Abstract
Objectives
To evaluate the biocompatibility, physical and chemical properties of three pre-mixed calcium silicate–based sealers and an epoxy resin–based material were assessed. Pre-mixed sealers supposedly obtain water from the root canal moist to hydrate and set.
Materials and methods
Polyethylene tubes were filled with the materials Bio-C Sealer Ion+, Bio-C Sealer, EndoSequence BC Sealer and AH Plus Jet, or left empty and surgically implanted in the subcutaneous tissue of Wistar rats. The animals were euthanised and the tubes and tissue were removed for histological analysis and scanning electron microscopy (SEM) coupled with energy-dispersive spectrometry (EDS). Materials’ surface chemical characterisation was assessed using Raman spectroscopy and SEM/EDS. Flow, setting time (in two conditions), solubility, radiopacity and pH were also analysed. ANOVA and Bonferroni correction were performed for comparisons (P < 0.05).
Results
Inflammatory response observed in the tissues subsided from 7 to 30 days. Tungsten migration could be detected in the surrounding tissue following AH Plus Jet implantation. All calcium silicate–based sealers exhibited zirconium oxide (radiopacifier) and tricalcium silicate peaks before and after implantation. All materials exhibited flow values above 17 mm. An approximately tenfold difference was observed between the plaster- and metal-mould setting times of the calcium silicate cements indicating its sensitivity to moist variations and solubility above 8% was also observed for these materials.
Conclusions
Pre-mixed materials exhibited variable setting time and solubility with a decreasing inflammatory response.
Clinical relevance
The variable moist-dependant setting time with high solubility poses a concern for the clinical use of these pre-mixed sealers.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The properties of newly introduced root canal sealers are important to verify in the literature with the intention of predicting material clinical behaviour for clinical use [1,2,3,4,5]. Biocompatibility is a crucial property that an endodontic sealer must present since the material is placed in close contact with periapical tissues [6]. Besides the material composition, its physico-chemical properties can be assessed using several techniques [7,8,9,10]. Properties of concern include biocompatibility [11], predictable setting time [2], long-term dimensional stability, material degradation [12], adequate radiopacity [13], colour stability [14] and removal during endodontic retreatment [15].
Two presentations of calcium silicate cement endodontic sealers are commercially available: pre-mixed [2] and powder/liquid [16]. The first presentation hydrates and set from the water obtained from the root canal residual moist [17], whereas the powder/liquid format starts the hydration during the material mixing.
Bio-C Sealer Ion+ (Angelus, Londrina, PR, Brazil) is a recently available pre-mixed endodontic sealer. According to the manufacturer, the calcium silicate–based formula was modified from Bio-C Sealer (Angelus, Londrina, PR, Brazil), also a pre-mixed sealer, by replacing alumina with magnesia in the cement crystals [18]. No studies have evaluated this composition. For Bio-C Sealer, previous studies demonstrated a short setting time (220 min), alkaline pH (around 9), radiopacity (5.5 mm Al) and adequate flow (31.2 mm) when tested using the ISO 6876/2012 methods [19]. EndoSequence BC Sealer (Brasseler, Savannah, Georgia, USA) is also a pre-mixed calcium silicate–based sealer marketed since 2008 with previously reported physical, chemical and biological properties [20, 21].
Long-term, three-dimensional sealing is necessary from endodontic sealers [22]. However, test reports have shown variable setting times [2] and high solubility [19, 23, 24] for pre-mixed calcium silicate cement sealers, which might impact long-term clinical success. Endodontic sealers must have a solubility less than 3% [25, 26] to comply with the ISO 6876:2012 standard.
Studies regarding endodontic sealers included AH Plus (Dentsply Sirona, Konstanz, Germany), an epoxy resin–based endodontic sealer, in a paste/paste presentation introduced in the 1990s [27]. AH Plus has been evaluated for solubility [28], adhesion to dentin [29], sealing ability [30,31,32], antimicrobial properties [33, 34], cytotoxicity [35] and long-term clinical outcome with tomographic evaluation [36]. This material is a well-researched endodontic sealer, which justifies its comparison with newly proposed compositions. Recently, the material presentation was altered by the manufacturer and the sealer renamed to AH Plus Jet for its convenient dual syringe format.
The present study aimed to evaluate in vivo and in vitro pre-mixed calcium silicate cement sealers in comparison to an epoxy resin–based sealer. The null hypothesis was that there would be no difference between the tested endodontic sealers considering the evaluated properties.
Materials and methods
Root canal endodontic sealers’ composition and batch are shown in Table 1.
The following materials were evaluated: Bio-C Sealer Ion+ (Angelus, Londrina, PR, Brazil), Bio-C Sealer (Angelus, Londrina, PR, Brazil), EndoSequence BC Sealer (Brasseler, Savannah, Georgia, EUA) and AH Plus Jet (Dentsply, Konstanz, Germany).
Bio-C Sealer Ion+, Bio-C Sealer and EndoSequence BC Sealer are pre-mixed materials in a single syringe. AH Plus Jet in its two-part syringe was mixed according to the manufacturer instructions. During analysis, the materials were kept in an oven at 37°C and 95% humidity, according to ISO 6876 test methods [25]. A previous study [2] was used as reference for the number of samples for the analysis of flow, setting time, solubility, radiopacity and pH.
Subcutaneous implants
The manuscript of this animal study has been written according to Preferred Reporting Items for Animal Studies in Endodontology (PRIASE) 2021 guidelines [37]. The sample size for the in vivo part was established based on previous studies [38, 39] calculated using the G*Power 3.1 programme for Mac (Hein Heine, University Dusseldorf) and the comparison test between more than 2 methods with independent groups (ANOVA). An estimate difference from the standard of 0.6 was performed and a minimum to be performed without a value of 1.6, test power (β) of 0.80 and alpha (α) of 0.05, resulting in 8 animals for each group (n = 8). A total of 32 male Wistar albino rats (approximately 300 g and 3-month-old) were used. All procedures were performed in accordance with guidelines in the Guide to the Care and Use of Laboratory Animals (US National Institutes of Health) and the principles of the 3Rs (‘replacement, reduction, and refinement’) [40]. This study was approved by the institutional ethics committee (CEUA 5387-1/2019).
Polyethylene tubes (Abbott Labs of Brazil, São Paulo, SP, Brazil) (1.0 mm internal diameter, 1.6 mm external diameter and 10.0 mm length) were filled with the sealers and empty tubes served as control. For the surgical procedure, the animals were anesthetised and shaved dorsally, and a 2.0-cm incision was made in a head-to-tail orientation. Tissue was reflected to implant three tubes with one test sealer material and one control and sutured with a 4/0 silk tread. Two tubes with material served for the histological analysis and the remaining tube served for scanning electron microscope (SEM) and energy-dispersive spectroscopy (EDS) analysis. Animals were observed the following day and bedding was changed every 3 days by a technician trained in animal care.
Subcutaneous implants and histological analysis
Rats were euthanised with an overdose of the anaesthetic solution at 7 days (16 animals) and 30 days (16 animals). Tubes for histological analysis along with the surrounding tissues were removed and immediately fixed in 10% buffered formalin at neutral pH. The specimens were processed and embedded in paraffin. Histological slices were performed in 5-μm-thick sections for haematoxylin-eosin staining.
The number of inflammatory cells was manually counted for each specimen image (n = 35 slides for each material/time point or control/time point) at 40× magnification by a single calibrated operator, blinded to the condition, using light microscopy (DM 4000 B; Leica Microsystem, Wetzlar, Germany).
For the SEM/EDS sample preparation, fixation in 70% ethanol and ascending dehydration up to 100%, followed by drying in a vacuum desiccator for 24 h, and carbon-coated for analysis.
Raman spectroscopy
Freshly aliquoted sealers, without animal implantation, and 30-day implanted materials were compared using Raman spectroscopy. Samples were separately placed on an aluminium-pan sample holder (TS1500; Linkam Scientific Instruments, Tadworth, UK) and real-time Raman spectra were obtained using an adjustable laboratory-made spectrometer [41]. A 785-nm laser beam (Cobolt 08 series 078508-11-0500-200; Hubner Photonics, Kassel, Germany) was used, adjusted to a power of 20 mW. The spectrometer was equipped with a 500-mm focal length monochromator (Andor/Oxford SR-500iC-SIL; Shamrock, Belfast, UK) and a charge-coupled device (CCD) camera (Andor/Oxford iDUS 416 DU416A - LDC - DD; Shamrock). The spectral resolution was approximately 2 cm−1 and the range was set from 100 to 1200 cm−1. Raman spectra were acquired by using a spot size of 1000 μm and integration time of 3 s. Data were processed by using a baseline correction algorithm (Asymmetric Least Squares, ALS) [42] to minimise the fluorescence background.
SEM/EDS characterisation
The material surface characterisation prior to implantation was carried out using SEM/EDS microscopy (JSM 5600, JEOL, Japan) in back-scatter mode. After setting for 24 h of storage at 37 °C in 95% relative humidity, the specimens were polished and separately carbon-coated, and representative micrographs were obtained at 500×, followed by EDS analysis of the elements.
Flow
For the flow analysis, 0.05 mL of each sealer (n = 6) was dosed in the centre of a flat smooth glass plate (40 × 40 × 5 mm) using a 1-mm graduated syringe (BD-Luer-Lok, MG, Brazil). After 3 min, a second 20-g plate along with an additional 100-g weight was placed centrally on top of each sample. Ten minutes after mixing or placement, the additional weight was removed, and the minimum and maximum diameters of the sample were measured using a digital calliper (500-463; Mitutoyo Corporation, Kanagawa, Japan). Flow was defined as the average value between the two diameters (in mm).
Setting time
The setting time was determined using two different methods in a controlled-temperature room (20 ± 2°C). The plaster-mould method used round-type IV plaster-moulds (Durone-IV, Dentsply, RJ, Brazil) (10 × 1 mm), according to ISO 6876:2012, that were previously humidified by overnight immersion in distilled water, and filled with the sealers (n = 6) according to a previously reported method [2]. The metal-mould method used stainless steel (10 × 2 mm) rings, placed on a glass plate. The sealers (n = 6) were inserted into the rings, and cotton moistened with distilled water was placed around the glass plate, but without contact with the sealers to provide the moist environment needed for this setting. Both methods used a storage at 37 °C and 95% humidity during the analysis.
Periodically, a 113.4-g Gilmore needle with a 2-mm tip was placed vertically on the sample surface to determine the initial setting time. After no indentations were seen, the 453.6-g Gilmore needle with a 1.06-mm tip was used (ASTM-C266-07) to determine the final setting time. When the heavier needle mark could no longer be observed on the material surface, the final setting time was considered to be achieved.
Solubility
For the solubility analysis, a previous study [16] was used as reference. Round plastic moulds (7.75 × 1.5 mm) (n = 6) were filled with the materials, incubated at 37°C, and a 3-fold final setting time wait was used. A waterproof nylon thread was attached to the plastic moulds, and the specimens were transferred to a vacuum desiccator for 24 h and weighed on an analytical balance (Ohaus-Adventurer AR2140, SP, Brazil). The samples were separately immersed in 50 mL of distilled water without touching the flask walls and kept in an oven at 37°C for 30 days. After this period, the samples were placed into the desiccator and re-weighed after 24 h. Solubility was obtained by calculating the weight difference after immersion (in percentage).
Radiopacity
For radiopacity assessment, round samples (10 × 1 mm) were formed, and after the material set, 600-grit sandpaper was gently used for uniform sample thickness. Samples were radiographed (60 kV, 10 mA, 0.3 s and a focus-film distance of 30 cm) using a digital sensor (Micro-Image, Sao Paulo, Brazil) with a 16-mm aluminium step-wedge. Radiopacity assessment was performed by analysing the grey levels (from 0 to 256) of the images obtained using Image Tool software (Version 3.0, University of Texas, TX, USA) in a standardised area of 3.000 pixels and compared to the aluminium steps. The radiopacity values were expressed in millimetres of aluminium.
pH
The pH values of the sealers were determined after 1, 7, 14 and 21 days of immersion [19]. Polyethylene tubes (10 × 1.6 internal mm) containing the materials were immersed in a plastic container with 10 mL of deionised water and stored in an oven at 37°C and relative humidity of 95%. The pH evaluation was performed using a previously calibrated digital pH-metre (Digimed, São Paulo, Brazil).
Statistical analysis
JASP (University of Amsterdam, Amsterdam, The Netherlands), version 0.9.2. (2020) with ANOVA software was used: one-way (flow, setting time, solubility and radiopacity); repeated measures and between-subject factors (pH). The sample size was determined for each test to express a test power of at least 80%. The assumptions of this method, normal distribution and homogeneity of variances were validated through the tests Shapiro-Wilk and Levene. To check the differences between the groups, a post hoc analysis with Bonferroni correction was performed, adopting a 5% significance level (P < 0.05).
Results
Subcutaneous implants and histological analysis
No visual behaviour or morphological alterations were observed in the animals throughout the experimental period. The PRIASE 2021 flowchart is shown in Fig. 1.
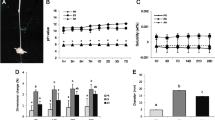
Representative images from the histological analysis of the tissue response after 7 and 30 days of implantation are shown in Fig. 2. The graphical representation of the number of inflammatory cells counted in the histological analysis is shown in Fig. 3. At 7 days, large numbers of inflammatory cells were observed; Bio-C Sealer and AH Plus Jet exhibited a higher number of inflammatory cells than the control (P < 0.05). At 30 days, the inflammation subsided to a mild for the cement-based sealer materials compared to the control; however, AH Plus Jet showed a significantly higher number of inflammatory cells in comparison to all others (P < 0.05).
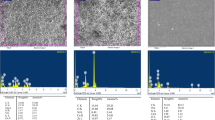
Tubes analysed using SEM/EDS after implantation for 7 and 30 days are shown in Figs. 4 and 5. Tungsten was detected in EDS at both time points from AH Plus Jet into the connective tissue, indicative of the migration of the calcium tungstate radiopaque component of AH Plus Jet. For all materials, zirconium overlapped with phosphorus, which limited the EDS analysis of these sealers’ components.
SEM/EDS analysis of the implanted tubes along with surrounding tissues after 7 days. Zirconium overlaps phosphorus, a limitation of EDS technique. Magnesium was not detectable in Bio-C Sealer Ion+ after implantation. EndoSequence BC Sealer exhibited quantifiable aluminium. Tungsten migrated from AH Plus Jet into the tissue but was not detectable in the material surface. Control tissue exhibited peaks of iron
Raman spectroscopy
The average Raman spectra for sealers based on calcium silicate cement or resin are shown in Fig. 6. All calcium silicate cement sealers contained zirconium oxide and tricalcium silicate (C3S) both before and after implantation. AH Plus Jet peaks obtained a better fit between before and after implantation indicative of the material stability; peaks of its radiopacifier and calcium tungstate were present.
Raman spectra of sealers. For calcium silicate–based sealers, peaks of zirconium dioxide (ZrO2) and tricalcium silicate (C3S) were detected with slight peak changes after implantation. For AH Plus Jet, peaks of the radiopacifier calcium tungstate (CaWO4) were evident and implantation not seemed to influence the Raman shift for this material
SEM/EDS characterisation
SEM microphotographs and EDS mapping from the materials are shown in Fig. 7. Peaks of calcium, silicon and the respective radiopacifier were observed for the hydraulic calcium silicate cement sealers. In Bio-C Sealer Ion+ samples, small peaks of magnesium, sulphur and potassium were also observed. AH Plus had a different EDS spectra indication of its epoxy resin–based composition; the peak for tungsten was not quantified.
SEM micrographs and corresponding EDS peaks of the tested materials used for material characterisation. a Bio-C Sealer Ion+ exhibited peaks of calcium, silicon and zirconium (radiopacifier). Small peaks of magnesium, sulphur and potassium were also observed. b Bio-C Sealer showed peaks of calcium, silicon, aluminium and zirconium (radiopacifier). c EndoSequence BC Sealer exhibited peaks of calcium, silicon and zirconium (radiopacifier); the former element overlapped with phosphorus, as an EDS limitation of analysis. d AH Plus showed peaks of carbon and silicon. An overlap of phosphorus over the zirconium (radiopacifier) peak was also observed. Tungsten (radiopacifier) peak was present along with a small peak of vanadium. Differences between the characterisation of EDS spectra and after implantation (Figs. 4 and 5) could be an indicative of chemical material alterations after contact with subcutaneous tissue
Flow, setting time, solubility, radiopacity and pH analysis
Flow, setting time, solubility and radiopacity results are shown in Table 2. All materials exhibited average flow values above 17 mm, fulfilling the ISO 6876:2012 standard. An approximately tenfold difference was observed between the plaster- and metal-mould setting times of the calcium silicate cement sealers, indicating the sensitivity to moisture of these cement materials’ solubility after 30 days was larger than 8%. AH Plus Jet exhibited a 3.2-fold difference between the two setting time methods but had a solubility below 0.2%. Radiopacity was above 7 mm Al for all the materials and the pH analysis (Table 3), up to 21 days, indicated initial alkalinity above 8 for the calcium silicate cement materials with a gradual decrease (P < 0.05). AH Plus Jet exhibited pH of around 7 throughout the analysis.
Discussion
This study aimed to evaluate the pre-mixed ‘ready-to-use’ hydraulic calcium silicate cements Bio-C Sealer Ion+, Bio-C Sealer and EndoSequence BC sealers compared to AH Plus Jet, an epoxy resin–based sealer. The analysis methods included were performed aiming to understand properties of these materials under in vivo and in vitro conditions. Some test methods of ISO 6876:2012 [25] were used along with methods of other studies [2, 16, 43] to obtain the present results. Studies comparing properties of calcium silicate cements and resin-based ones were previously reported [36, 44, 45] supporting the plausibility of this comparison. Based on the results here obtained, the null hypothesis that there would be no difference between the tested endodontic sealers was rejected.
The Wistar rat is a well-established animal model widely used for biocompatibility tests [46] and its biological complexity can provide useful information on the behaviour of a substance. Using polyethylene tubes filled with the materials for implantation made the histological processing possible, without tissue collapse, and to observe the location of the contact site. These conditions of tissue imaging (shown in Fig. 2 in lower magnification) allowed the counting of the number of inflammatory cells at both experimental time points. Previous studies have also used implanted tubes [47]. At 7 days, higher numbers of inflammatory cells for all conditions were counted, indicating an early reaction to the materials compared to 30 days, when inflammation had subsided. No previous studies had implantation of Bio-C Sealer Ion+. Regarding the two other calcium silicate–based sealers tested, previous studies have reported similar inflammatory responses after implantation [39, 48]. However, AH Plus Jet still induced at least 2-fold higher number of inflammatory cells after 30 days of implantation; this corroborates a previous study using the same time point for this material, reporting a mild inflammation after a month [49].
A study analysing a radiopacified (with bismuth oxide) calcium silicate cement (ProRoot MTA) reported the migration of this radiopacifier into the adjacent subcutaneous tissues following implantation [50]. In the present study, tungsten, presumed to be calcium tungstate, was detected by EDS—as in the referenced previous study [50]—as a chemical element present in the connective tissue surrounding AH Plus Jet. Calcium tungstate was confirmed by Raman spectroscopy in non-implanted samples and 30-day implanted ones. No previous studies have reported the calcium tungstate migration into biological tissues from AH Plus Jet; future studies ought to explore potential systemic implications.
Endodontic sealers contain radiopacifiers to enable its visualisation periapical and panoramic x-rays, and cone-beam computed tomography [3, 51, 52]. The radiopacity of the tested sealers (above 7 mm Al) exceeded the minimum required (3 mm) of the ISO 6876:2012 standard. AH Plus Jet contains calcium tungstate and zirconium oxide and had the highest radiopacity here. Previous studies reported a range of radiopacity for AH Plus [2] and this property was maintained after simulated heat application [16] for this material. The presence of zirconium in the material is interfered in EDS analysis with phosphorus due to overlap of their peaks [53, 54]; thus, it is crucial to use other methods of characterisation for the same material to verify its composition and radiopacifier(s). Similar values were reported for AH Plus (9.2 mm Al), but lower values for Bio-C Sealer (5.5 mm Al) [19]. Another study [55] reported the radiopacity of EndoSequence BC Sealer as 4-mm Al, lower than that observed in the present study. The Bio-C Sealer Ion+ exhibited significantly higher radiopacity than Bio-C Sealer. These differences in radiopacity between the materials can be explained by their composition and the amount of radiopacifier that is included in the sealer. Worryingly, there is no information by the manufacturers regarding the exact percentage of added components serving as radiopacifier.
SEM coupled with EDS is a well-established method for characterisation of the elements present in a material [4]. Calcium silicate cements had EDS spectra with peaks of calcium, silicon and the respective radiopacifier, as stated by the manufacturers. Bio-C Sealer Ion+ exhibited magnesium and no aluminium. Potassium was also detected and quantifiable by EDS for Bio-C Sealer Ion+, as disclosed by the manufacturer. The Raman shift peaks for the two Angelus materials were similar before and after implantation, probably due to the very small amount of magnesium detected by the EDS characterisation. No previous studies have characterised this material composition using these techniques.
The material flow is a crucial property to perform the aimed sealing of the root canal complex anatomic structures [56]. In the present study, all materials had flow values that met the requirement (> 17 mm) of the ISO 6876:2012 standard. Similar flow was reported in a previous study [57] for EndoSequence BC Sealer (23.1 mm) and AH Plus (21.2 mm). Another previous study [19] reported a similar flow for AH Plus (around 20 mm) but a higher value for Bio-C Sealer (around 31 mm). This result discrepancy might be associated with a different batch of Bio-C Sealer used once the same weight (120 g) and material amount were used in both flow analysis; besides, the methodological feasibility of precisely dosing 0.05 mL of material for this test ought to be regarded when comparing flow values by different studies. In the present study, Bio-C Sealer Ion+ exhibited a significantly lower average flow (17.1 mm) in comparison to Bio-C Sealer (20 mm), which may be attributed to the differences in their compositions, material batch and raw material used for these sealers’ manufacturing. No other studies have evaluated this property of Bio-C Sealer Ion+.
When pre-mixed calcium silicate cement sealers are used clinically, the hydration reaction starts when the sealer contacts the moisture present inside the root canal [17]. Therefore, it is essential to evaluate in vitro their setting time in conditions simulating the hydration that occurs inside the root canal in providing moist conditions [2]. In the present study, Bio-C Sealer had the shortest setting time in either methods. The present results for setting time widely vary from those of previous studies [19, 57,58,59]. This fact poses a crucial concern considering the clinical use of pre-mixed water-dependent sealers, especially regarding the root canal drying performed, which is fully operator-dependent, before obturation in which the residual moist interferes in the sealers’ set and consequently in its long-term sealing [17].
The choice of a stable, long-term endodontic sealer for obturation with gutta-percha points is essential to preventing bacterial leakage and providing a successful endodontic treatment outcome [60]. The ISO 6876:2012 requirement for solubility of the endodontic sealer is that the material solubility would not exceed 3%. In the present study, using a previous study [16] as methodological reference, AH Plus Jet exhibited the lowest solubility, which corroborates with previous studies [19, 28, 60] that evaluated AH Plus. All pre-mixed materials evaluated in the present study obtained solubility values above 8% after 30-day immersion, representing a crucial concern for the long-term stability of these materials.
An alkaline pH was measured after hydration of the calcium silicate cement sealers due to calcium and hydroxyl ions release, which is a desirable property to induce local repair after endodontic treatments [61]. All the cement-based sealers had alkaline pH, decreasing during the 21-day analysis. Our results corroborate with previous studies [19, 55] that also reported an alkaline pH of pre-mixed cements, using the same periods of analysis. AH Plus was previously reported [19] to exhibit an acidic pH, but our results exhibited a neutral pH ranging from 7.8 and decreasing to 7.1 after 21 days. The significantly highest pH decrease was observed for Bio-C Sealer Ion+ during analysis; no previous studies evaluated this sealer regarding pH.
Taken together, the results here reported indicated an unpredictable setting time of calcium silicate cement sealers. Besides, a high solubility could be associated with these materials’ compositions where the release of calcium and hydroxyl ions from the material exerts biological interactions. AH Plus Jet set exhibited negligible solubility, but this material provoked higher inflammation. Taken together, the results here discussed agree with a previously reported systematic review that also compared endodontic sealers’ properties [21].
Conclusion
The three pre-mixed calcium silicate cement sealers were variable regarding their setting times, and had solubility but all exhibited a decreasing inflammatory response over time, less than AH Plus Jet. The clinical use of these soluble compositions is not indicated.
References
Viapiana R, Flumignan DL, Guerreiro-Tanomaru JM et al (2014) Physicochemical and mechanical properties of zirconium oxide and niobium oxide modified Portland cement-based experimental endodontic sealers. Int Endod J 47:437–448. https://doi.org/10.1111/iej.12167
Janini ACP, Pelepenko LE, Gomes BPFA, Marciano MA (2022) Physico-chemical properties of calcium silicate-based sealers in powder/liquid and ready-to-use forms. Braz Dent J 33:18–25. https://doi.org/10.1590/0103-6440202204832
Sanz JL, Guerrero-Gironés J, Pecci-Lloret MP et al (2021) Biological interactions between calcium silicate-based endodontic biomaterials and periodontal ligament stem cells: a systematic review of in vitro studies. Int Endod J. https://doi.org/10.1111/iej.13600
Marciano MA, Duarte MAH, Camilleri J (2016) Calcium silicate-based sealers: assessment of physicochemical properties, porosity and hydration. Dent Mater 32:e30–e40. https://doi.org/10.1016/j.dental.2015.11.008
Duarte MAH, Marciano MA, Vivan RR, Tanomaru Filho M, Tanomaru JMG, Camilleri J (2018) Tricalcium silicatebased cements: properties and modifications. Braz Oral Res 18:32(suppl 1):e70. https://doi.org/10.1590/1807-3107bor-2018.vol32.0070
Scarparo RK, Grecca FS, Fachin EVF (2009) Analysis of tissue reactions to methacrylate resin-based, epoxy resin-based, and zinc oxide-eugenol endodontic sealers. J Endod 35:229–232. https://doi.org/10.1016/j.joen.2008.10.025
Kebudi Benezra M, Schembri Wismayer P, Camilleri J (2017) Influence of environment on testing of hydraulic sealers. Sci Rep 7:17927. https://doi.org/10.1038/s41598-017-17280-7
Khalil I, Naaman A, Camilleri J (2016) Properties of tricalcium silicate sealers. J Endod 42:1529–1535. https://doi.org/10.1016/j.joen.2016.06.002
Marciano MA, Guimarães BM, Ordinola-Zapata R et al (2011) Physical properties and interfacial adaptation of three epoxy resin-based sealers. J Endod 37:1417–1421. https://doi.org/10.1016/j.joen.2011.06.023
Generali L, Prati C, Pirani C et al (2017) Double dye technique and fluid filtration test to evaluate early sealing ability of an endodontic sealer. Clin Oral Investig 21:1267–1276. https://doi.org/10.1007/s00784-016-1878-0
Okamoto M, Matsumoto S, Moriyama K et al (2022) Biological evaluation of the effect of root canal sealers using a rat model. Pharmaceutics 14. https://doi.org/10.3390/pharmaceutics14102038
De-Deus G, Souza EM, Silva EJNL et al (2022) A critical analysis of research methods and experimental models to study root canal fillings. Int Endod J 55(Suppl 2):384–445. https://doi.org/10.1111/iej.13713
Tagger M, Katz A (2003) Radiopacity of endodontic sealers: development of a new method for direct measurement. J Endod 29:751–755. https://doi.org/10.1097/00004770-200311000-00016
van der Burgt TP, Mullaney TP, Plasschaert AJ (1986) Tooth discoloration induced by endodontic sealers. Oral Surg Oral Med Oral Pathol 61:84–89. https://doi.org/10.1016/0030-4220(86)90208-2
Zhang W, Liu H, Wang Z et al (2022) Long-term porosity and retreatability of oval-shaped canals obturated using two different methods with a novel tricalcium silicate sealer. Clin Oral Investig 26:1045–1052. https://doi.org/10.1007/s00784-021-04088-z
Antunes TBM, Janini ACP, Pelepenko LE et al (2021) Heating stability, physical and chemical analysis of calcium silicate-based endodontic sealers. Int Endod J 54:1175–1188. https://doi.org/10.1111/iej.13496
Ozlek E, Gündüz H, Akkol E, Neelakantan P (2020) Dentin moisture conditions strongly influence its interactions with bioactive root canal sealers. Restor Dent Endod 45:1–9. https://doi.org/10.5395/rde.2020.45.e24
Angelus (2019) Bio-C Sealer Ion+ brochure. 1–11. Available at: https://www.angelusdental.com/img/arquivos/bio_c_sealer_ion_angelus_dental.pdf
Zordan-Bronzel CL, Esteves Torres FF, Tanomaru-Filho M et al (2019) Evaluation of physicochemical properties of a new calcium silicate–based sealer, Bio-C Sealer. J Endod 45:1248–1252. https://doi.org/10.1016/j.joen.2019.07.006
Chen B, Haapasalo M, Mobuchon C et al (2020) Cytotoxicity and the effect of temperature on physical properties and chemical composition of a new calcium silicate–based root canal sealer. J Endod 46:531–538. https://doi.org/10.1016/j.joen.2019.12.009
Silva Almeida LH, Moraes RR, Morgental RD, Pappen FG (2017) Are premixed calcium silicate–based endodontic sealers comparable to conventional materials? A systematic review of in vitro studies. J Endod 43:527–535. https://doi.org/10.1016/j.joen.2016.11.019
Schilder H (2006) Filling root canals in three dimensions. J Endod 32:281–290. https://doi.org/10.1016/j.joen.2006.02.007
Poggio C, Dagna A, Ceci M et al (2017) Solubility and pH of bioceramic root canal sealers: a comparative study. J Clin Exp Dent:e1189–e1194. https://doi.org/10.4317/jced.54040
Donnermeyer D, Bürklein S, Dammaschke T, Schäfer E (2019) Endodontic sealers based on calcium silicates: a systematic review. Odontology 107:421–436. https://doi.org/10.1007/s10266-018-0400-3
ISO (2012) International Organization for Standardization - Dentistry - Root canal sealing materials. 6876:3–4
Materials D (1984) ANSI/ADA specification no. 57 for endodontic filling materials. J Am Dent Assoc 108:88. https://doi.org/10.14219/jada.archive.1984.0208
Zmener O, Spielberg C, Lamberghini F, Rucci M (1997) Sealing properties of a new epoxy resin-based root-canal sealer. Int Endod J 30:332–334. https://doi.org/10.1046/j.1365-2591.1997.00086.x
Schafer E, Zandbiglari T (2003) Solubility of root-canal sealers in water and artificial saliva. Int Endod J 36:660–669. https://doi.org/10.1046/j.1365-2591.2003.00705.x
Saleh IM, Ruyter IE, Haapasalo M, Orstavik D (2002) The effects of dentine pretreatment on the adhesion of root-canal sealers. Int Endod J 35:859–866. https://doi.org/10.1046/j.1365-2591.2002.00585.x
Adanir N, Çobankara FK, Belli S (2006) Sealing properties of different resin-based root canal sealers. J Biomed Mater Res Part B Appl Biomater 77B:1–4. https://doi.org/10.1002/jbm.b.30408
Miletic I, Anic I, Pezelj-Ribaric S, Jukic S (1999) Leakage of five root canal sealers. Int Endod J 32:415–418. https://doi.org/10.1046/j.1365-2591.1999.00254.x
Haïkel Y, Freymann M, Fanti V et al (2000) Apical microleakage of radiolabeled lysozyme over time in three techniques of root canal obturation. J Endod 26:148–152. https://doi.org/10.1097/00004770-200003000-00005
Saleh IM, Ruyter IE, Haapasalo M, Ørstavik D (2004) Survival of Enterococcus faecalis in infected dentinal tubules after root canal filling with different root canal sealers in vitro. Int Endod J 37:193–198. https://doi.org/10.1111/j.0143-2885.2004.00785.x
Siqueira JF, Favieri A, Gahyva SM et al (2000) Antimicrobial activity and flow rate of newer and established root canal sealers. J Endod 26:274–277. https://doi.org/10.1097/00004770-200005000-00005
Azar NG, Heidari M, Bahrami ZS, Shokri F (2000) In vitro cytotoxicity of a new epoxy resin root canal sealer. J Endod 26:462–465. https://doi.org/10.1097/00004770-200008000-00008
Zavattini A, Knight A, Foschi F, Mannocci F (2020) Outcome of root canal treatments using a new calcium silicate root canal sealer: a non-randomized clinical trial. J Clin Med 9:782. https://doi.org/10.3390/jcm9030782
Nagendrababu V, Kishen A, Murray PE et al (2021) PRIASE 2021 guidelines for reporting animal studies in Endodontology: a consensus-based development. Int Endod J 54:848–857. https://doi.org/10.1111/iej.13477
Benetti F, de Azevedo Queiroz ÍO, PHC de O et al (2019) Cytotoxicity and biocompatibility of a new bioceramic endodontic sealer containing calcium hydroxide. Braz Oral Res 33:e42. https://doi.org/10.1590/1807-3107bor-2019.vol33.0042
Alves Silva EC, Tanomaru-Filho M, da Silva GF et al (2020) Biocompatibility and bioactive potential of new calcium silicate–based endodontic sealers: Bio-C Sealer and Sealer Plus BC. J Endod. 46(10):1470–1477. https://doi.org/10.1016/j.joen.2020.07.011
Flecknell P (2002) Replacement, reduction and refinement. ALTEX Altern zu Tierexperimenten 19:73–78
Paiva EM, Ribessi RL, Pereira CF, Rohwedder JJR (2020) Low-frequency Raman spectrophotometer with wide laser illumination on the sample: a tool for pharmaceutical analytical analysis. Spectrochim Acta Part A Mol Biomol Spectrosc 228:117798. https://doi.org/10.1016/j.saa.2019.117798
Eilers PHC (2003) A perfect smoother. Anal Chem 75:3631–3636. https://doi.org/10.1021/ac034173t
Carvalho-Junior JR, Correr-Sobrinho L, Correr AB et al (2007) Solubility and dimensional change after setting of root canal sealers: a proposal for smaller dimensions of test samples. J Endod 33:1110–1116. https://doi.org/10.1016/j.joen.2007.06.004
Kim J-H, Cho S-Y, Choi Y et al (2022) Clinical efficacy of sealer-based obturation using calcium silicate sealers: a randomized clinical trial. J Endod 48:144–151. https://doi.org/10.1016/j.joen.2021.11.011
Bardini G, Casula L, Ambu E et al (2021) A 12-month follow-up of primary and secondary root canal treatment in teeth obturated with a hydraulic sealer. Clin Oral Investig 25:2757–2764. https://doi.org/10.1007/s00784-020-03590-0
Clause BT (1993) The Wistar rat as a right choice: establishing mammalian standards and the ideal of a standardized mammal. J Hist Biol 26:329–349. https://doi.org/10.1007/BF01061973
de Oliveira PHC, Gomes Filho JE, MJ da S R et al (2022) Influence of supplement administration of omega-3 on the subcutaneous tissue response of endodontic sealers in Wistar rats. Int Endod J 55:1026–1041. https://doi.org/10.1111/iej.13795
Almeida LH, Gomes APN, Gastmann AH et al (2019) Bone tissue response to an MTA-based endodontic sealer, and the effect of the addition of calcium aluminate and silver particles. Int Endod J 52:1446–1456. https://doi.org/10.1111/iej.13135
Marques Costa VS, Emerenciano Bueno C, Valentim D et al (2021) Biocompatibility and immunolabeling of fibronectin and tenascin of resinous root canal sealers. J Conserv Dent 24:323. https://doi.org/10.4103/jcd.jcd_628_20
Wismayer PS, Lung CYK, Rappa F et al (2016) Assessment of the interaction of Portland cement-based materials with blood and tissue fluids using an animal model. Nat Publ Gr 1–9. https://doi.org/10.1038/srep34547
Húngaro Duarte MA, de Oliveira El Kadre GD, Vivan RR et al (2009) Radiopacity of Portland cement associated with different radiopacifying agents. J Endod 35:737–740. https://doi.org/10.1016/j.joen.2009.02.006
Salineiro FCS, Talamoni IP, Velasco SK et al (2019) Artifact induction by endodontic materials. Clin Lab Res Dent. https://doi.org/10.11606/issn.2357-8041.clrd.2019.155624
Pelepenko LE, Saavedra F, Antunes TBM et al (2021) Physicochemical, antimicrobial, and biological properties of White-MTAFlow. Clin Oral Investig 25:663–672. https://doi.org/10.1007/s00784-020-03543-7
Moinzadeh AT, Aznar Portoles C, Schembri Wismayer P, Camilleri J (2016) Bioactivity potential of endo sequence BC RRM putty. J Endod 42:615–621. https://doi.org/10.1016/j.joen.2015.12.004
GT de M C, Correia FC, MAH D et al (2012) Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J Endod 38:842–845. https://doi.org/10.1016/j.joen.2012.02.029
Estrela C, Pécora JD, Estrela CRA et al (2017) Common operative procedural errors and clinical factors associated with root canal treatment. Braz Dent J 28:179–190. https://doi.org/10.1590/0103-6440201702451
Zhou H, Shen Y, Zheng W et al (2013) Physical properties of 5 root canal sealers. J Endod 39:1281–1286. https://doi.org/10.1016/j.joen.2013.06.012
Loushine BA, Bryan TE, Looney SW et al (2011) Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J Endod 37:673–677. https://doi.org/10.1016/j.joen.2011.01.003
Xuereb M, Vella P, Damidot D et al (2015) In situ assessment of the setting of tricalcium silicate–based sealers using a dentin pressure model. J Endod 41:111–124. https://doi.org/10.1016/j.joen.2014.09.015
Ersahan S, Aydin C (2013) Solubility and apical sealing characteristics of a new calcium silicate-based root canal sealer in comparison to calcium hydroxide-, methacrylate resin- and epoxy resin-based sealers. Acta Odontol Scand 71:857–862. https://doi.org/10.3109/00016357.2012.734410
Koutroulis A, Kuehne SA, Cooper PR, Camilleri J (2019) The role of calcium ion release on biocompatibility and antimicrobial properties of hydraulic cements. Sci Rep 9:19019. https://doi.org/10.1038/s41598-019-55288-3
Acknowledgements
The authors wish to thank Ana Cristina do Amaral Godoy for her technical assistance during histological processing, Adriano Luis Martins for his technical assistance in SEM/EDS and Rafael Soares de Sousa for his technical assistance in animal care.
Funding
This study was supported by the Sao Paulo Research Foundation (FAPESP 2019/22098-9). This study was also financed in part by the Coordination for the Improvement of Higher Education Personnel (CAPES) – Finance Code 001.
Author information
Authors and Affiliations
Contributions
Conceptualization: L.E.P., A.C.P.J. and M.A.M.; methodology: L.E.P., A.C.P.J., V.A.B.S, N.A.S, I.M.R. Jr., B.P.F.A.G., J.M.B and M.A.M.; software: A.C.P.J. and M.A.M.; validation: L.E.P., A.C.P.J.; formal analysis: L.E.P. and M.A.M.; investigation: L.E.P., A.C.P.J. and M.A.M.; resources: M.A.M.; data curation: L.E.P., A.C.P.J., V.A.B.S, N.A.S, I.M.R. Jr., B.P.F.A.G. and M.A.M.; writing—original draft preparation: L.E.P.; writing—review and editing: M.A.M., B.P.F.A.G. and. I.M.R. Jr.; visualisation: L.E.P. and M.A.M.; supervision: M.A.M., B.P.F.A.G. and I.M.R. Jr.; project administration: M.A.M.; funding acquisition: M.A.M. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study was approved by the institutional ethics committee animal research of the Piracicaba Dental School (code CEUA 5387-1/2019).
Consent to participate
This study did not involve humans.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Janini, A.C.P., Pelepenko, L.E., Boldieri, J.M. et al. Biocompatibility analysis in subcutaneous tissue and physico-chemical analysis of pre-mixed calcium silicate–based sealers. Clin Oral Invest 27, 2221–2234 (2023). https://doi.org/10.1007/s00784-023-04957-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-04957-9