Abstract
Objectives
The main objective of this study was to enhance the blockage of dentinal tubules using nanobioglass in the presence of diode (980 nm) and Nd:YAG lasers in order to reduce permeability and dentin hypersensitivity.
Materials and methods
Thirty-six dentinal samples were randomly divided into 6 subgroups (n = 6): (A) control, (B) diode laser (980 nm, 3-W), (C) Nd:YAG laser (1064, 1.0-W), (D) nanobioglass, (E) nanobioglass + diode laser (980 nm), (F) nanobioglass + Nd:YAG laser. The average number of open dentinal tubules was qualitatively and quantitatively evaluated by scanning electron microscopy (SEM). Data were evaluated by SPSS software version 22, Kruskal–Wallis test, and Mann–Whitney tests with Bonferoni’s correction (α = 0.008).
Results
Based on the results of Mann–Whitney test, there was a significant difference in the mean number of open dentinal tubules between the control group and the other groups (p < 0.008). However, the difference among the other groups was not statistically significant (p > 0.008).
Conclusions
Findings of this study showed that high-power laser radiation, such as Nd:YAG and diode (980 nm) alone or with nanobioglass, has a significant effect on the blockage of dentinal tubules.
Clinical relevance
Introduction of non-invasive methods with long-term and lasting effect on reducing pain and discomfort caused by dentin hypersensitivity
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
One of the most common issues in dentistry is that patients have to deal with dentin hypersensitivity (DH). The importance of this issue is evident as around 8–57% of adults suffer from increased dentin hypersensitivity [1]. Also, the prevalence of DH in patients with periodontal disease or those receiving basic therapies (such as scaling and root planning), as well as periodontal surgeries, is much higher (60–98%) [2]. Therefore, DH can be considered as one of the most painful chronic conditions with the least predictive value in terms of success in dental sciences. This is a painful response due to a variety of chemical, thermal, or osmotic stimuli on the uncovered dentin [1]. The success of various therapeutic methods and agents, such as fluoride and nitrate toothpastes, topical desensitizers (e.g., fluoride salts, potassium nitrate (KNO3), strontium chloride, and arginine), and resins, has been evaluated in the past studies. Also, the use of laser technology for treating tooth sensitivity has received attention [1].
More than 90% of the areas involved in DH are dentinal surfaces adjacent to the gingiva [2]. Recent studies have shown that healthy and non-sensitive dentin surfaces often have tubules with closed openings, while sensitive tissues have a large cumulative volume of open tubules [3,4,5,6,7]. The most important causes of uncovered root surfaces and exposure of dentinal tubules can be due to erosion, attrition, abrasion, or gingival recession following scaling and root planning [5].
The primary goal of DH treatment is changes in diameter and tubular content, and to this end, various desensitizer compounds and non-pharmacological treatments have been introduced. Generally, desensitizer agents can reduce dentinal sensitivity in two ways. The first method is to reduce the flow of tubular fluid, and the second one involves reducing the neuronal function through ion interactions affecting the transmission of neural signals. Consequently, compounds such as arginine 8% with calcium phosphate or ammonium hexafluorosilicate are substances that are primarily involved in the first method, while steronium chloride, potassium nitrate, and varnish fluoride are the compounds used in the second mechanism for the treatment of dentin hypersensitivity [5, 8, 9].
Laser technology is one of the most important non-pharmacological treatments that have been widely employed for treating DH. In this regard, various types of lasers, such as CO2, Nd:YAG, Er:YAG, and He-Ne, have been used [2, 5]. The effect of laser radiation on reducing dentinal hypersensitivity can be due to tubular obstruction or a change in the irritability threshold of pulpal neurons. However, several studies have been conducted on the ability of different lasers to obstruct tubules and to compare their function with desensitizer compounds [2, 10,11,12].
Another method involves using nanoparticles of various compounds to improve the quality of tubular obstruction by reducing the size and facilitating the penetration of particles into dentinal tubules. Bioglass with the ability to stimulate crystallization and to create a new mineral layer can be one of the most important agents in this field [13].
Although previous studies have introduced various therapeutic and non-drug therapies and some of them were somewhat successful, treating dentin hypersensitivity with long-term success is still considered as a major problem. Hence, the purpose of this study was to treat tooth sensitivity by enhancing the blockage of dentinal tubules using nanobioglass in the presence of diode (980nm) and Nd:YAG lasers.
Materials and methods
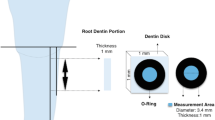
In this study, 36 dentinal samples were selected from third molar teeth without decay or restoration. Periodontal tissues were removed and placed in 0.1% thymol for 1 month and then kept in distilled water at 4 °C [14]. Then, the enamel of the occlusal surface was removed and dentin disks with dimensions of 4 * 4 * 2 mm were prepared by the microtome machine (Leica SP1600, Nussloch, Germany). Following this, the dentin disks were immersed in acrylic polymethylmethacrylate (Acropars, Iran). Leveling and polishing the dentin surface was carried out using silicone waterproof sandpaper (APC, Iran) with sequences of 150, 240, 400, and 600 grits [15]. Each sample with cracks or structural defects was excluded from the study. In order to open the dentinal tubules in a more effective way, we put 14% ethylenediaminetetraacetic acid (EDTA pH 7.4) on the dentin slabs for 2 min and then rinsed thoroughly with water spray [16].
Synthesis of nanobioglass
The nanobioglass was synthesized using the sol-gel method [17]. Briefly, in a solution of water/ethanol (2:1), tetraethyl orthosilicate (TEOS 98%, Merck, Germany) was mixed with calcium nitrate tetrahydrate (Merck, Germany). By adding citric acid (1 M, Merck, Germany), the pH of solution was adjusted to 2.0. The process was followed by stirring until a clear solution was obtained (solution A). A solution of 2% polyethylene glycol (MW: 2000, Merck, Germany) and diammonium hydrogen orthophosphate (Sigma-Aldrich Canada Ltd.) was prepared and through adding ammonia (25% Merck, Germany), its pH was fixed to 10 (solution B). Both solutions A and B were mixed under the stirring for 10 h to obtain a homogenous gel. After being washed with deionized water and filtering, the obtained white gel was dried and lyophilized and finally it was calcined at 650 °C for 10 h with a heating rate of 5 °C/min.
Characterization of nanobioglass
XRD examination
X-Ray diffraction pattern (XRD) was used to investigate the crystal structure of nanobioglass. Panalytical Xpert PRO X Ray Diffractometer (Panalytical, Netherlands) model Xpert Pro MPD with wavelength 1.5405 Å and power 40 kV/30 mA was used to study the structure and crystal phase of the nanobioglass by scanning it in the range of 20 to 80°.
SEM examination
The surface morphology, shape, and size of synthesized nanobioglasses were assessed by scanning electron microscope (SEM) model XL30 (Philips, Netherlands).
Subsequently, the prepared samples were randomly divided into 6 subgroups (n = 6):
-
A.
Control: No use of nanobioglass or laser radiation.
-
B.
Diode laser (980 nm): With a specific radiation characteristics (980-nm wavelength, 3-W power, SSP mode, 5-mm non-contact), scanning in the occluso-apical and mesio-distal directions and vice versa, 1 min/cm2 total radiation time [18].
-
C.
Nd:YAG laser: With specific radiation characteristics (wavelength 1064 nm, perpendicular to the surface, contact, 1.0-W power, 10-Hz frequency, 85 J/cm2 per pulse energy density), scanning in the occluso-apical and mesio-distal directions and vice versa, 1 min/cm2 total radiation time [16].
-
D.
Nanobioglass: A 50% solution of bioglass nanoparticles and distilled water was prepared. Then, ultrasonic probe with the characteristics of 20-kHz was used for 20 s for 20 cycles in order to homogenize the solution. Subsequently, the solution was applied using an applicator brush for 20 s and was applied to the surfaces in two steps.
-
E.
Nanobioglass + diode laser radiation (980 nm): According to the group B method.
-
F.
Nanobioglass + Nd:YAG laser radiation: According to the group C method.
Evaluation of the interaction pattern between dentin and nanobioglass with SEM
Scanning electron microscopy (SEM) was used to study the morphology and surface topology of dentin, and also the interaction pattern between dentin and nanobioglass. The surface of the dentin disks was covered with a 100 A0 gold layer using the Bal-Tec (Swiss) instrument, and then the prepared specimens were carefully examined with the XL30 SEM (Philips, Netherlands) microscope at 250, 500, 1000, and 2000 magnifications. The average number of open dentinal tubules at 2000 magnification was counted twice by a skillful operator.
Statistical analysis
Then, the average number of open dentinal tubules in different groups was statistically evaluated by SPSS software version 22, Kruskal-Wallis test, and Mann-Whitney tests with Bonferoni’s correction (α = 0.008).
Results
Nanobioglass SEM examination
The SEM image was used to analysis the surface topology and morphology of nanobioglass. The results of this study show the presence of silica in the glassy phase of a spherical-shaped structure with the average size of 50 nm (Figure 1).
Nanobioglass XRD examination
XRD pattern shows a clear apatite phase (Figure 2). Both SEM and XRD data indicate the deposition of sodium and calcium ions over the crystal structure from the silica matrix which is due to the calcinations process. The XRD pattern of synthesized nanobioglass is consistent with the Larnite crystalline mode corresponding to Ca2SiO4 (JCPDS # 33-0302) [19]. A sharp peak shown in Figure 2 at 2θ=32.16 is indexed as (300).
Based on the data presented in Table 1, frequency distribution of the averages of the open dentinal tubules in all 6 groups of control (Figure 3), nanobioglass (Figure 4), diode, nanobioglass-diode (Figure 5), Nd:YAG, and nanobioglass-Nd:YAG (Figure 6) is 62.16, 19.00, 26.00, 14.16, 12.80, and 8.00, respectively.
According to the results of Mann-Whitney test (Table 2), there is a significant difference in the mean number of open dentinal tubules between the control group and the other groups (p < 0.008). However, the difference among the other groups is not statistically significant (p > 0.008).
In the following, SEM images represent the dentinal tubule obstruction and the surface coverage of control and study groups.
Discussion
Dentinal hypersensitivity (DH) can be considered as a type of pain caused by thermal, chemical, or osmotic stimulations on the uncovered dentin that does not match the causes and origins of other dental pains. However, it should be noted that the presence of uncovered dentin cannot be simply a reason for the painful feelings resulting from the stimuli. Since, according to several studies, the number and diameter of dentinal tubules play an important role in the emergence of DH, many therapeutic approaches focus on reducing the number of open dentinal tubules and diameter of their openings [1, 20, 21]. Thus, this study aims to reduce the permeability of dentin surfaces using nanotechnology to improve penetration of particles through tubular openings and employing high-power laser radiation to melt minerals (Figure 7).
A Completely open dentinal tubule with an average diameter of 3–5 µm. B Theory of using nanoparticles with a diameter of about 40–60 nm for the relative occlusion of the dentinal tubule. C High power laser radiation with the aim of melting mineral compounds and creating complete occlusion of the dentinal tubule
In this study, nonparametric tests (Kruskal-Wallis and Mann-Whitney) were used due to the lack of normal distribution of data and the impossibility of using parametric methods. According to the results of this study, all the surface treatments, such as diode (980nm) and Nd:YAG lasers with specific radiation characteristics as well as the use of nanobioglass with or without laser radiation, significantly reduce the number of open dentinal tubules compared with the control group (p<0.008). On the other hand, the highest reduction in open dentinal tubules is observed in the nanobioglass-Nd:YAG laser group.
The use of laser Nd:YAG is the best type of laser radiation in the treatment of dentinal hypersensitivity because it is able to obstruct the tubules by melting and resolidifying the dentin while there is no palpation and cracks. In addition, the formation of an obstructive layer with a thickness of about 4 μm following the laser radiation of Nd:YAG has a significant role in the immediate reduction of dentinal hypersensitivity [22, 23]. According to a clinical study by Suri et al., laser diode (980nm) with 2-W power, and also with desensitizer compounds such as 5% NaF varnish, had a significant effect on dental sensitivity reduction. As the authors argued, the combination of laser irradiation and desensitizer agents has synergistic effect on DH reduction [24]. In this study, the inefficiency of laser radiation in creating uniform tubular obstruction was one of the important reasons why we decided to use laser treatment in combination with nanoparticles. The results of SEM images indicate that the quality of blockage of dentinal tubules in nanobioglass-diode (980 nm) and nanobioglass-Nd:YAG groups is much better than that of laser radiation alone.
As mentioned above, nanobioglass was another method used in this study. The composition of bioglass has been studied extensively due to their high ability for reconstructing and remineralizing hard tissues by creating a layer of carbonate apatite [25,26,27,28].
One of the most crucial challenges in treating DH has been finding a long-lasting treatment. Therefore, one of the most important properties of bioactive glass is the continuous remineralization as well as its contribution to the deposition of mineral particles. This can help to improve the obstruction of dentinal tubules and reduce DH over time. This theory was evaluated and confirmed by Mitchell et al. [13]. According to the findings of their laboratory study, Abbassy et al. believe that a mixture of bioglass powder (45S5) and 50% phosphoric acid can release calcium, phosphate, and sodium and the penetration of these compounds into the outer layer of the enamel cause remineralization of white spot lesions [28]. Also, according to the study of Lopez et al., the proximity of bioglass compounds with stem cells derived from deciduous teeth (SHEDs) can play an important role in cellular differentiation, thereby helping to form tertiary dentin and to increase the mineral matrix deposition [29].
The results of the present study show that a radiation of 980-nm diode with 3-W power significantly decreases the number of open dentinal tubules, which is consistent with the clinical findings of Tabibzadeh et al. In addition, according to the other clinical results of Tabibzadeh et al., no pulpal damage was observed due to thermal changes of diode (980nm, 3-W) laser radiation [18].
Also, in this study, Nd:YAG laser radiation with 1-W power was used. According to the findings of the study by Zapletalová et al., Nd:YAG laser radiation with a power of more than 1.5-W can cause microcracks and carbonization and also increase intrapulpal temperature, resulting in irreversible damage [30]. Although in the clinical study by Lopes et al. the use of Nd:YAG laser with 1-W led to significant reduction in DH pain, some patients still had degrees of pain after treatment. Lopes et al. considered the cause of this phenomenon to be an uncompleted blockade of dentinal tubules [22]. Our findings in this study confirm their hypothesis. The study of Gholami et al. was another study that has consistent results with our findings. They also found significant differences in reducing the diameter of dentinal tubules after Nd:YAG laser radiation at 1-W [5]. The White et al. study was another investigation that examined the appropriate radiation characteristics with the aim of preventing intrapulpal damages due to temperature changes of Nd:YAG pulsed laser. They believe that Nd:YAG laser radiation with 0.3 to 3.0-W and frequencies of 10 and 20 Hz cannot cause excessive temperature rise in the pulpal tissue [31]. Also, based on clinical findings, Birang et al. stated that the thermal changes caused by the 1-W Nd:YAG laser irradiation have no effect on the pulp [32]. Moritz et al. used a 1.5-W Nd:YAG laser to minimize thermal damage to periodontal tissues [33]. The radiant power used in the study was 1-W, which is the peak power, and in pulsed radiation, this power will be less than 1-W. On the other hand, the radiation method was scanning and the laser radiation was not concentrated in one point. Both factors, together with blood circulation in the vital pulp, reduce thermal damages.
Although laboratory findings indicated that the therapeutic methods used in this study may have a potential effect on reducing DH, the need for long-term clinical studies for investigating the durability of DH therapy is necessary. Given that many existing therapies meet the immediate needs of patients, the main problem of these methods is that their effects are unstable. Furthermore, laser irradiation has biological properties that can only be examined under in vivo conditions.
Conclusions
According to the results of this study, the use of high-power laser radiation, such as Nd:YAG and diode (980nm) alone or with nanobioglass, has a significant effect on tubular obstruction. However, high-power Nd:YAG laser radiation accompanied by nanobioglass seems to have a higher ability to obstruct and reduce dentinal permeability compared to their separate application.
References
Al-Saud L, Al-Nahedh H (2012) Occluding effect of Nd: YAG laser and different dentin desensitizing agents on human dentinal tubules in vitro: a scanning electron microscopy investigation. Oper Dent 37(4):340–355
García-Delaney C, Abad-Sánchez D, Arnabat-Domínguez J, Valmaseda-Castellón E, Gay-Escoda C (2017) Evaluation of the effectiveness of the photobiomodulation in the treatment of dentin hypersensitivity after basic therapy. A randomized clinical trial. J Clin Exp Dent 9(5):e694
Al-Maliky MA, Mahmood AS, Al-Karadaghi TS, Kurzmann C, Laky M, Franz A, Moritz A (2014) The Effects of CO2 Laser with or without Nanohydroxyapatite Paste in the Occlusion of Dentinal Tubules. The Scientific World Journal 2014:8
Cummins D (2009) Dentin hypersensitivity: from diagnosis to a breakthrough therapy for everyday sensitivity relief. J Clin Dent 20(1):1
Gholami GA, Fekrazad R, Esmaiel-Nejad A, Kalhori KA (2011) An evaluation of the occluding effects of Er; Cr: YSGG, Nd: YAG, CO2 and diode lasers on dentinal tubules: a scanning electron microscope in vitro study. Photomed Laser Surg 29(2):115–121
Addy E, Addy M, Adams D (1987) Dentin hypersensitivity, a study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentin. J Clin Periodontol 14:280–284
Cuenin MF, Scheidt MJ, O’Neal RB, Strong SL, Pashley DH, Horner JA et al (1991) An in vivo study of dentin sensitivity: the relation of dentin sensitivity and the patency of dentin tubules. J Periodontol 62(11):668–673
Mockdeci H, Polonini H, Martins I, Granato A-P, Raposo N (2017) Evaluation of ex vivo effectiveness of commercial desensitizing dentifrices. J Clin Exp Dent 9(4):e503
Lena K, Marianne K (2017) Ozone treatment on dentin hypersensitivity surfaces–a pilot study. Open Dent J 11:65
Chavez EM, Taylor GW, Borrell LN, Ship JA (2000) Salivary function and glycemic control in older persons with diabetes. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 89(3):305–311
Pandey R, Koppolu P, Kalakonda B, Lakshmi BV, Mishra A, Reddy PK et al (2017) Treatment of dentinal hypersensitivity using low-level laser therapy and 5% potassium nitrate: a randomized, controlled, three arm parallel clinical study. Int J Appl Basic Med Res 7(1):63
Asnaashari M, Fekrazad R, Mozayeni MA, Mozayeni M (2010) An invitro study on the temperature changes of dentin, irradiated by CO2 and Er: Cr; YSGG lase. J Lasers Med Sci 1(1):1–7
Mitchell JC, Musanje L, Ferracane JL (2011) Biomimetic dentin desensitizer based on nano-structured bioactive glass. Dent Mater 27(4):386–393
Altunsoy M, Botsali MS, Sari T, Onat H (2015) Effect of different surface treatments on the microtensile bond strength of two self-adhesive flowable composites. Lasers Med Sci 30(6):1667–1673
Ding J-f, He Y-j, Chen Y, Jiang Q-z (2015) Effect of pretreatment on Er: YAG laser-irradiated dentin. Lasers Med Sci 30(2):753–9
Palazon MT, Scaramucci T, Aranha ACC, Prates RA, Lachowski KM, Hanashiro FS et al (2013) Immediate and short-term effects of in-office desensitizing treatments for dentinal tubule occlusion. Photomed Laser Surg 31(6):274–282
Moorthi A, Saravanan S, Srinivasan N, Partridge N, Zhu J, Qin L et al (2012) Synthesis, characterization and biological action of nano-bioglass ceramic particles for bone formation. J Biomater Tissue Eng 2(3):197–205
Tabibzadeh Z, Fekrazad R, Esmaeelnejad A, Shadkar MM, Khalili Sadrabad Z, Ghojazadeh M (2018) Effect of combined application of high- and low-intensity lasers on dentin hypersensitivity: a randomized clinical trial. J Dent Res Dent Clin Dent Prospects 12(1):49–55
Liu J, Miao X (2004) Sol–gel derived bioglass as a coating material for porous alumina scaffolds. Ceram Int 30(7):1781–1785
Gaffar A (1999) Treating hypersensitivity with fluoride varnish. Compend Contin Educ Dent 20(1 Suppl):27–33 (quiz 5)
Absi E, Addy M, Adams D (1987) Dentine hypersensitivity. J Clin Periodontol 14(5):280–284
Lopes AO, de Paula EC, Aranha ACC (2017) Evaluation of different treatment protocols for dentin hypersensitivity: an 18-month randomized clinical trial. Lasers Med Sci 32(5):1023–1030
Lan W-H, Liu H-C (1995) Sealing of human dentinal tubules by Nd: YAG laser. J Clin Laser Med Surg 13(5):329–333
Suri I, Singh P, Shakir QJ, Shetty A, Bapat R, Thakur R (2016) A comparative evaluation to assess the efficacy of 5% sodium fluoride varnish and diode laser and their combined application in the treatment of dentin hypersensitivity. J Indian Soc Periodontol 20(3):307–314
Lin C-P, Lin F-H, Tseng Y-C, Kok S-H, Lan W-H, Liao J-D (2000) Treatment of tooth fracture by medium energy CO2 laser and DP-bioactive glass paste: compositional, structural, and phase changes of DP-bioglass paste after irradiation by CO2 laser. Biomaterials 21(6):637–643
Efflandt S, Magne P, Douglas W, Francis L (2002) Interaction between bioactive glasses and human dentin. J Mater Sci - Mater Med 13(6):557–565
Forsback AP, Areva S, Salonen J (2004) Mineralization of dentin induced by treatment with bioactive glass S53P4 in vitro. Acta Odontol Scand 62(1):14–20
Abbassy MA, Bakry AS, Almoabady EH, Almusally SM, Hassan AH (2021) Characterization of a novel enamel sealer for bioactive remineralization of white spot lesions. J Dent 109:103663
Lopez TC, Diniz IM, Ferreira LS, Marchi J, Borges R, de Cara SP et al (2017) Bioactive glass plus laser phototherapy as promise candidates for dentine hypersensitivity treatment. J Biomed Mater Res B Appl Biomater 105(1):107–116
Zapletalová Z, Peřina J Jr, Novotný R, Chmelíčková H (2007) Suitable conditions for sealing of open dentinal tubules using a pulsed Nd: YAG laser. Photomed Laser Surg 25(6):495–499
White JM, Fagan MC, Goodis HE (1994) Intrapulpal temperatures during pulsed Nd: YAG laser treatment of dentin, in vitro. J Periodontol 65(3):255–259
Birang R, Poursamimi J, Gutknecht N, Lampert F, Mir M (2007) Comparative evaluation of the effects of Nd: YAG and Er: YAG laser in dentin hypersensitivity treatment. Lasers Med Sci 22(1):21–24
Moritz A, Doertbudak O, Gutknecht N, Goharkhay K, Schoop U, Sperr W (1997) Nd: YAG laser irradiation of infected root canals in combination with microbiological examinations. J Am Dent Assoc 128(11):1525–30
Acknowledgements
The authors would like to extend their gratitude to the Deputy of Research at AJA University of Medical Sciences for its contribution to this study. We also thank Hamadan University of Medical Sciences and Laser Research Center in Dentistry of Tehran University of Medical Sciences for providing technical supports.
Funding
The work was supported by the Dental Faculty, AJA University of Medical Sciences, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Solati, M., Fekrazad, R., Vahdatinia, F. et al. Dentinal tubule blockage using nanobioglass in the presence of diode (980 nm) and Nd:YAG lasers: an in vitro study. Clin Oral Invest 26, 2975–2981 (2022). https://doi.org/10.1007/s00784-021-04279-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04279-8