Abstract
Gels with high concentrations of hydrogen peroxide (H2O2) have been associated with cytotoxicity and consequent post-bleaching tooth sensitivity. This study assessed the bleaching efficacy (BE) and cytotoxicity (CT) of bleaching gels with low concentrations of H2O2 containing manganese oxide (MnO2) and photocatalyzed with violet LED (LEDv). The following groups were established: G1: no treatment (negative control, NC); G2: 35% H2O2 (positive control, PC); G3: LEDv; G4: 10% H2O2; G5: 6% H2O2; G6: 10% H2O2 + MnO2 + LEDv; G7: 6% H2O2 + MnO2 + LEDv. To analyze BE, standardized enamel/dentin discs (E/DDs) were subjected to the bleaching procedures for 45 min (1 session). The color change was determined before and after performing the bleaching protocols (ΔE00; ΔWI). To analyze CT, the E/DDs were adapted to artificial pulp chambers, and the extracts (culture medium + diffused gel components) were applied to cultured odontoblast-like MDPC-23 cells. Then, the cells were assessed concerning their viability (VB), oxidative stress (OxS), and Live/Dead. The amount of H2O2 diffused was also determined (ANOVA/Tukey; p < 0.05). Cell viability decreased in all bleached groups compared to G1 (NC; p < 0.05). The cells in G6 and G7 presented higher viability than in G2, G4, and G5 (p < 0.05). The BE in G7 was similar to G2 (PC; p < 0.05). The lowest OxS and H2O2 diffusion values were found in G6 and G7, compared to the other bleached groups (G2, G4, and G5; p < 0.05). The 6% H2O2 bleaching gel (G7) submitted to both methods of catalysis (MnO2 + LEDv) caused only a mild cytotoxicity and maintained the excellent esthetic outcome promoted by in-office conventional tooth bleaching.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tooth bleaching, one of the esthetic procedures most performed in the dental office, has been effective in reducing color pigments responsible for tooth darkening [1]. For this professional therapy, bleaching gels with high concentrations of hydrogen peroxide (H2O2) have been used to achieve fast and satisfactory clinical outcomes [2, 3]. However, a number of studies has shown that the in-office dental bleaching results in a high amount of H2O2 that does not react with the chromophores present in teeth [4, 5]. The authors showed that the residual H2O2, termed as free-H2O2 can diffuse through dentinal tubules to damage pulp cells. Hypothetically, the intense toxic effects caused by free-H2O2 to pulp cells would explain, at least partially, the post-bleaching tooth sensitivity reported by most patients subjected to conventional in-office tooth bleaching [6, 7]. Previous in vitro investigations showed that the intensity of toxic effect of such professional treatment to pulp cells is directly proportional to the time of contact of the bleaching gel with the enamel and concentration of H2O2 in the product [5]. Thus, performing in-office dental bleaching with low-concentrated gels seems to be an interesting strategy to ensure a safer and painless professional esthetic therapy for patients [8, 9]. A clinical trial performed by Bersezio et al. (2019) [10] showed that bleaching gels with low concentration of H2O2 may provide a satisfactory esthetic outcome. The authors reported that the bleaching effect achieved when using a gel with 6% H2O2 remained for longer periods of time compared to gels containing 35% H2O2.
The decomposition of the H2O2 molecules and consequent production of other reactive oxygen species (ROS), such as the hydroxyl ions (OH•), favor the esthetic efficacy of tooth bleaching and reduce the amount of free-H2O2 that can reach the pulp space [4, 11,12,13]. Thus, the chemical catalysis and/or photocatalysis of H2O2 in bleaching gels seem to be promising strategies for in-office tooth bleaching [14,15,16]. The current literature has shown the effective H2O2 decomposition in other ROS through different transition metals, such as catalyst agents derived from manganese [11, 13, 14]. However, among the chemical catalysts used for H2O2 decomposition, manganese oxide (MnO2) stands out because of its low cost [17] as well as because its chemical reaction does not produce by-products in the oxidation process, contrary to the chemical reaction mediated by iron derivatives [14]. A recent study by Chiam et al. (2020) [18] showed the catalyst potential of MnO2 when associated with visible light. Therefore, this oxide would work as a chemical catalyst and a photocatalyst of organic pigments and could be employed promisingly in bleaching therapies associated with the use of light sources.
The photocatalysis of H2O2 with violet LED has been investigated as a strategy to optimize the tooth bleaching outcome [19]. The violet LED wavelength can penetrate the enamel and dentin to react with local chromophores, causing tooth bleaching by photolysis [20]. Thus, violet LED would potentially cause chromatic changes in dental tissues even when not used in association with bleaching gels containing H2O2 [20, 21].
Based on the potential benefits of the chemical catalysis and photocatalysis of H2O2 to professional tooth bleaching, this study aimed to assess the influence of the association of manganese oxide (MnO2) and violet LED on the esthetic efficacy and trans-amelodentinal cytotoxicity of bleaching gels with 6%- and 10% H2O2. The null hypothesis of this study was that reducing H2O2 concentrations in the gels, associated with different types of catalysis of this ROS, does not affect the esthetic efficacy and cytotoxicity of bleaching treatments.
Materials and methods
Sample size calculation
The number of biological replicates for the response variables “esthetic efficacy” and “cytotoxicity” was calculated using the G*Power software (version 3.1; University Dusseldorf, Dusseldorf, Germany). Power (1-β error probability) and α error probability were 0.80 and 0.05, respectively. The effect size for esthetic efficacy was 4.03 and the trans-amelodentinal cytotoxicity was 3.35. The estimated sample size of 8 specimen/group was defined for all response variables. Each protocol was conducted on two separate experimental occasions (duplicates) to minimize systematic errors and allow the observation of reproducibility.
Formulation of the experimental bleaching gels (n = 8)
The Carbopol thickener (polyacrylic acid Mv ~ 3,000,000, Sigma-Aldrich, St. Louis, MO, USA) was obtained by preparing a solution at 1% (v/m). The liquid phase containing 6%, 10%, or 35% H2O2 was prepared by diluting a stock solution with 35% H2O2 (35% hydrogen peroxide P.A.; Neon, Suzano, SP, Brazil). Next, 10 mg/mL of MnO2 (Sigma-Aldrich, St. Louis, MO, USA) was incorporated in the bleaching gel aliquots corresponding to the groups that received the catalyst agent (MnO2) associated with H2O2.
Achievement and standardization of enamel/dentin discs (E/DDs; n = 8)
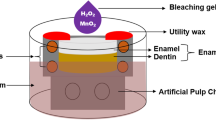
One hundred and twelve standardized E/DDs (5.6 mm of diameter) were obtained from intact bovine incisors with a trephine diamond bur (Dinser Brocas Diamantadas Ltda., São Paulo, SP, Brazil) attached to a bench drill (FSB 16 Pratika, Schμltz, Joinville, SC, Brazil). The dentin surface was corrected by wear with sandpapers of 400 and 600-granulations (T469-SF-Noton, Saint-Gobam Abrasivos Ltda, Jundiaí, SP, Brazil) and manual rotary movements for each granulation until obtaining a standardized thickness of 2.3 mm of E/DD, simulating the thickness of lower human incisors [5, 22].
Assessment of bleaching efficacy (n = 8)
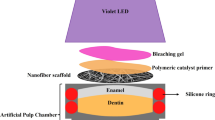
The E/DDs were subjected to laboratory staining with black tea, as previously described by de Oliveira Duque et al. (2020) [26], and distributed into groups (Table 1). The bleaching agents were manipulated immediately before their application to enamel, and the bleaching protocol was standardized in one session of 45 min. For the groups that used violet LED (Bright Max Whitening, MMOptics, São Carlos, SP, Brazil), irradiation was applied for 20 min without an interval during the bleaching session [15]; the device has 4 LED diodes emitting in the spectral range of 405 ± 10 nm, output power of 350 mW each LED, totaling 1.5 W of optical power and irradiance of 140.2 mW/cm2. After placing the discs in a white silicone matrix, bleaching efficacy was analyzed with a UV-reflective spectrophotometer (Reflection Spectrophotometer ΜL Color Guide, BYK Gardner GmbH Gerestsried, Germany). The values of L*a*b* obtained before (baseline) and 72 h after the bleaching procedures were used to assess color changes (CIEDE 2000, ΔE00). The whitening index (ΔWI) was determined according to the following equations:
Next, the color parameters (L*, a*, b*, ΔE00, and ΔWI) obtained for all groups were analyzed statistically (two-way ANOVA; Tukey’s test). The values of perceptibility threshold (PT) and acceptability threshold (AT) were used as 50:50%, ΔE00 values were 0.8 (PT) and 2.7 (AT), and ΔWI values were 0.72 (PT) and 2.60 (AT) [24].
MDPC-23 cell culture
Immortalized odontoblast-like MDPC-23 cells maintained in the Laboratory of Experimental Pathology and Biomaterials of the São Paulo State University (UNESP), School of Dentistry, Araraquara, Brazil, were cultivated in sterilized plates of 75 cm2 (KASVI Imp., Curitiba, PR, Brazil) with Dulbecco's Modified Eagle's medium (DMEM; GIBCO. Grand Island, NY, USA) containing 10% fetal bovine serum (FBS; GIBCO); 100 and 100 μg/mL, respectively, of penicillin and streptomycin; and 2 mmol/L of glutamine (GIBCO), in a wet atmosphere at 37 °C, 5% CO2, and 95% air. The cells were passed until obtaining an adequate number for experimenting.
Experimental procedure
The E/DDs were individually adapted to artificial pulp chambers (APCs), and the disc/APC sets were sterilized in ethylene oxide (Acecil, Central de Esterilização Comércio e Indústria LTDA, Campinas, SP, Brazil). Then, each set was individually placed in the compartment of 24-well sterilized acrylic plates (KASVI Imp.) containing 1 mL of DMEM [5,6,7]. Immediately after concluding the bleaching procedures, the extracts (DMEM + bleaching gel components diffused across the E/DDs) were collected and stored in aliquots of 100 µL in cryogenic tubes for later use in cytotoxicity tests [22]
Trans-amelodentinal cytotoxicity assessment
To determine the indirect cytotoxic effects caused by the bleaching protocols, the MDPC-23 cells were subjected to three specific analyses:
Cell viability (Alamar Blue assay; n = 8)
Aliquots of 100 µL of each extract were applied to the MDPC-23 cells previously cultivated (10,000 cells) in compartments of 96-well sterilized acrylic plates (KASVI Imp.). One hour after incubation, the extracts were aspirated and the MDPC-23 cells that remained adhered to the bottom of the compartments were incubated for 4 h in contact with a solution composed of 90 µL of DMEM and 10 µL of Alamar Blue solution (Life Technologies; Grand Island, NY, USA). Then, the solution was collected and transferred to a 96-well plate (KASVI Imp.), and fluorescence was measured at 540 nm excitation and 590 nm emission with a spectrophotometer (Synergy H1).
Oxidative stress (n = 8)
Cellular oxidative stress was assessed by estimating the reactive oxygen species (ROS) produced by the MDPC-23 cells, immediately after bleaching. For this purpose, cells previously cultivated in compartments of 96-well sterilized plates were exposed for 30 min to the carboxy-H2DCFDA probe (Invitrogen, San Francisco, CA, USA) at a concentration of 10 μg/mL. This probe is permeable to the cell membrane and emits fluorescence when in contact with ROS. Next, the aliquots of 100 µL of the extracts from each group were applied to the cells for 1 h, and fluorescence intensity was assessed at 592 nm excitation and 517 nm emission (Synergy H1) after the time of exposure to the extracts. The data were normalized by the negative control group [22].
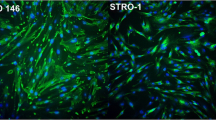
Live/Dead (n = 4)
One hour after exposure to the extracts, the MDPC-23 cells were assessed with the Live/Dead assay (Live/Dead cell viability/cytotoxicity kit; Invitrogen, Carlsbad, CA, USA). Therefore, the cells were incubated (n = 8) for 30 min in contact with 100 µL of DMEM, supplemented with Calcein AM and Ethidium Homodimer markers (Invitrogen) at the concentration of 1:1000. Next, the cells were assessed in a fluorescence microscope (Leica DM 5500B, Nussloch GmbH, Nussloch, Germany) to obtain representative images of each group.
Quantification of H2O2 in the extracts (n = 8)
Aliquots of 100 μL of the extracts obtained from each group were transferred to 5-Ml-plastic tubes (KASVI Imp.) containing 900 μL of acetate buffer solution (2 mol; pH 4.5), which allows stabilizing H2O2. Then, 500 μL of this solution was transferred to new 5-mL-plastic tubes (KASVI Imp.) containing water and leucocrystal violet dye (0.5 mg/mL, Sigma-Aldrich). The tubes were agitated in a vortex (Vortex Mixer VM300, Vixar; Plymouth, MN, USA), and 50 μL of a solution of 1 mg/mL of horseradish peroxidase enzyme (Sigma-Aldrich) was added. The absorbance of solutions was measured in a spectrophotometer (Synergy H1) at a 596-nm wavelength. The standard curve of the known amounts of H2O2 was used for converting the optical density values obtained in the samples into μg of H2O2 per mL of extract [4, 5, 22].
Statistical analysis
Data were subjected to the Shapiro–Wilk test to assess normality, the Levene test to assess homoscedasticity, followed by a two-way analysis of variance (two-way ANOVA), considering the independent variables “concentration of H2O2” and “presence or absence of MnO + LED,” complemented by Tukey’s post-test. The analyses were performed using the SPSS (version 26.0; IBM, Chicago, IL, USA) and GraphPad Prism (version 9.0; GraphPad, San Diego, CA, USA) and statistical inferences were based on a 5% significance level.
Results
Bleaching efficacy
According to the results presented in Fig. 1, the ΔE00 and ΔWI values significantly increased in G2, G3, G4, G5, G6, and G7, compared to the negative control (G1) (p < 0.05). The highest ΔE00 and ΔWI values were found in G6 (10% H2O2 + MnO2 + LEDv) and G7 (6% H2O2 + MnO2 + LEDv) compared to G2 (35% H2O2), G3 (LEDv), G4 (10% H2O2), and G5 (6% H2O2) (p < 0.05). The G4 and G5 presented statistically similar ΔE00 and ΔWI values (p > 0.05).
Statistical analysis of CIEDE 2000 (ΔE00) and ΔWI values for each group at different H2O2 concentrations, associated or not with MnO2 + LED. The numbers are mean values of CIEDE 200 (ΔE00) and ΔWI corresponding to each H2O2 concentration used. Lower-case letters allow comparing H2O2 concentrations for each group (two-way ANOVA; Tukey’s test). Different letters show statistically significant differences (p < 0.05)
Trans-amelodentinal cytotoxicity
Cell viability (VB) significantly decreased in all bleached groups, compared to G1 (negative control) and G3 (LEDv) (p < 0.05). The VB in G6 and G7 was higher than in G2, G3, G4, and G5 (p < 0.05). Overall, the groups that used MnO2 + LEDv to catalyze the bleaching gels (G6 and G7) presented higher MDPC-23 cell viability (Fig. 2A). Higher oxidative stress (OxS) occurred in G2, G4, G5, G6, and G6 than in G1 and G3 (Fig. 2B; p < 0.05). However, the H2O2 catalysis performed with the association of MnO2 + LEDv (G6 and G7) caused lower OxS than in G4, G5, and G2 (p < 0.05) (Fig. 2B). Figure 3 shows the photomicrographs obtained with the Live/Dead assay. There was a lower number of living cells (marked with Calcein AM — green) and a higher number of dead cells (marked with EthD-1 — red) in all experimental groups (G2 to G7) than in G1 (negative control). However, there was a higher number of living cells adhered to the acrylic substrate in G6 and G7 than in G2, G3, G4, and G5.
Lower trans-amelodentinal diffusion of H2O2 occurred in G6 and G7 than in G2, G4, and G5, in which gels with 35, 10, and 6% H2O2 were applied directly to the enamel (p < 0.05; Fig. 2C). In G6 and G7, in which 10 and 6% H2O2 bleaching gels were associated with MnO2 + LED, the trans-amelodentinal diffusion of H2O2 decreased by around 42% and 65%, respectively, compared to G2 (positive control).
Discussion
Researchers have demonstrated the severe pulpal damage caused by conventional in-office tooth bleaching therapies [7, 25]. This adverse effect has been related to the high amount of H2O2 that do not react with the chromophores of teeth (free-H2O2), and thus diffuse through the enamel and dentin to reach the pulpal space. When in contact with pulp cells, free-H2O2 triggers oxidative stress associated with lipid peroxidation, which invariably cause in irreversible cell damage [26]. As aforementioned, the intensity of pulp cell damage is directly related to the H2O2 concentration in gels and the application time on teeth [4]. To prevent or at least minimize the adverse effects caused by gels with high concentrations of H2O2, which are widely used for in-office tooth bleaching, innovative strategies have been proposed [4, 13,14,15,16, 22]. The present study assessed the influence of the photocatalysis (LEDv) and chemical catalysis (MnO2) of H2O2 in gels containing 6 and 10% of this reactive molecule on the esthetic efficacy and cytotoxicity of the treatment. Overall, associating LEDv + MnO2 in bleaching procedures with gels containing low H2O2 concentrations (G6 and G7) reduced cell viability by around 30%. However, the 35% H2O2 gel (G2 — positive control) reduced MDPC-23 cell viability by around 70%. Besides presenting the lowest indices of H2O2 diffusion and cellular OxS, G6, and G7 also showed ΔE00 and ΔWI values similar statistically to G2, which represented conventional in-office tooth bleaching. Based on these data, the null hypothesis of the present study was rejected.
Bovine teeth have been extensively employed in dental research, especially due to the ethical questions of using human teeth [27]. Moreover, these teeth exhibit structure and chemical composition similar to human teeth [28, 29]. In this way, the present study used bovine teeth to obtain enamel/dentin discs (E/DDs) standardized for thickness (2.3 mm) to simulate human lower incisors, which allow a higher trans-amelodentinal diffusion of free-H2O2 [4, 5] Therefore, the methodology selected for this in vitro study characterizes the ultimate challenge of indirect cytotoxicity tests of experimental gels and new bleaching therapy proposals [4, 5, 25]. To simulate clinical conditions, the present study used artificial pulp chambers (APCs) to analyze trans-amelodentinal cytotoxicity and quantify free-H2O2 that can reach the pulpal space [30]. Bovine E/DDs adapted to APCs represent a well-established laboratory methodology that has been used for almost a decade in studies seeking to develop and analyze more effective and safer bleaching strategies for patients [4, 5, 14,15,16, 22, 30].
Using enzymes (peroxidase) and transition metals (ferrous sulfate and manganese gluconate) as chemical activators of the bleaching gel accelerates H2O2 decomposition, reducing the amount of free-H2O2 that can diffuse through hard tissues and increasing the bleaching efficacy of the treatment [11, 13, 14]. In a study by Soares et al. (2019) [14], the heme peroxidase duplicated the bleaching potential of a bleaching gel after a single 45-min session of applying the product to the enamel. However, the high cost of the enzyme and the specific temperature and pH conditions required for peroxidase to catalyze H2O2 have turned this bleaching strategy inadequate for clinical application. Manganese oxide (MnO2) is abundantly found in nature and inexpensive, compared to enzymes [17]. Based on Fenton reactions, the mechanism of interaction of MnO2 with H2O2 might occur through the process of manganese reduction by the reactive molecule [32]: MnO2 + H2O2 → MnO (or Mn2O3) + H2O [18, 33]. Considering these factors and based on previous studies that showed the positive effects of associating manganese-derived agents with in-office bleaching therapy, this oxide was selected for the present study.
To optimize the bleaching procedure and reduce the adverse effects of professional bleaching, the photoactivation of bleaching gels with different light sources was initially proposed some years ago [34]. Among the most recently studied light sources, violet LED stands out due to its ability to minimize the harmful effects commonly caused by in-office tooth bleaching [20]. This is because the violet LED wavelength promotes chromatic changes in dental tissues even without associating it with H2O2 gels. However, the isolated use of violet LED provided a mild bleaching efficacy, which characterizes the limitation of this specific esthetic therapy [20, 21]. Hence, researchers have attempted to use violet LED associated with bleaching gels at different H2O2 concentrations, and the results have been as promising [19,20,21]. The present study analyzed bleaching efficacy with a UV-reflective spectrophotometer. This first phase of the study determined the color parameters according to methods established in the literature, in which ΔE00 (CIEDE 2000) and ΔWI (whitening index) values are considered to assess the final color result after bleaching. Different from G2 (35% H2O2 — positive control), G4 (10% H2O2), and G5 (6% H2O2), only G6 and G7 subjected the bleaching gels to the chemical catalysis (MnO2) and photocatalysis (LEDv) of H2O2. Overall, the 10% (G4) and 6% (G5) H2O2 gels reduced by approximately 50% the bleaching efficacy and whitening index, compared to G2. However, associating both catalysis methods applied to the 10% (G6) and 6% (G7) H2O2 gels determined an esthetic result similar to G2. These interesting scientific data indicate that catalyzing the H2O2 in bleaching gels with low concentrations of this toxic molecule may be an effective strategy to optimize tooth bleaching, potentially reducing pulp cell damage.
The trans-amelodentinal cytotoxicity of tooth bleaching protocols assessed in the present study was initially determined with the Alamar Blue assay. This analysis showed the lowest cell viability values in all groups bleached with H2O2 gels (G2, G4, G5, G6, and G7), compared to the negative control group (G1). However, among the bleached groups, the highest cell viability values were found in G6 and G7, which catalyzed H2O2 with MnO2 + LEDv. Therefore, besides achieving the same esthetic efficacy as conventional in-office bleaching (G2), the association of H2O2 catalysis strategy reduced the cytotoxic effects of the treatment by around 40%. A recent study by Gallinari et al. (2020) [35] reported that violet LED favored the chromatic change of teeth when combined with a 10% carbamide peroxide gel. However, this bleaching protocol contributed to post-treatment tooth sensitivity. Perhaps the warming of dental tissues caused by the light source determined this negative clinical outcome presented by Gallinari et al. (2020) [35], considering that the increased dental pulp temperature may result in harmful effects on cells of this specialized connective tissue [36]. High temperatures in the pulp caused by light devices such as violet LED [37] may potentiate tooth sensitivity, which is an adverse effect reported by most patients subjected to tooth bleaching [36]. However, the present study showed that the isolated application of violet LED to the enamel (G3) did not change MDPC-23 cell viability, which remained morphologically and functionally similar to G1 (control), the group characterized as 100% of cell viability. These interesting results determined that violet LED applied for 20 min to the enamel does not indirectly damage pulp cells. Therefore, considering the limitations of this laboratory research, using violet LED might not damage the pulp or induce tooth sensitivity. However, further investigations are required to explain and clarify this issue.
This study analyzed cellular oxidative stress (OxS) to complement the results of cytotoxicity caused by bleaching gels with low H2O2 concentrations, subjected or not to catalysis with MnO2 + LEDv. In this type of test, the amount of free radicals produced by cells is directly proportional to the cellular OxS promoted by the toxic molecule [22]. Overall, lower OxS values were observed in those groups in which gels with reduced H2O2 concentrations (G4 to G7) were used in comparison with G2 (positive control). It is worth noting that the cells in G6 and G7 presented the lowest OxS index, compared to G2, G4, and G5. According to previous studies, the data on cellular OxS explain, at least partially, the higher cell viability values when subjecting the H2O2 in the bleaching gels to different types of catalysis [14, 22]. Certainly, the low cell damages in groups G6 and G7 occurred because of the decreased trans-amelodentinal diffusion of free-H2O2, as shown in previous studies [4, 5, 14]. This is usually due to H2O2 decomposition by chemical [4, 11, 13, 14] and enzyme [22] catalysts, or even by the photocatalysis promoted by violet LED [15, 19].
Despite the interesting findings obtained in this in vitro study, which guide the establishment of bleaching strategies that are more effective and biocompatible with the dentin-pulp complex, further in vivo investigations and detailed clinical trials are still needed. As previously reported, the scientific data of laboratory research should be carefully interpreted and not extrapolated immediately to clinical conditions [30, 38].
Conclusion
Chemical catalysis (MnO2) associated with the photocatalysis (LEDv) of H2O2 present in a bleaching gel containing 6% of this reactive molecule maintains the bleaching efficacy achieved with conventional in-office tooth bleaching but significantly reduces trans-amelodentinal cytotoxicity usually caused by this professional esthetic therapy.
Data Availability
All procedures that involved the use of bovine teeth for scientific research purposes are in compliance with the precepts of Law No.11.794, of October 8, 2008, of Decree No 6.899, of July 15, and with the rules edited by the National Council of Control of Animal Experimentation (“Conselho Nacional de Controle da Experimentação Animal”) (CONCEA).
References
Suty H, De Traversay C, Cost M (2004) Applications of advanced oxidation processes: present and future. Water Sci Technol 49(4):227–233
Reis A, Kossatz S, Martins GC, Loguercio AD (2013) Efficacy of and effect on tooth sensitivity of in-office bleaching gel concentrations: a randomized clinical trial. Oper Dent 38(4):386–393. https://doi.org/10.2341/12-140-C
De Paula EA, Nava JA, Rosso C, Benazzi CM, Fernandes KT, Kossatz S, Loguercio AD, Reis A (2015) In-office bleaching with two- and seven-day intervals between clinical sessions: a randomized clinical trial on tooth sensitivity. J Dent 43(4):424–429. https://doi.org/10.1016/j.jdent.2014.09.009
De Oliveira Duque CC, Soares DG, Basso FG, Hebling J, de Souza Costa CA (2014) Bleaching effectiveness, hydrogen peroxide diffusion, and cytotoxicity of a chemically activated bleaching gel. Clin Oral Investig 18(6):1631–1637. https://doi.org/10.1007/s00784-013-1147-4
De Oliveira Duque CC, Soares DG, Basso FG, Hebling J, De Souza Costa CA (2017) Influence of enamel/dentin thickness on the toxic and esthetic effects of experimental in-office bleaching protocols. Clin Oral Investig 21(8):2509–2520. https://doi.org/10.1007/s00784-017-2049-7
Markowitz K (2010) Pretty painful: Why does tooth bleaching hurt? Med Hypotheses 74(5):835–840. https://doi.org/10.1016/j.mehy.2009.11.044
Roderjan DA, Stanislawczuk R, Hebling J, de Souza Costa CA, Reis A, Loguercio AD (2015) Response of human pulps to different in-office bleaching techniques: preliminary findings. Braz Dent J 26(3):242–248. https://doi.org/10.1590/0103-6440201302282
Bersezio C, Estay J, Jorquera G, Peña M, Araya C, Angel P, Fernández E (2019) Effectiveness of dental bleaching with 37.5% and 6% hydrogen peroxide and its effect on quality of life. Oper Dent 44(2):146–155. https://doi.org/10.2341/17-229-C
Bersezio C, Pardo C, Miranda S, Medeiros Maran B, da JorqueraSilva GRA Jr, Tonetto RodriguesFernández ME (2021) Evaluation of the effectiveness in teeth whitening of a single session with 6% hydrogen peroxide Laser/LED system. Photodiagnosis Photodyn Ther 36:102532. https://doi.org/10.1016/j.pdpdt.2021.102532
Bersezio C, Martín J, Angel P, Bottner J, Godoy I, Avalos F, Fernández E (2019) Teeth whitening with 6% hydrogen peroxide and its impact on quality of life: 2 years of follow-up. Odontology 107(1):118–125. https://doi.org/10.1007/s10266-018-0372-3
Torres CR, Wiegand A, Sener B, Attin T (2010) Influence of chemical activation of a 35% hydrogen peroxide bleaching gel on its penetration and efficacy - in vitro study. J Dent 38(10):838–846. https://doi.org/10.1016/j.jdent.2010.07.002
Travassos AC, Torres CR, Borges AB, Barcellos DC (2010) In vitro assessment of chemical activation efficiency during in office dental bleaching. Oper Dent 35(3):287–294. https://doi.org/10.2341/09-256-L
Torres CR, Souza CS, Borges AB, Huhtala MF, Caneppele TM (2013) Influence of concentration and activation on hydrogen peroxide diffusion through dental tissues in vitro. Sci World J 2013:193241. https://doi.org/10.1155/2013/193241
Soares DG, Marcomini N, Duque CCO, Bordini EAF, Ortecho-Zuta U, Basso F, Hebling J, de Souza Costa CA (2019) Increased whitening efficacy and reduced cytotoxicity are achieved by the chemical activation of a highly concentrated hydrogen peroxide bleaching gel. J Appl Oral Sci 27:20180453. https://doi.org/10.1590/1678-7757-2018-0453
Martins BV, Dias MF, de Oliveira Ribeiro RA, Leite MLAES, Hebling J, de Souza Costa CA (2022) Innovative strategy for in-office tooth bleaching using violet LED and biopolymers as H2O2 catalysts. Photodiagnosis Photodyn Ther 38:102886. https://doi.org/10.1016/j.pdpdt.2022.102886
Ortecho-Zuta U, de Oliveira Duque CC, de Oliveira Ribeiro RA, Leite ML, Soares DG, Hebling J, Briso ALF, de Souza Costa CA (2022) Strategy for reducing cytotoxicity and obtaining esthetic efficacy with 15 min of in-office dental bleaching. Clin Oral Investig 26(5):4099–4108. https://doi.org/10.1007/s00784-022-04379-z
Sun B, Guan X, Fang J, Tratnyek PG (2015) Activation of manganese oxidants with bisulfite for enhanced oxidation of organic contaminants: the involvement of Mn (III). Environ Sci Technol 49(20):12414–12421. https://doi.org/10.1021/acs.est.5b03111
Chiam SL, Pung SY, Yeoh FY (2020) Recent developments in MnO2- based photocatalysts for organic dye removal: a review. Environ Sci Pollut Res 27(6):5759–5778. https://doi.org/10.1007/s11356-019-07568-8
Gallinari MO, Fagundes TC, da Silva LM, de Almeida Souza MB, Barboza A, Briso ALF (2019) A new approach for dental bleaching using violet light with or without the use of whitening gel: study of bleaching effectiveness. Oper Dent 44(5):521–529. https://doi.org/10.2341/17-257-L
Kury M, Wada EE, Silva DP, Tabchoury CPM, Giannini M, Cavalli V (2020) Effect of violet LED light on in-office bleaching protocols: a randomized controlled clinical trial. J Appli Oral Sci 28:e20190720. https://doi.org/10.1590/1678-7757-2019-0720
Daltro TWS, Almeida SAG, Dias MF, Lins-Filho PC, Silva CHV, Guimarães RP (2020) The influence of violet LED light on tooth bleaching protocols: in vitro study of bleaching effectiveness. Photodiagnosis Photodyn Ther 32:102052. https://doi.org/10.1016/j.pdpdt.2020.102052
Ortecho-Zuta U, de Oliveira Duque CC, de Oliveira Ribeiro RA, Leite ML, Soares DG, Hebling J, Briso ALF, de Souza Costa CA (2021) Polymeric biomaterials maintained the esthetic efficacy and reduced the cytotoxicity of in-office dental bleaching. J Esthet Restor Dent 33(8):1139–1149. https://doi.org/10.1111/jerd.12805
de Oliveira Duque CC, Soares DG, Briso A, Ortecho-Zuta U, de Oliveira Ribeiro RA, Hebling J, de Souza Costa CA (2020) Influence of tooth pigmentation on H2O2 diffusion and its cytotoxicity after in-office tooth bleaching. Oper Dent 45(6):632–642. https://doi.org/10.2341/19-013-L
Paravina RD, Pérez MM, Ghinea R (2019) Acceptability and perceptibility thresholds in dentistry: a comprehensive review of clinical and research applications. J Esthet Restor Dent 31(2):103–112. https://doi.org/10.1111/jerd.12465
de Souza Costa CA, Riehl H, Kina JF, Sacono NT, Hebling J (2010) Human pulp responses to in-office tooth bleaching. Oral Surg Oral Med Oral Pathol Oral Radiol 109(4):59–64. https://doi.org/10.1016/j.tripleo.2009.12.002
Marson FC, Gonçalves RS, Silva CO, Cintra LT, Pascotto RC, Santos PH, Briso ALF (2015) Penetration of hydrogen peroxide and degradation rate of different bleaching products. Oper Dent 40(1):72–79. https://doi.org/10.2341/13-270-L
Enrich-Essvein T, Benavides-Reyes C, Álvarez-Lloret P, Bolaños-Carmona MV, Rodríguez-Navarro AB, González-López S (2021) Influence of de-remineralization process on chemical, microstructural, and mechanical properties of human and bovine dentin. Clin Oral Investig 25(3):841–849. https://doi.org/10.1007/s00784-020-03371-9
Schmalz G, Hiller KA, Nunez LJ, Stoll J, Weis K (2001) Permeability characteristics of bovine and human dentin under different pretreatment conditions. J Endod 27(1):23–30. https://doi.org/10.1097/00004770-200101000-00007
Teruel Jde.D, Alcolea A, Hernández A, Ruiz AJ, (2015) Comparison of chemical composition of enamel and dentine in human, bovine, porcine and ovine teeth. Arch Oral Biol 60(5):768–775. https://doi.org/10.1016/j.archoralbio.2015.01.014
de Souza Costa CA, Hebling J, Scheffel DL, Soares DG, Basso FG, Ribeiro AP (2014) Methods to evaluate and strategies to improve the biocompatibility of dental materials and operative techniques. Dent Mater 30(7):769–784. https://doi.org/10.1016/j.dental.2014.04.010
Soares DG, Marcomini N, Basso FG, Pansani TN, Hebling J, de Souza Costa CA (2014) Indirect cytocompatibility of a low-concentration hydrogen peroxide bleaching gel to odontoblast-like cells. Int Endod J 49(1):26–36. https://doi.org/10.1111/iej.12426
Yao S, Yuan S, Xu J, Wang Y, Luo J, Hu S (2006) A hydrogen peroxide sensor based on colloidal MnO2/Na-montmorillonite. Appl Clay Sci 33(1):35–42. https://doi.org/10.1016/j.clay.2006.03.006
Watts RJ, Sarasa J, Loge FJ, Teel AL (2005) Oxidative and reductive pathways in manganese-catalyzed Fenton’s reactions. J Environ Eng 131(1):158–164. https://doi.org/10.1061/(ASCE)0733-9372(2005)131:1(158)
Baroudi K, Hassan NA (2014) The effect of light-activation sources on tooth bleaching. Niger Postgrad Med J 55(5):363–368. https://doi.org/10.4103/0300-1652.140316
Gallinari MO, Cintra LTA, Barboza ACS, da Silva LMAV, de Alcantara S, Dos Santos PH, Fagundes TC, Briso ALF (2020) Evaluation of the color change and tooth sensitivity in treatments that associate violet LED with carbamide peroxide 10%: a randomized clinical trial of a split-mouth design. Photodiagnosis Photodyn Ther 30:101679. https://doi.org/10.1016/j.pdpdt.2020.101679
Eldeniz AU, Usumez A, Usumez S, Ozturk N (2005) Pulpal temperature rise during light-activated bleaching. J Biomed Mater Res Part B: Appl Biomater 72(2):254–259. https://doi.org/10.1002/jbm.b.30144
Guanaes BKA, Duarte TN, Correr GM, Kaizer MDR, Gonzaga CC (2022) In-office dental bleaching with violet light emitting diode: bleaching efficacy and pulpal temperature rise. Restor Dent Endod 47(1):e7. https://doi.org/10.5395/rde.2022.47.e7
de Souza Costa CA (2020) Biological aspects of dental materials. J Adhes Dent 22(5):540–544. https://doi.org/10.3290/j.jad.a45409
Funding
This study was partially supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP: grants 2021/01184–4, and 2020/09095–8), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; grants 302047/2019–0, and 408721/2018–9), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Finance Code 001).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dias, M.F., Martins, B.V., de Oliveira Ribeiro, R.A. et al. Improved esthetic efficacy and reduced cytotoxicity are achieved with a violet LED irradiation of manganese oxide-enriched bleaching gels. Lasers Med Sci 38, 2 (2023). https://doi.org/10.1007/s10103-022-03688-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10103-022-03688-9