Abstract
Objective
To investigate the treatment efficacy of low-level light therapy on dentin hypersensitivity.
Materials and methods
Following the PRISMA guideline, six electronic databases supplemented with bibliographies were searched till December 2020. Two reviewers performed the screenings independently with a reliability assessment. Studies fulfilling the pre-registered eligibility criteria were included for risk-of-bias assessment and data synthesis.
Results
Thirty-five articles ultimately informed this systematic review based on the eligibility criteria and underwent risk-of-bias assessment (ĸ = 0.86). Quantitative results were deduced by meta-analysis of 20 randomised controlled trials: LLLT showed favourable outcomes compared to placebos for immediate (SMD: 1.09, 95% CI: 0.47 to 1.70), interim (SMD: 1.32, 95% CI: 0.41 to 2.23), and persistent efficacies (SMD: 2.86, 95% CI: 1.98 to 3.74). However, substantial heterogeneity existed among included studies (I2: 64–95%). Regarding comparisons with other desensitising strategies, LLLT showed no significant benefits in DH alleviation over others except fluorides for interim efficacy (SMD: 0.31, 95% CI: 0.10 to 0.52) and persistent efficacy (SMD: 0.45, 95% CI: 0.03 to 0.86).
Conclusions
This systematic review shows that LLLT has positive immediate, interim, and persistent DH-treatment efficacies compared with placebo. No superior treatment effects of LLLT were observed except fluoride agent use. Further studies are warranted—RCTs with low risk of bias, consistent technical settings, comprehensive assessments, and long follow-up periods.
Clinical relevance
This systematic review bridges a critical research gap by analysing clinical evidence in the DH-alleviating efficacy of LLLT in comparison with placebo and other in-office desensitising strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dentin hypersensitivity (DH) is an unpleasant experience characterised by short and sharp dental pain in response to external stimuli that cannot be attributed to specific forms of dental defect or pathology [1, 2]. Twenty-five to 35% of the adult population have experienced DH [3, 4], and among those who suffer from periodontal diseases, the prevalence may be as high as 84% [5]. Although DH does not directly deteriorate tooth vitality or life expectancy, it is closely related to oral health–related functionality and may lead to physical, psychological, or social disability [6]. In recent decades, strategies to alleviate DH have been developed based on at-home management or professional clinical treatment [7]. However, none of these has met the criteria proposed by Grossman for an ideal DH treatment that addresses all aspects [8]: pulp integrity, rapid in action, permanent efficacy, comfortable and easy application, and no pigmentation on tooth structures [2, 9].
Home management with desensitising toothpaste is often the first-choice treatment for DH due to its wide availability and convenience for patients. However, the effects of this treatment usually take 4 to 8 weeks to develop [10]. Patients suffering from severe DH who desire immediate relief are highly recommended to seek professional care [11]. To date, a wide range of professional DH treatments has been introduced. The available modalities are typically classified in terms of their characteristics: varnishes and precipitants (e.g. fluorides, oxalates, calcium compounds, and bioactive glasses), restorative materials (e.g. adhesives, glass ionomers, and resins), agents for nerve desensitisation (such as potassium nitrates and guanethidine), light therapy, and periodontal surgery [9, 11, 12]. Despite this wide range of treatment choices, there is no consensus on which professional treatment is most effective or which treatment-application technique is most efficient [9].
Low-level light therapy (LLLT) refers to using red or near-infrared light to regulate biological activities without provoking thermal changes [13,14,15,16]. It is valued for its non-invasiveness, safety, comfort, precision, reproducibility, and rapid action [2, 17,18,19]. Chung et al. [14] suggested that the settings of LLLT are within 600–1070 nm wavelength and 1–1000 mW output power for good tissue penetration and promising treatment efficacy. Many clinical studies have reported the abilities of LLLT in DH alleviation. Yet, the effectiveness is still under debate: some studies corroborated findings that LLLT more effectively relieves DH than other strategies [20, 21], whereas others concluded that reductions in DH, especially those resulting in immediate relief, are substantially attributable to the placebo effect [22, 23]. A significant reason for the above inconsistency is the large variance in the technical parameters of light wavelength, beam size, output power, wave mode, exposure time, application frequency and irradiation method, and the periods of observation across studies [11, 19, 24]. The diversity of the comparators may also explain the inconsistent findings: some studies used negative controls, whereas others used positive controls since no gold-standard treatment has been established for DH management [11, 25, 26]. All above hinder the determination of the true efficacy of LLLT and its translation into clinical practice.
Therefore, this systematic review was conducted to analyse current evidence regarding the effects of LLLT on DH management. The primary outcome was treatment efficacies compared to placebo, based on the observed changes in patients’ subjective perceptions of DH at immediate (< 1 month), interim (1 to < 6 months), and persistent (≥ 6 months) time points. The secondary outcomes were the effects of LLLT on DH alleviation relative to those of other in-office desensitisation strategies, based on the evidence from previous clinical studies.
Materials and methods
This systematic review was performed and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [27, 28]. The protocol was prospectively registered on the International Prospective Register of Systematic Reviews online database (CRD42020162721).
Search strategy
Two reviewers (ZYS and JJJ) independently and systematically searched six major electronic databases (MEDLINE, EMBASE, PubMed, Scopus, ProQuest, and the Cochrane Central Register of Controlled Trials) from their date of establishment until December 2020 for manuscripts with English abstracts but no language restriction for the main text. The search terms used were medical subject headings, free text words, and their synonyms, and included ‘tooth/dentine/pulp’, ‘sensitivity/hypersensitivity/irradiation/discomfort/pain’. and ‘low-level light/low-intensity light/soft laser/cold laser/photobiomodulation’. Full details of this electronic searching strategy are presented in Appendix 1. Supplementary manual searching was performed by screening the bibliographies of all the included publications.
Study selection
The eligibility criteria were as follows (in population, intervention, control, and outcomes format).
Population
Inclusion criteria
-
1.
Patients who self-reported DH.
-
2.
Patients who had teeth with intact and vital pulps.
-
3.
Systemically healthy patients with permanent dentition.
Exclusion criteria
-
1.
Patients who had teeth containing cervical caries, defective restorations, premature contacts, cracked enamel, fluorotic damage, or any other factor that could be responsible for more exposed dentin tubules and DH.
-
2.
Patients with teeth displayed any indication of pulpitis, pulp necrosis, or acute and chronic inflammation of the periapical and periodontal areas.
-
3.
Patients who had teeth that had been subject to trauma, surgery, or invasive periodontal treatment within the past 3 months.
-
4.
Patients who had DH while using desensitising toothpaste or receiving other dental treatments, such as dental bleaching, cavity or restorative preparation, or orthodontic treatment.
-
5.
Patients who were pregnant or lactating were taking systemic medications or had severe craniofacial abnormalities, temporomandibular diseases, trigeminal neuralgia, or migraine that could affect their subjective judgement.
Intervention
LLLT at a light wavelength between 600 and 1070 nm and an output power between 1 and 1000 mW [14].
Comparison
Placebo or other in-office desensitisation strategies.
Outcomes
Scores rated by patients for DH in response to external (thermal, chemical, tactile, electrical, or osmotic) stimuli.
Study
Randomised controlled trials (RCTs) and non-randomised controlled studies (NRSs).
For literature management, all the titles and abstracts obtained from the electronic database searches were imported into EndNote X9.3.3 software [29]. Two reviewers independently screened all the literature based on the eligibility criteria. Potentially relevant studies were retrieved for full-article assessment and final data synthesis. During the entire process, any disagreement between the two reviewers was resolved by discussion or consultation with a third reviewer (YQY). Cohen’s κ-values were computed to verify inter-reviewer reliability, and κ 0.6 was considered to indicate acceptable reliability [30].
Data extraction and analysis
The following data were extracted: general information (first author, nationality, and year of publication), study type and design, participants (number, age, and sex) and target teeth, intervention (light’s type, wavelength, wave mode, output power, energy density, time of exposure, irradiation session, total dosage, and method of irradiation), comparators, and outcome assessment (stimulus, numeric scale, and observation period).
Risk-of-bias assessment
The risk-of-bias assessment was performed in RevMan.5.4 [31], according to the Cochrane Handbook [32]. RCTs were evaluated using the revised Cochrane risk-of-bias tool for randomised trials (RoB 2) [33] in the following five domains: bias from the randomisation process, bias due to deviations from the intended intervention, bias due to missing outcome data, bias in the measurement of the outcome, and bias in the selection of the reported result. NRSs were assessed using the Risk of Bias in Non-randomised Studies of Interventions (ROBINS-I) tool [34] in the following seven domains: bias due to confounding, bias in the selection of participants for the study, bias in the classification of interventions, bias due to deviations from the intended intervention, bias due to missing data, bias in the measurement of outcomes, and bias in the selection of the reported result. Following the assessment of all domains, each study’s overall risk of bias was graded according to the Handbook as ‘low, some concerns, or high’ (for RCTs) and ‘low, moderate, serious, or critical’ (for NRSs). The two reviewers (ZYS and JJJ) conducted this process independently, and any disagreements were resolved by discussion.
Data synthesis and statistical analysis
Quantitative syntheses of data from RCTs and NRSs with a low risk of bias were performed according to the guidelines in the Cochrane Handbook [35]. Based on the results of data extraction, the effects of LLLT on the changes in DH, as indicated by patients’ self-rated scores on a visual analogue scale (VAS; 0 to 100) immediately after LLLT sessions (first assessment post-treatment), at interim follow-ups (last assessment within 1 month and up to 6 months) and persistent follow-ups (last observation at 6 months or beyond), were collected and pooled. The results of studies that used other numeric scales were transformed proportionally to VAS scores using a standard formula: \(VAS score=\frac{{x}_{i}}{\mathrm{max}\left({x}_{i}\right)}\times 100\), where \({x}_{i}\) were readings of \(i\)-th numeric scale and \(\mathrm{max}(\cdot )\) denoted the maximum element of the scale. Ultimately, this yielded all data on one generic VAS (0 to 100; 0 = no pain, 100 = worst possible pain) for meta-analysis. Since there are considerable clinical-setting variations in participants’ age and gender, LLLT’s technical parameters, and DH assessment approaches, the outcomes were analysed using RevMan5.4 [31] by pooling standard mean differences (SMDs) and 95% confidence intervals (CIs) of individual studies based on a random-effects model to minimise the impact of precision variance among studies [36]. The results are presented in forest plots and a summary-of-findings table. Statistical heterogeneity was evaluated using the I2 statistic, and I2 values > 50% were considered to indicate substantial or critical heterogeneity. Based on the sufficiency of pooled data, a multiple meta-regression was conducted using Stata 15 software [37] to analyse the efficacy of LLLT on DH alleviation, with adjustment for factors associated with study quality and interventional settings.
Results
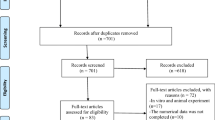
The electronic searches of the six databases, supplemented with manual searching, yielded 1558 records. Following the removal of duplicates, the titles and abstracts of 1387 records were screened according to our pre-registered eligibility criteria. This yielded 99 articles for full-text assessment (ĸ = 0.78). Following assessment of these articles according to the eligibility criteria, 64 studies were excluded, and 35 articles were included in the qualitative data synthesis (ĸ = 0.86), comprising 27 RCTs [20,21,22,23, 38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60] and eight NRSs [17, 61,62,63,64,65,66,67]. Subsequent quantitative data syntheses were performed using data from 20 RCTs that reported the same outcome for DH alleviation, as measured by numeric scales according to patients’ self-perceptions in response to chair-side air blast stimuli. All eight NRSs [17, 61,62,63,64,65,66,67] were excluded due to a moderate-to-serious risk of bias. In addition, two RCTs that contained duplicated data [49, 59] and five RCTs that had incomplete data [38, 42, 50, 52, 53] were excluded. The entire study-selection procedure is illustrated in the PRISMA flow diagram depicted in Fig. 1.
Characteristics of included studies
All the study samples comprised subjects of both sexes over a wide age range (12–70 years). The interventions consisted of a diode laser [17, 21, 22, 38, 39, 42,43,44,45,46,47, 49,50,51,52, 54,55,56,57,58,59,60,61,62,63,64,65, 67] or a neodymium-doped yttrium aluminum garnet laser (Nd:YAG) [21, 23, 40, 41, 43, 44, 51, 53, 65, 66] and were delivered using a wide range of parameters in terms of wavelength (630–1067 nm), output power (1.5–1000 mW), total dosage (0.1–300 J), energy density (2–100 J/cm2), exposure time (10–180 s) and number of irradiation sessions (1–6). Overall, 12 studies compared the effects of LLLT with placebo [22, 23, 39, 40, 44, 47, 52, 53, 55, 57, 59, 60], and the other comparators were fluorides [21, 38, 42, 45, 52, 58, 60, 61, 64], adhesives [20, 38, 45, 46, 49,50,51, 55, 56], potassium nitrate or oxalate [22, 38, 54, 56, 57], and dentifrices (arginine-calcium carbonate [39] and calcium sodium phosphosilicate [23]). Most studies examined the outcome of DH treatment by the patient’s subjective response to an air blast as determined by VAS or other numeric scales, namely a 3-point [49], 4-point [41, 53, 57, 65, 67], or 5-point scale [64]. The other DH investigations included response to mechanical [22, 23, 40,41,42, 47,48,49,50,51, 53, 54, 57, 58, 62], ice-cold [20, 46, 54, 63, 66], and electric [54] stimuli. The detailed characteristics of all included RCTs and NRSs are illustrated in Table 1 and Table 2, respectively.
Risks of bias
The risks of bias in the 27 RCTs was evaluated in five domains using the RoB 2 Tool [33]. As shown in Fig. 2, nine studies had a high risk of bias that was mainly arising from outcome measurements [38, 39, 41, 42, 47, 50,51,52, 58]. A further 12 studies were rated as having ‘some concerns’ in the overall risk of bias, as they possessed an unclear risk of bias in at least one domain arising from randomisation or selection of reported results [20, 21, 40, 43,44,45, 48, 53, 55, 56, 59, 60], and six studies presented a low risk of bias across all domains [22, 23, 46, 49, 54, 57].
The overall risks of bias in the eight NRSs were assessed in seven domains with four levels (low, moderate, serious and critical) using the ROBINS-I Tool [34]. As shown in Fig. 3, one NRS had a moderate risk of bias [63] and the remaining seven studies had a serious risk of bias [17, 61, 62, 64,65,66,67]. All eight NRSs were excluded from the subsequent meta-analysis.
Meta-analysis
When processing meta-analysis, we noticed that the included studies were various in stimuli devices and application methods, making it challenging to synthesise studies using other external stimuli than air blasts quantitatively. Therefore, only studies using air blast stimuli were included in quantitative meta-analysis. Quantitative analysis of LLLT’s effect on DH was based on the changes in VAS score (0 to 100; 0 = no pain, 100 = worst possible pain). Eighteen studies [20,21,22,23, 39, 40, 43,44,45,46,47,48, 51, 54,55,56, 58, 59] used VAS, and only two studies [41, 57] evaluated DH by a 4-degree scale and needed proportional transformation to a VAS. For these two studies, a standard formula was used. Since this conversion may bring up some precision variances in the data extracted, we presented the results in SMDs based on the random-effects model, which is a classical way to minimise the influence of precision variances [36].
DH-alleviating efficacy of LLLT compared to placebo
The results show that compared to placebo, LLLT alleviated DH at all stages. In terms of immediate efficacy, the SMD between LLLT and placebo was 1.09 (95% CI: 0.47 to 1.70, p < 0.001). In terms of interim efficacy and persistent efficacy, the SMD between LLLT and placebo was 1.32 (95% CI: 0.41 to 2.23, p = 0.005) and 2.86 (95% CI: 1.98 to 3.74, p < 0.001), respectively (Table 3). Interestingly, there was a significant difference between the immediate and interim efficacy SMDs in a subgroup analysis of studies categorised by the risk-of-bias level (i.e. low, moderate, or high) (p < 0.001). No study with persistent efficacy had a low risk of bias. The statistical heterogeneity was assessed by determining the I2 values for all included studies in terms of immediate, interim, and persistent efficacies, which were 92%, 95%, and 64%, respectively (Fig. 4). Funnel plots show that publication bias existed for all periods. Due to the high I2 and considerable variability in the technical parameters used in different studies regarding wavelength, output power, wave mode, exposure time, application frequency, and irradiation method, a meta-regression was conducted to determine the true ability of LLLT to alleviate DH and the related factors (covariates).
The meta-regression of immediate and interim efficacies was performed using Stata 15 Software [37], and five factors were assessed: ‘risk of bias’, ‘wavelength’, ‘wave mode’, ‘energy density’, and ‘total dosage’. Due to data insufficiency for long-term follow-ups, a meta-regression of persistent efficacy could not be conducted. In addition, three factors—‘output power’, ‘time of exposure’, and ‘irradiation sessions’—were not individually investigated, as they have multiplicative relationships with the ‘total dosage’, according to the following equation:
The results of a random-effect model analysis using a forward method reveal that only ‘energy density’ is significantly correlated with the immediate and interim treatment effects of LLLT, as demonstrated by the adjusted R2 values of 34.71% and 60.11%, respectively. The residual variances (I2res) due to heterogeneity are 83.89% for immediate efficacy and 49.11% for interim efficacy. Based on the regression models, the predicted treatment effects of LLLT, as indicated by the mean reduction in VAS scores, are equal to 37.47–0.213 × (energy density) for immediate post-treatment observations and 44.45–0.166 × (energy density) for evaluations 1–3 months after treatment. Each unit of increase in ‘energy density’ contributes to a 0.213 or 0.166 decrease in the VAS score of the LLLT-based alleviation of DH in terms of the immediate or interim efficacy, respectively (Table 4).
DH-alleviating efficacy of LLLT compared to other in-office desensitisation strategies
In addition to placebo, the VAS changes in response to air blasts were also compared between LLLT and other in-office desensitisation agents, namely fluorides, adhesives, potassium compounds, and dentifrices. To make it align with the other groups for consistency of statistical analysis method, we still performed a subgroup analysis for these outcomes. Compared to fluorides, LLLT had no DH-alleviating effect in terms of immediate efficacy (SMD: 0.11, 95% CI: − 0.31 to 0.54, p = 0.60) but yielded slightly higher interim (p = 0.003) and persistent efficacies (p = 0.03) (Fig. 5). Interestingly, we noticed that when comparing immediate and interim efficacies between LLLT and fluorides, the heterogeneity in the moderate RoB subgroup (I2: 87% and 17% for immediate and interim efficacy, respectively) was even more considerable than the total heterogeneity (I2: 79% and 9% for immediate and interim efficacy, respectively). This result could relate to the minimal number of studies (n = 6) addressing fluorides comparator and no study with low RoBs. Compared to adhesives, LLLT had no DH-alleviating effect at any stage (p > 0.05) (Fig. 6). Similar results were obtained for comparisons with potassium compounds and dentifrices; for these, the SMDs of LLLT range from − 0.02 to 0.19 for immediate and interim DH-alleviating efficacy, with no statistically significant difference (p > 0.05), and no persistent efficacy data could be synthesised (Fig. 7). However, these results must be interpreted with caution, given the considerable heterogeneity within subgroups and the inclusion of few RCTs with a low risk of bias and few studies that addressed persistent efficacy.
Discussion
One novelty of this systematic review and meta-analysis is that we conducted stage-based analysis on LLLT’s desensitising effects. Although the biomolecular and cellular PBM activities have not been entirely determined, there were three perspectives referring to different stages how LLLT alleviates DH. First, LLLT may immediately change patients’ self-perception by modulating neuronal physiology in terms of varying the axonal flow, cytoskeletal organisation, and adenosine triphosphate production in sensory nerves [68,69,70]. Second, the effect of LLLT on inflammation may play a role in the interim alleviation of DH since studies suggested there is a potential relationship between DH and micro-inflammation within dentine-pulp complexes [71,72,73,74]. The third theory is more explicable for persistent DH relief, as light irradiation may help increase blood vasculature in pulp tissues and stimulate the viability of odontoblasts; they both contribute to the deposition of secondary dentine and reduction of dentin permeation [75,76,77,78]. Based on the above three theories, we investigated the DH-alleviating efficacy of LLLT treatment in a stage-based manner and separately extracted data for immediate, interim, and persistent outcomes. Another intention of using the stage-based data synthesis is to reduce the clinical heterogeneity of included studies and avoid correlation-associated overestimations. Marto et al. [25] adopted the same strategy; unfortunately, they included all laser types as one desensitising approach and did not elaborate on the effects of LLLT. Another two systematic reviews did examine different types of laser therapies [19, 79]; yet they only retrieved data of the earliest and latest time points without consideration of the association between clinical performance and biological activities underneath.
Another novelty of our systematic review is that we performed a methodological subgroup analysis to investigate the causes and type of heterogeneity [80]. Specifically, the analysis of LLLT’s efficacies was based on the quality assessment of included studies. Intuitively, studies with low RoBs provide the highest quality and should play the dominant role in generalisation. However, prerequisites should be sufficient high-quality evidence and acceptable heterogeneity to avoid loss of power or dilution of efficacy estimates [80]. Among the included studies for immediate efficacy, only three RCTs had low RoBs but presented high heterogeneity (I2: 49%), while seven studies had moderate or high RoBs with relatively mild heterogeneity (I2: 36% and 0%, respectively). Therefore, we also included studies with moderate and high RoBs for meta-analysis to obtain a more general overview of the results, and demonstrated outcomes by their quality.
In addition, this systematic review further conducted a meta-regression to examine the causes of heterogeneity and explore confounding factors [81]. Out of five factors that potentially relate to VAS changes, we only found ‘energy density’ was significantly associated with immediate and interim efficacies. Energy density (J/cm2), also called ‘fluency’, is a crucial parameter in LLLT and represents the energy absorbed by tissues per unit area [82]. In vitro and in vivo studies have reported a close relationship between the energy density of irradiation and the biphasic responses of a patient in terms of the stimulation or inhibition of biological activities [83,84,85]; an optimal energy density generates the maximum desired PBM [13]. Notably, our meta-regression results support their findings: LLLT has a higher immediate and interim DH-alleviating efficacy under low energy density (2–10 J/cm2) in comparison with those under higher energy density (> 40 J/cm2). However, a lack of data prevented us from determining the optimal DH-alleviating energy density, as many reports lacked detailed information on LLLT settings [42,43,44, 53, 54, 57]. Also, the negative correlations of regression models should be interpreted with great caution, as substantial residual variances of 83.39% and 49.11% were observed for immediate and interim efficacy, respectively.
Overall, this systematic review bridges a critical research gap by analysing current clinical evidence in the DH-alleviating efficacy of LLLT. Despite striving for a pertinent data synthesis plan and meta-analysis method, the following limitations exist. First, the number of well-conducted RCTs with high quality was quite insufficient. There were only three studies with low RoBs available for comparison between LLLT and placebo, which presents relative high heterogeneity, i.e. 49% and 64% for immediate and interim efficacy, respectively. In addition, the absence of studies with low RoBs on the efficacy difference between LLLT and fluorides indicates that more studies are required to warrant convincing evidence in the future. Second, there is a great inconsistency in the age range for recruited subjects and intervention/assessment methods for LLLT and its comparators. Third, quantitative analysis on DH was only conducted on the air blast–stimulated response due to insufficient and inconsistent data for other clinical outcomes. Finally, and there is a shortage of studies that cover long-term follow-ups. These may bring substantial bias in evaluating persistent efficacy when the technical settings of LLLT were divergent [6]. Therefore, we advocate more well-conducted RCTs with low RoBs, consistent settings, comprehensive assessments, and long follow-up periods in the future to generate high-quality evidence regarding the DH-alleviating effects of LLLT.
Conclusion
This systematic review analysed clinical evidence regarding the DH-alleviating efficacy of LLLT. The immediate, interim, and persistent efficacy results show that, compared to placebo, LLLT generally alleviated DH in the included studies. Energy density appears to be a critical factor for the successful treatment of DH with LLLT, as higher immediate and interim efficacy was achieved under low-energy–density conditions. The evidence does not suggest that the DH-alleviating effects of LLLT are superior to those of other in-office desensitisation strategies, except fluorides in terms of interim and persistent efficacy. Future RCTs with low RoBs, consistent settings, comprehensive assessments, and long follow-up periods are highly recommended.
References
West NX, Lussi A, Seong J, Hellwig E (2013) Dentin hypersensitivity: pain mechanisms and aetiology of exposed cervical dentin. Clin Oral Invest 17(1):9–19
Kimura Y, Wilder-Smith P, Yonaga K, Matsumoto K (2000) Treatment of dentine hypersensitivity by lasers: a review. J Clin Periodontol 27(10):715–721
Splieth CH, Tachou A (2013) Epidemiology of dentin hypersensitivity. Clin Oral Invest 17(S1):3–8
Ye W, FENG XP, Li R (2012) The prevalence of dentine hypersensitivity in Chinese adults. J Oral Rehabil 39(3):182–187
Chabanski M, Gillam D, Bulman J, Newman H (1996) Prevalence of cervical dentine sensitivity in a population of patients referred to a specialist Periodontology Department. J Clin Periodontol 23(11):989–992
Bekes K, John MT, Schaller HG, Hirsch C (2009) Oral health-related quality of life in patients seeking care for dentin hypersensitivity. J Oral Rehabil 36(1):45–51
Dababneh R, Khouri A, Addy M (1999) Dentine hypersensitivity—an enigma? A review of terminology, mechanisms, aetiology and management, British dental journal 187(11):606–611
Grossman LI (1935) A systematic method for the treatment of hypersensitive dentin. J Am Dent Assoc 22(4):592–602
West NX, Seong J, Davies M (2015) Management of dentine hypersensitivity: efficacy of professionally and self-administered agents. J Clin Periodontol 42:S256–S302
Chu C-H, Lo EC-M (2010) Dentin hypersensitivity: a review. Hong Kong Dent J 7(1):15–22
Lin PY, Cheng YW, Chu CY, Chien KL, Lin CP, Tu YK (2013) In-office treatment for dentin hypersensitivity: a systematic review and network meta-analysis. J Clin Periodontol 40(1):53–64
West NX (2007) The dentine hypersensitivity patient - a total management package. Int Dent J 57(S6):411–419
De Freitas LF, Hamblin MR (2016) Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron 22(3):348–364
Chung H, Dai T, Sharma SK, Huang Y-Y, Carroll JD, Hamblin MR (2012) The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng 40(2):516–533
Hamblin MR (2017) Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS biophysics 4(3):337–361
Hamblin MR (2018) Mechanisms and mitochondrial redox signaling in photobiomodulation. Photochem Photobiol 94(2):199–212
Marsilio AL, Rodrigues JR, Borges AB (2003) Effect of the clinical application of the GaA1As laser in the treatment of dentine hypersensitivity. J Clin Laser Med Surg 21(5):291–296
Lier B, Rösing C, Aass A, Gjermo P (2002) Treatment of dentin hypersensitivity by Nd: YAG laser. J Clin Periodontol 29(6):501–506
Y. Kong, Y. Lei, S. Li, Y. Zhang, J. Han, M. Hu, (2019) Network meta-analysis of the desensitizing effects of lasers in patients with dentine hypersensitivity, Clinical Oral Investigations
Praveen R, Thakur S, Kirthiga M, Narmatha M (2018) Comparative evaluation of a low-level laser and topical desensitizing agent for treating dentinal hypersensitivity: a randomized controlled trial. Journal of conservative dentistry: JCD 21(5):495
Soares ML, Porciúncula GB, Lucena MI, Gueiros LA, Leão JC, Carvalho AA (2016) Efficacy of Nd: YAG and GaAlAs lasers in comparison to 2% fluoride gel for the treatment of dentinal hypersensitivity. Gen Dent 64(6):66–70
Vieira AHM, Passos VF, De Assis JS, Mendonca JS, Santiago SL (2009) Clinical evaluation of a 3% potassium oxalate gel and a GaAlAs laser for the treatment of dentinal hypersensitivity. Photomed Laser Surg 27(5):807–812
Maximiano V, Machado AC, Yoshida ML, Pannuti CM, Scaramucci T, Aranha ACC (2019) Nd: YAG laser and calcium sodium phosphosilicate prophylaxis paste in the treatment of dentin hypersensitivity: a double-blind randomized clinical study. Clin Oral Invest 23(8):3331–3338
Hu M-L, Zheng G, Han J-M, Yang M, Zhang Y-D, Lin H (2019) Effect of lasers on dentine hypersensitivity: evidence from a meta-analysis. Journal of Evidence Based Dental Practice 19(2):115–130
Marto CM, Baptista Paula A, Nunes T, Pimenta M, Abrantes AM, Pires AS, Laranjo M, Coelho A, Donato H, Botelho MF, Marques Ferreira M, Carrilho E (2019) Evaluation of the efficacy of dentin hypersensitivity treatments—a systematic review and follow‐up analysis. J Oral Rehabil 46(10):952–990
Shiau HJ (2012) Dentin hypersensitivity, Journal of Evidence Based. Dent Pract 12(3):220–228
D. Moher, A. Liberati, J. Tetzlaff, D. Altman, The Preferred Reporting Items for Systematic Reviews and Meta-Analyses Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62:1006–1012
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62(10):e1–e34
T.E. Team, EndNote, Clarivate, Philadelphia, PA, 2013.
McHugh ML (2012) Interrater reliability: the kappa statistic. Biochemia medica 22(3):276–282
R.M. (RevMan), The Cochrane Collaboration, 2020.
Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J (2019) Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 10:ED000142
J.A. Sterne, J. Savović, M.J. Page, R.G. Elbers, N.S. Blencowe, I. Boutron, C.J. Cates, H.-Y. Cheng, M.S. Corbett, S.M. Eldridge, (2019) RoB 2: a revised tool for assessing risk of bias in randomised trials, bmj 366
Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I, Carpenter JR, Chan AW, Churchill R, Deeks JJ, Hróbjartsson A, Kirkham J, Jüni P, Loke YK, Pigott TD, Ramsay CR, Regidor D, Rothstein HR, Sandhu L, Santaguida PL, Schünemann HJ, Shea B, Shrier I, Tugwell P, Turner L, Valentine JC, Waddington H, Waters E, Wells GA, Whiting PF, Higgins JP (2016) ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355:i4919
J.P. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M.J. Page, V.A. Welch, 2019 Cochrane handbook for systematic reviews of interventions, John Wiley & Sons.
Takeshima N, Sozu T, Tajika A, Ogawa Y, Hayasaka Y, Furukawa TA (2014) Which is more generalizable, powerful and interpretable in meta-analyses, mean difference or standardized mean difference? BMC Med Res Methodol 14(1):30
T.S.L. StataCorp. 2017. Stata Statistical Software: Release 15. College Station.
Aranha ACC, Pimenta LAF, Marchi GM (2009) Clinical evaluation of desensitizing treatments for cervical dentin hypersensitivity. Braz Oral Res 23(3):333–339
Bal MV, Keskiner İ, Sezer U, Açıkel C, Saygun I (2015) Comparison of low level laser and arginine-calcium carbonate alone or combination in the treatment of dentin hypersensitivity: a randomized split-mouth clinical study. Photomed Laser Surg 33(4):200–205
Birang R, Poursamimi J, Gutknecht N, Lampert F, Mir M (2007) Comparative evaluation of the effects of Nd: YAG and Er: YAG laser in dentin hypersensitivity treatment. Lasers Med Sci 22(1):21–24
BouChebel F, Zogheib CM, Baba NZ, Corbani KA (2018) Clinical comparative evaluation of Nd: YAG laser and a new varnish containing casein phosphopeptides-amorphous calcium phosphate for the treatment of dentin hypersensitivity: a prospective study. J Prosthodont 27(9):860–867
Dantas EM, Amorim FKdO, Nóbrega FJdO, Dantas PMC, Vasconcelos RG, Queiroz LMG (2016) Clinical efficacy of fluoride varnish and low-level laser radiation in treating dentin hypersensitivity. Braz Dent J 27(1):79–82
Dilsiz A, Canakci V, Ozdemir A, Kaya Y (2009) Clinical evaluation of Nd: YAG and 685-nm diode laser therapy for desensitization of teeth with gingival recession. Photomed Laser Surg 27(6):843–848
Dilsiz A, Aydin T, Canakci V, Gungormus M (2010) Clinical evaluation of Er: yAG, Nd: yAG, and diode laser therapy for desensitization of teeth with gingival recession. Photomed Laser Surg 28(Suppl 2):S11–S17
Femiano F, Femiano R, Lanza A, Festa MV, Rullo R, Perillo L (2013) Efficacy of diode laser in association to sodium fluoride vs Gluma desensitizer on treatment of cervical dentin hypersensitivity A double blind controlled trial. Am J Dent 26(4):214–218
Flecha OD, Azevedo CG, Matos FR, Vieira-Barbosa NM, Ramos-Jorge ML, Gonçalves PF, Koga Silva EM (2013) Cyanoacrylate versus laser in the treatment of dentin hypersensitivity: a controlled, randomized, double-masked and non-inferiority clinical trial. J Periodontol 84(3):287–294
Gentile LC, Greghi SLE (2004) Clinical evaluation of dentin hypersensitivity treatment with the low intensity Gallium-Aluminum-Arsenide laser - AsGaAl. Journal of applied oral science : revista FOB 12(4):267–272
Gerschman JA, Ruben J, Gebart-Eaglemont J (1994) Low level laser therapy for dentinal tooth hypersensitivity. Aust Dent J 39(6):353–357
Lima TC, Vieira-Barbosa NM, Grasielle de SáAzevedo C, de Matos FR, Douglas de Oliveira DW, de Oliveira ES, Ramos-Jorge ML, Gonçalves PF, Flecha OD (2017) Oral health-related quality of life before and after treatment of dentin hypersensitivity with cyanoacrylate and laser. J Periodontol 88(2):166–172
Lopes AO, de Paula Eduardo C, Aranha ACC (2015) Clinical evaluation of low-power laser and a desensitizing agent on dentin hypersensitivity. Lasers Med Sci 30(2):823–829
Lopes AO, de Paula Eduardo C, Aranha ACC (2017) Evaluation of different treatment protocols for dentin hypersensitivity: an 18-month randomized clinical trial. Lasers Med Sci 32(5):1023–1030
Lund RG, Silva AFD, Piva E, Da Rosa WLDO, Heckmann SS, Demarco FF (2013) Clinical evaluation of two desensitizing treatments in southern Brazil: a 3-month follow-up. Acta Odontol Scand 71(6):1469–1474
A. Mogharehabed, H. Khademi, Z.Z. ABDI, S.A. Bouraima, J. Yaghini, B. Poormoradi, (2012) Comparative evaluation of the effects of 5% sodium fluoride varnish and neodymium-doped yttrium aluminium garnet (Nd: YAG) laser and their combined application on dentin hypersensitivity treatment
Narayanan R, Prabhuji MLV, Paramashivaiah R, Bhavikatti SK (2019) Low-level laser therapy in combination with desensitising agent reduces dentin hypersensitivity in fluorotic and non-fluorotic teeth - a randomised, controlled, double-blind clinical trial. Oral Health Prev Dent 17(6):547–556
Orhan K, Aksoy U, Can-Karabulut DC, Kalender A (2011) Low-level laser therapy of dentin hypersensitivity: a short-term clinical trial. Lasers Med Sci 26(5):591–598
Osmari D, Fraga S, Ferreira ACO, Eduardo CP, Marquezan M, Silveira BLD (2018) In-office treatments for dentin hypersensitivity: a randomized split-mouth clinical trial. Oral Health Prev Dent 16(2):125–130
Sicilia A, Cuesta-Frechoso S, Suárez A, Angulo J, Pordomingo A, De Juan P (2009) Immediate efficacy of diode laser application in the treatment of dentine hypersensitivity in periodontal maintenance patients: a randomized clinical trial. J Clin Periodontol 36(8):650–660
R. Umberto, R. Claudia, P. Gaspare, T. Gianluca, D.V. (2012) Alessandro, Treatment of dentine hypersensitivity by diode laser: a clinical study, International journal of dentistry 2012
Yilmaz HG, Kurtulmus-Yilmaz S, Cengiz E, Bayindir H, Aykac Y (2011) Clinical evaluation of Er, Cr:YSGG and GaAlAs laser therapy for treating dentine hypersensitivity: a randomized controlled clinical trial. J Dent 39(3):249–254
Yilmaz HG, Kurtulmus-Yilmaz S, Cengiz E (2011) Long-term effect of diode laser irradiation compared to sodium fluoride varnish in the treatment of dentine hypersensitivity in periodontal maintenance patients: a randomized controlled clinical study. Photomed Laser Surg 29(11):721–725
Corona SAM, Do Nascimento TN, Catirse ABE, Lizarelli RFZ, Dinelli W, Palma-Dibb RG (2003) Clinical evaluation of low-level laser therapy and fluoride varnish for treating cervical dentinal hypersensitivity. J Oral Rehabil 30(12):1183–1189
Hashim NT, Gasmalla BG, Sabahelkheir AH, Awooda AM (2014) Effect of the clinical application of the diode laser (810 nm) in the treatment of dentine hypersensitivity. BMC Res Notes 7:31
Ladalardo TCCGP, Pinheiro A, Campos RADC, BrugneraJúnior A, Zanin F, Albernaz PLM, Weckx LLM (2004) Laser therapy in the treatment of dentine hypersensitivity. Braz Dent J 15(2):144–150
Pesevska S, Nakova M, Ivanovski K, Angelov N, Kesic L, Obradovic R, Mindova S, Nares S (2010) Dentinal hypersensitivity following scaling and root planing: comparison of low-level laser and topical fluoride treatment. Lasers Med Sci 25(5):647–650
Tabatabaei MH, Chiniforush N, Hashemi G, Valizadeh S (2018) Efficacy Comparison of Nd:YAG laser, diode laser and dentine bonding agent in dentine hypersensitivity reduction: a clinical trial. Laser Ther 27(4):265–270
Talesara K, Kulloli A, Shetty S, Kathariya R (2014) Evaluation of potassium binoxalate gel and Nd:YAG laser in the management of dentinal hypersensitivity: a split-mouth clinical and ESEM study. Lasers Med Sci 29(1):61–68
Tengrungsun T, Sangkla W (2008) Comparative study in desensitizing efficacy using the GaAlAs laser and dentin bonding agent. J Dent 36(6):392–395
Yadav BK, Jain A, Rai A, Jain M (2015) Dentine hypersensitivity: a review of its management strategies. Journal of International Oral Health 7(10):137–143
Chow RT, Armati PJ (2016) Photobiomodulation: implications for anesthesia and pain relief. Photomed Laser Surg 34(12):599–609
M.S.M. Alayat, M.A. Basalamah, W.G.E.A.-E. Elbarrany, N.A.M. El-Sawy, E.M. Abdel-Kafy, A.A.-R. El-Fiky, (2020) Dose-dependent effect of the pulsed Nd:YAG laser in the treatment of crushed sciatic nerve in Wister rats: an experimental model, Lasers in Medical Science
Terayama AM, Benetti F, de Araújo Lopes JM, Barbosa JG, Silva IJP, Sivieri-Araújo G, Briso ALF, Cintra LTA (2020) Influence of low-level laser therapy on inflammation, collagen fiber maturation, and tertiary dentin deposition in the pulp of bleached teeth. Clin Oral Investig 24(11):3911–3921
Moosavi H, Arjmand N, Ahrari F, Zakeri M, Maleknejad F (2016) Effect of low-level laser therapy on tooth sensitivity induced by in-office bleaching. Lasers Med Sci 31(4):713–719
de Santana DA, Fonseca GF, Ramalho LMP, Rodriguez TT, Aguiar MC (2017) Effect of low-level laser therapy (λ780 nm) on the mechanically damaged dentin-pulp complex in a model of extrusive luxation in rat incisors. Lasers Med Sci 32(9):1995–2004
Närhi M, Yamamoto H, Ngassapa D, Hirvonen T (1994) The neurophysiological basis and the role of inflammatory reactions in dentine hypersensitivity. Arch Oral Biol 39:S23–S30
Oliveira CF, Basso FG, Lins EC, Kurachi C, Hebling J, Bagnato VS, de Souza Costa CA (2010) Increased viability of odontoblast-like cells subjected to low-level laser irradiation. Laser physics 20(7):1659–1666
Pereira LB, Chimello DT, Wimmers Ferreira MR, Bachmann L, Rosa AL, Bombonato-Prado KF (2012) Low-level laser therapy influences mouse odontoblast-like cell response in vitro. Photomed Laser Surg 30(4):206–213
Fornaini C, Brulat-Bouchard N, Medioni E, Zhang S, Rocca J-P, Merigo E (2020) Nd:YAP laser in the treatment of dentinal hypersensitivity: an ex vivo study. J. Photochem. Photobiol. B 203:111740
Masuda Y, Yokose S, Sakagami H (2017) Gene expression analysis of cultured rat-endothelial cells after Nd:YAG laser irradiation by Affymetrix GeneChip Array. In Vivo 31(1):51–54
Hu ML, Zheng G, Han JM, Yang M, Zhang YD, Lin H (2019) Effect of lasers on dentine hypersensitivity: evidence from a meta-analysis. J Evid Based Dent Pract 19(2):115–130
Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ (Clinical research ed) 327(7414):557–560
Thompson SG, Higgins JPT (2002) How should meta-regression analyses be undertaken and interpreted? Stat Med 21(11):1559–1573
Matys J, Dominiak M, Flieger R (2015) Energy and power density: a key factor in lasers studies. JCDR 9(12):ZL01-ZL2
Alghamdi KM, Kumar A, Moussa NA (2012) Low-level laser therapy: a useful technique for enhancing the proliferation of various cultured cells. Lasers Med Sci 27(1):237–249
E.K.M. Ziago, V.P.S. Fazan, M.M. Iyomasa, L.G. Sousa, P.Y. Yamauchi, E.A. Da Silva, E. Borie, R. Fuentes, F.J. Dias, (2017) Analysis of the variation in low-level laser energy density on the crushed sciatic nerves of rats: a morphological, quantitative, and morphometric study
Limpanichkul W, Godfrey K, Srisuk N, Rattanayatikul C (2006) Effects of low-level laser therapy on the rate of orthodontic tooth movement. Orthod Craniofac Res 9(1):38–43
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shan, Z., Ji, J., McGrath, C. et al. Effects of low-level light therapy on dentin hypersensitivity: a systematic review and meta-analysis. Clin Oral Invest 25, 6571–6595 (2021). https://doi.org/10.1007/s00784-021-04183-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04183-1