lozano A, Forner L, Llena C, Rodriguez-Lozano FJ (2017) Biocompatibility of three new calcium silicate-based endodontic sealers on human periodontal ligament stem cells. Int Endod J 50:875–884. https://doi.org/10.1111/iej.12703
Chen B, Haapasalo M, Mobuchon C, Li X, Ma J, Shen Y (2020) Cytotoxicity and the effect of temperature on physical properties and chemical composition of a new calcium silicate-based root canal sealer. J Endod 46:531–538. https://doi.org/10.1016/j.joen.2019.12.009
Alsubait SA, Al Ajlan R, Mitwalli H, Aburaisi N, Mahmood A, Muthurangan M, Almadhri R, Alfayez M, Anil S (2018) Cytotoxicity of different concentrations of three root canal sealers on human mesenchymal stem cells. Biomolecules 8. https://doi.org/10.3390/biom8030068
Wang Y, Zhou Y, Jin L, Pang X, Lu Y, Wang Z, Yu Y, Yu J (2018) Mineral trioxide aggregate enhances the osteogenic capacity of periodontal ligament stem cells via NF-kappaB and MAPK signaling pathways. J Cell Physiol 233:2386–2397. https://doi.org/10.1002/jcp.26110
Li J, Zhang F, Zhang N, Geng X, Meng C, Wang X, Yang Y (2019) Osteogenic capacity and cytotherapeutic potential of periodontal ligament cells for periodontal regeneration in vitro and in vivo. PeerJ 7:e6589. https://doi.org/10.7717/peerj.6589
Komori T (2018) Runx2, an inducer of osteoblast and chondrocyte differentiation. Histochem Cell Biol 149:313–323. https://doi.org/10.1007/s00418-018-1640-6
Komaki M, Iwasaki K, Arzate H, Narayanan AS, Izumi Y, Morita I (2012) Cementum protein 1 (CEMP1) induces a cementoblastic phenotype and reduces osteoblastic differentiation in periodontal ligament cells. J Cell Physiol 227:649–657. https://doi.org/10.1002/jcp.22770
Han P, Ivanovski S, Crawford R, Xiao Y (2015) Activation of the canonical Wnt signaling pathway induces cementum regeneration. J Bone Miner Res 30:1160–1174. https://doi.org/10.1002/jbmr.2445
Olcay K, Tasli PN, Guven EP, Ulker GMY, Ogut EE, Ciftcioglu E, Kiratli B, Sahin F (2020) Effect of a novel bioceramic root canal sealer on the angiogenesis-enhancing potential of assorted human odontogenic stem cells compared with principal tricalcium silicate-based cements. J Appl Oral Sci 28:e20190215. https://doi.org/10.1590/1678-7757-2019-0215
Funding
This work was supported by the Spanish Network of Cell Therapy (TerCel), RETICS subprograms of the I+D+I 2013–2016 Spanish National Plan, project “RD16/0011/0001” funded by the Instituto de Salud Carlos III to JMM and co-funded by the European Regional Development Fund.
Author information
Authors and Affiliations
Contributions
Investigation and methodology: Sergio López-García, Francisco Javier Rodríguez Lozano, and José Luis Sanz; supervision, visualization, conceptualization, and data curation: Adrián Lozano and Julia Guerrero-Gironés; investigation, methodology, and writing—original draft: José Luis Sanz and Francisco Javier Rodríguez-Lozano; conceptualization, formal analysis, project administration, supervision, validation, and writing—review and editing: Leopoldo Forner, Carmen Llena, and Maria Pilar Pecci-Lloret; investigation, methodology, project administration, resources, writing—original draft, and writing—review and editing: José Luis Sanz, Francisco Javier Rodríguez-Lozano, and Leopoldo Forner. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
The study protocol was approved by the Clinical Research Ethics Committee of the Universidad de Murcia (ID: 2199/2018). Likewise, permission was obtained from the Health Department authorities to use the information contained in the CDHs, previously anonymized by one of the investigators belonging to the medical staff of the Health Department in order to protect patient confidentiality. All the information was processed in abidance with the confidentiality regulations defined under Act 15/1999 referred to as personal data protection.
Informed consent
Informed consent was obtained from the parents of all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sanz, J.L., López-García, S., Lozano, A. et al. Microstructural composition, ion release, and bioactive potential of new premixed calcium silicate–based endodontic sealers indicated for warm vertical compaction technique. Clin Oral Invest 25, 1451–1462 (2021). https://doi.org/10.1007/s00784-020-03453-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03453-8