Abstract
Conventional therapies have aimed to try to help individuals suffering with dentine hypersensitivity (DH/DHS). A relatively new approach, laser therapy claims to be beneficial while having immediate and long-lasting effect. Therefore, our analysis aims to explore the immediate and 1-month efficacy of near-infrared laser (NIR) therapy in treating dentinal hypersensitivity. A systematic literature search conducted in databases, and analysis was undertaken utilizing a meta-analysis approach. Randomized controlled clinical trials comparing near-infrared lasers and placebo/no treatment in patients (> 18 years) were included. The risk of bias for included studies was assessed using Cochrane RoB tool (for randomized studies). Random effects meta-analyses model of standardized mean differences and 95% confidence intervals were performed using RevMan 5.4 software. A comprehensive electronic and manual search yielded a total of 1081 potential articles. Following the implementation of the inclusion and exclusion criteria, a total of 6 studies were included in the analysis. Near-infrared laser therapy led to statistical significant reduction in immediate and 1-month follow-up VAS (visual analog scale) scores compared to placebo/no treatment (p < 0.05). Statistical heterogeneity across the studies was high (I2-96%). The findings suggest that near-infrared laser therapy does have a significant immediate effect in reducing dentine hypersensitivity compared to placebo/no treatment. Furthermore, this effect is not diminished and endured at 1-month follow-up.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dentinal hypersensitivity (DH/DHS) is a frequently encountered condition by dentists. Clinically it is described as an exaggerated response to non-noxious stimuli, satisfying all the criteria to be classified as a true pain syndrome [1]. According to Canadian Advisory Board on Dentine Hypersensitivity, it is defined as short sharp pain arising from exposed dentine in response to stimuli typically thermal, evaporative, tactile, osmotic, or chemical and which cannot be ascribed to any other form of dental defect or disease [2].
It is commonly reported by patients in the 4th and 5th decades of life causing discomfort during eating, drinking, and breathing. Pain associated with exposed dentine often leads to change in dietary behaviours and sometimes results in deterioration of quality of life. The prevalence of DH ranges from 3.8 to 74.0% [3].
Dentin is inherently sensitive attributed to its close structural and functional relationship with the dental pulp. However, this inherent sensitivity usually is not an issue because it is covered by enamel on the crown and by the cementum on the root dentin. DH is associated with dentin exposure leading to open dentinal tubules and dental pulp nerve response to external stimuli. Various physical, chemical, pathological, and biological challenges and/or developmental abnormalities that result in dental and/or periodontal damage or defects can cause dentine exposure. Various clinical conditions that cause DH include enamel attrition, erosion, corrosion, abrasion, and abfraction. Another major predisposing factor that leads to exposure of cervical and root dentin is the gingival recession. Other factors such as aggressive brushing, soft tissue dehiscence, and ageing can also cause gingival recession, leading to exposure of dentin [4].
Various theories have been proposed attempting to explain the biological mechanism of dentine hypersensitivity, with the hydrodynamic theory being the most widely accepted and has remained the most popular explanation to date. As per this theory, when dentin is exposed to a non-noxious stimulus, it induces fluid flow in the dentinal tubules and consequent nociceptor activation in the pulp/dentin border area. Intra-dental myelinated A-β and some A-δ fibres are thought to respond to stimuli, resulting in the characteristic short, sharp pain of DH [5].
Evidence from literature indicates that external stimuli-induced mechanosensitive responses from odontoblasts and consequent nociceptive transduction in pulpal nerve fibres may present a novel explanation as to how odontoblasts participate in a mechanosensory mechanism leading to the pain associated with DH [6].
A variety of treatment modalities are available to manage dentine hypersensitivity. These include at-home and in-office treatment options. Alteration of fluid flow in the dentinal tubules and modification or blocking of the pulpal nerve response are the main therapeutic modalities used to alleviate pain associated with DH. As the first line of approach, it is recommended to use a dentifrice with potassium salts, strontium salts, fluoride, or remineralizing agents such as CPP-ACP (casein phosphopeptide-stabilized amorphous calcium phosphate) and TCP (tricalcium phosphate). In-office therapies may also help address dentine hypersensitivity. Desensitizing prophylaxis paste formulated with 8% arginine and calcium carbonate to occlude tubules with plugs of arginine, calcium, phosphate, and carbonate can also be applied [7].
Traditional DH treatments mentioned above have shown disadvantages such as they are not effective in all patients, do not resist oral stresses, decrease action with time, and the need for reapplication in a short time. Hence, there is a need for alternative treatment strategies promising immediate, safe, and long-term efficiency [8, 9].
Due to the reported high prevalence of dentine hypersensitivity in the general population, it has become a major topic for research focus. Consequently, it led to advancements in developing innovative measures for its effective and safe treatment. Recently use of lasers in treating DH has attracted significant attention.
LASER is an acronym for light amplification by stimulated emission of radiation. Lasers were used to treat dentine hypersensitivity for the first time in 1985. Various lasers were mentioned in the literature for the treatment of dentine hypersensitivity and include He–Ne (helium–neon, 632 nm), diode (660 nm, 810 nm, 940 nm, 980 nm), Er:YAG (erbium-doped yttrium aluminium garnet, 2940 nm), Er,Cr:YSGG (erbium, chromium-doped yttrium scandium gallium garnet, 2780 nm), Nd:YAG (neodymium-doped yttrium aluminium garnet, 1064 nm), KTP (potassium-titanyl-phosphate, 532 nm), and CO2 (carbon dioxide, 10, 600 nm) lasers. Since their introduction to treating dentine hypersensitivity, these lasers were used in both low- and high-power settings. Among the near-infrared (diode, Nd:YAG) lasers are routinely used in dental practice due to their affordability and versatility. Various studies in literature reported not only their effectiveness but also their unique ability to provide instant relief of dentine hypersensitivity. Several in vitro and in vivo studies have been conducted on their efficacy to treat dentine hypersensitivity, and several mechanisms of action have been hypothesized and proposed thereof. However, proposed mechanisms remain unclear owing to the variability, questioning the reproducibility and safety of their use. Evidence in this context remains limited which makes clinical decision-making difficult [10].
Results of previous systematic reviews and meta-analysis on dentine hypersensitivity have shown a significant clinical effect of lasers on dentinal hypersensitivity when compared to placebo [11, 12]. However, these reviews analysed lasers in general. It is also noteworthy to understand that different lasers with their specific wavelengths have different effects owing to their specific chromophore absorption. Moreover, therapeutic effects significantly vary with the delivery parameters. Therefore, the purpose of this meta-analysis was to evaluate the in vivo efficacy of near-Infrared lasers in the treatment of dentinal hypersensitivity compared to placebo/no treatment.
Materials and methods
A systematic literature search conducted in databases and analysis was undertaken utilizing a meta-analysis approach. The review protocol is registered in PROSPERO (CRD42021232841).
Research question
In order to perform this systematic review and meta-analysis, the following question was noted:
“Are Near-infrared lasers effective in treating dentinal hypersensitivity compared to placebo/no treatment?”
Objective: To compare immediate and 1-month post-treatment VAS scores of near-infrared laser group and placebo/no treatment group in patients with dentine hypersensitivity.
Inclusion and exclusion criteria
The PICO strategy was applied. Intervention studies in adult humans with dentinal hypersensitivity (P) that compared near-infrared lasers (I) to placebo/no treatment group (C) in order to assess reduction in dentinal hypersensitivity (O) were included.
The eligibility criteria were as follows:
-
◦
Inclusion criteria
Studies published between January 2000 and February 2021
Studies that included systemically healthy adult patients with dentine hypersensitivity, age > 18 years
Only in vivo randomized controlled trials (parallel and split-mouth study designs)
Studies that compared lasers versus placebo/no treatment
Follow-up period should be at least 4 weeks/1 month
Studies using VAS scale to assess dentine hypersensitivity (outcome)
-
◦
Exclusion criteria
Studies related to dentine hypersensitivity due to bleaching, dental caries, dental trauma, cracked tooth, and restorations
Studies on other treatment modalities like oxalates, fluorides, nitrates, dentine bonding agents, and restorations
Studies published in other than English language
Studies that did not assess DH by scale/score
Systematic search strategy
The electronic searches were carried out in the following databases: Medline, Pub med, Cochrane, Science Direct, ProQuest, and Open Grey Literature. Additionally, searches were made through the Google scholar. The reference lists of included studies in the review and previously published review articles on dentine hypersensitivity were checked and screened to identify eligible studies. Data bases were searched using the combination of text and mesh terms (Table 1).
Study selection and data extraction
The studies were screened by two independent reviewers to determine whether they met the eligibility criteria. Data extraction form was used to systematically extract the information required from selected articles. Disagreements were resolved following discussions with the primary supervisor. The information and data form included the first author of the study, publication year, country, first follow-up time, other follow-up time, intervention and comparator group, number of interventions and comparisons, laser parameters, and measurement scale.
Study quality assessment
All the included studies were screened to assess the methodological quality of the research. “The Cochrane Collaboration’s tool for assessing risk of bias” tool through the Review Manager software (version 5.4, Review Manager (RevMan) (computer program) Copenhagen: the Nordic Cochrane Centre, the Cochrane Collaboration, 2014) was used for randomized intervention studies [13]. For each included study, the risk of bias was judged for each domain, and the overall assessment as low risk, high risk, or uncertain risk was given.
Meta-analysis
Data from included studies were analysed using RevMan soft-ware (Version 5.4, the Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). The standard mean difference was applied with 95% confidence interval. The I2 statistic was used to explore heterogeneity as it provides a measure of variability across studies that is due to heterogeneity rather than chance [14]. RCTs are conducted by a range of researchers using a wide and varied array of laser wavelengths, parameters, and methodologies which ultimately results in variability in effect size. Hence, a random effect model was employed as the studies were not functionally the same [15]. Forest plots were used to graphically represent the meta-analysis outcome [16]. Funnel plots were used to evaluate the possibility of publication bias [17].
Results
Literature search outcome
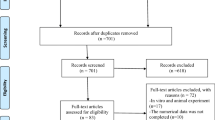
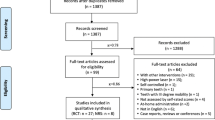
The literature search resulted in a preliminary database of articles from the electronic databases and additional searches. The titles and abstracts of the articles were reviewed to determine the eligibility of the studies. Selected studies were subjected to the inclusion and exclusion criteria resulting in a final group of included studies. The systematic search was established in respect of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines [18] (Fig. 1).
Of the 1262 published studies, 94 studies were identified for potential inclusion. The articles excluded during the initial screen included books, review articles, meta-analysis, in vitro studies, studies that used other desensitizing agents, studies that used other laser wavelengths, and studies that did not have any placebo/no treatment group. Several articles were not randomized trials, and some studies were not done on dentinal hypersensitivity. After the full review of 94 studies, 6 RCTs were included in the meta-analysis based on eligibility criteria.
Study characteristics
Summary of basic characteristics of the 6 included studies is shown in Table 2 and Table 3. All the final included studies were between January 2000 and February 2021. Among included studies, 3 studies used Nd:YAG laser (Lier et al., Birang et al., Maximiano et al.) [19,20,21], and the other 3 studies used diode lasers (Bal et al., Yilmaz et al. (a), Yilmaz et al. (b)) [22,23,24]. The post-treatment follow-up time in included studies varied and ranged from the immediately after treatment to 6-month post-treatment with evaluation at different time points. As per the inclusion criteria, we chose to take results at immediate and 1-month follow-up. The initial follow-up time of all the selected studies was immediately after laser treatment, excluding Maximiano et al.’s which is 5 min after treatment. Few studies had groups other than near-infrared lasers and placebo/no treatment. We have extracted data of only the groups/arms relevant to our analysis. All the included studies neither had similarity in laser parameters used nor in technique/points of application of laser for dentine hypersensitivity. None of the studies reported any adverse outcomes after laser treatment for dentine hypersensitivity in follow-up evaluation. Mean and standard deviation values of respective groups are extracted from the selected studies for meta-analysis.
Risk of bias
A total of 6 randomized clinical trials were assessed for risk of bias using the Cochrane tool. The evaluation of risk of bias of included studies showed that only 1 study showed high risk, 4 studies showed moderate risk, and 1 study showed low risk (Fig. 2a, b).
Findings of meta-analysis
For immediate post-treatment effect
Laser treatment led to a reduction in VAS scores across all controlled studies except a study conducted by Lier et al. A significant effect size of − 1.49, Z = 2.22, p = 0.03, (95% CI [− 2.80, − 0.18]) was found (Fig. 3). A measure of between-study heterogeneity (Tau2 = 2.60) was large indicating that the variance is high. A high level of heterogeneity or significant inconsistency was observed across the included studies (I2 = 96%, p < 0.0001).
The confidence interval of the combined effect does not include zero; i.e. in the case of a confidence level of 95%, the P-value is smaller than 0.05 and implies that the immediate efficacy is statistically significant.
For 1-month post-treatment effect
At 1-month post-treatment, laser therapy led to reduction in VAS scores across all controlled studies except the study conducted by Lier et al. A significant effect size of g = − 2.28, Z = − 2.64, and p = 0.008 (95% CI [− 3.98, − 0.5]) was found (see Fig. 4). Tau2 (2.60) was large indicating that between-study variance is high. I2 = 97%, p < 0.00001, showing a high level of heterogeneity across the included studies. The confidence interval of combined effect in Fig. 4 does not include zero; i.e. in the case of a confidence level of 95%, the P-value is smaller than 0.05 and implies that 1-month efficacy is statistically significant.
Publication bias
Publication bias of these studies is graphically represented using funnel plots (Figs. 5 and 6). According to the meta-analysis, there was no significant asymmetry or evidence of significant publication bias among the selected studies. The figures display symmetrical distribution of studies around the effect size implying no evidence of publication bias. As can be seen in the funnel plots, all the studies are more widely dispersed around the combined effect size. However, because of a high level of heterogeneity, the results of the funnel plot in the present meta-analysis cannot be interpreted as publication bias should be performed if only the results are homogenous.
Discussion
The literature supports the hypothesis about lasers having an immediate and long-term analgesic effect on pain associated with dentine hypersensitivity [25]. However, it requires strong support by evidence-based clinical decisions regarding the use of therapeutic lasers in the treatment of dentine hypersensitivity. Management of DH is generally considered difficult due to the highly subjective nature of pain and the variation of this pain response between individuals. Moreover, it is crucial that before any treatment is undertaken, a definitive diagnosis should be made. This involves the exclusion of all other conditions with a similar presentation of dentin hypersensitivity. Our quantitative analysis excluded all the studies that treated DH associated with dental caries, tooth bleaching, trauma, restorations, and periodontal flap surgery.
Various measures have been proposed to both qualify and quantify pain associated with DH which includes both mechanical and thermal stimulation of exposed dentine to elicit a pain response. Clinical trials on this topic employed specialized devices such as controlled pressure probes, thermal probes, standardized pain stimulation techniques using a triple air syringe (air blast), or a simple explorer probe to elicit pain response [26]. The present analysis included studies that utilized air blast to elicit DH for it is most commonly used in several studies.
Dentine hypersensitivity being a subjective symptom seeks an objective measure to translate it. A variety of pain scales are being used in literature such as VAS (visual analog scale), VRS (verbal rating scale), NRS (numerical rating scale), and Schiff cold air sensitivity scale [27]. An opinion regarding these scales in the literature is controversial. VAS is widely used in clinical studies to measure DH for its reported validity. Hence, we included studies that used the VAS scale for dentine hypersensitivity measurement. However, owing to its physical structure and non-linearity, combined with the individual’s behaviour when confronted with the scale still casts doubts on its validity [28]. Studies that utilized the VAS scale to evaluate the reduction in DH have been included in our analysis for it being often used in clinical trials and to minimize the heterogeneity.
The present meta-analysis attempted to analyse all the randomized clinical trials that compared near-infrared lasers versus placebo/no treatment in the treatment of dentinal hypersensitivity. The studies included in the present meta-analysis used different wavelengths with varying parameters for treating dentine hypersensitivity. All the studies have done a single application of laser and evaluated at different time points. Among the included studies, though had multiple evaluation time points, we confined to immediate and long-term effect at 1 month for statistical analysis to maximally minimize the heterogeneity.
The results of the present meta-analysis revealed that using lasers for treating dentine hypersensitivity reduced the VAS scores demonstrating p values for the test of significance of the total overall estimate of 0.026 and 0.008 for immediate and 1-month efficacy, respectively. This data confirms that NIR laser therapy has been able to modulate pain associated with dentine hypersensitivity. This finding is consistent with previous reviews which reported significantly better outcomes in the laser group compared to placebo [10, 29].
The risk of bias assessment of our study showed that one study had a high risk and others had an unclear risk for most domains of internal validity particularly for allocation concealment which may distort the effect estimates. Grossman suggested that an ideal desensitizing agent should not endanger the integrity of dental pulp while being relatively painless on application and should have rapid action and be permanently effective. All the included studies reported no adverse outcomes/pulpal damage. Therefore, near-infrared laser treatment is regarded as safe and effective as it met most of the criteria of an ideal desensitizing effect [30].
Due to the diversity of laser wavelengths and parameters used in the selected studies, a separate analysis must be performed for each of the wavelengths utilized. Our analysis tried to reduce the heterogeneity by being confined to a near-infrared group of lasers compared to previous reviews that included all laser wavelengths. The results from the present study lend support to the claim that the use of near-infrared lasers for the treatment of dentine hypersensitivity does decrease VAS scores when compared to placebo/no treatment across included trials. This provides support to the underlying theoretical understanding of the mechanism of dentine hypersensitivity and the role of lasers in providing immediate and long-term desensitizing effects. However, the exact mechanism through which the laser exhibits its action remains unclear. Various theories have been proposed in an attempt to explain prolonged pain suppression when lasers (at low and high power) have been used in treating DH [31,32,33]. At low power (p < 0.5 W), the following are the theories proposed:
-
Inducing changes in neural transmission networks within dental pulp that may stimulate normal physiological cellular function
-
Stimulation of odontoblasts with the production of tertiary dentin and obliteration of dentinal tubules
-
Increasing PGF2, COX2, and growth factors
-
Stimulating circulation, cellular activity, and providing various effects such as analgesia, anti-inflammatory, and healing
-
Immediate analgesic effect by blocking the depolarization of C fibre afferents nerves
At high power (p > 0.5 W).
-
Sealing of dentinal tubules by melting from heat transmission leading to fusion and re-solidification of dentin
-
Evaporation of dentinal fluid
Based on the results from this meta-analysis, multiple concerns need to be addressed when considering future research. These include, but are not limited to, poor methodological standards, lack of evidence to suggest that a prior power calculation had been done, and reporting incomplete parameters. None of the studies included an evidence-based treatment approach and an unclear explanation of the technique of laser application and laser parameters.
This meta-analysis reviewed 6 articles as per the inclusion criteria that investigated the efficacy of NIR lasers in treating DH. Three studies utilized Nd:YAG laser, while the other three studies utilized diode lasers with varied wavelength. Unfortunately, all the included studies lacked complete information on laser protocol and parameters. Reviewed studies differed considerably in the parameters employed and the point of application of irradiance. For instance, a study conducted by Maximaiano et al. irradiated the vestibular area for treating DH, while the study conducted by Bal et al. irradiated the cervical area of the tooth in addition to the vestibular area. Despite these differences, results of both the studies showed a reduction in DH. In addition, none of the selected studies provided a complete set of parameters. This questions the validity and reproducibility of published research to great extent.
The use of a power metre to calibrate the exposure is critical to determine power loss, which is not reported in any of the included studies. Information on tip diameter, mode, pulse repetition time (continuous wave mode/gated mode or pulsed mode), tip-to-tissue distance (contact/non-contact), power, point of application, beam divergence, length of treatment, speed of movement, initiation technique if done, and irradiation surface area will help in calculating the dose (fluence) applied on the target tissue in question. It is well-known that power density and mainly energy density drive all laser tissue interactions [34]. Further, the calculated dose can be checked for whether it falls within the recommended dose range for treatment of a particular condition (e.g. analgesic effect in dentine hypersensitivity). This information is crucial to carry our future investigations and propose evidence-based guidelines. An important concern while using laser energy is that the heat produced at the irradiated root surface may diffuse to the pulp causing irreversible pulpal damage. Intra-pulpal temperatures increase as a function of power, frequency, and time [35]. The radiant exposure employed by Lier et al. [19] seemed to be higher than optimal where they used 4 W power for 2-min duration. With the use of such high-power parameters, inhibitory effects and deleterious thermal effects on dental pulp are expected. Despite the high-power parameters used, the vitality test and histological examination revealed no adverse effects with evident normal pulp. The study also reports a possible placebo effect at follow-up time periods.
The application of lasers for therapeutic purposes requires a thorough knowledge of optical physics and training. Lack of sufficiently good quality randomized controlled studies combined with inadequate parameter reporting of included studies reduces the power of our investigation and further impacts the integrated data to a considerable extent. Given the limitations, there is a need for researchers to conduct large-scale randomized controlled clinical trials. Further, the inclusion criteria should specify clear cut-off ages with a smaller age range and should confine to a single etiological factor (e.g. DH due to gingival recession, DH post-bleaching, DH after flap surgery) to target individuals that may benefit from an intervention. This further may reduce the chance of ceiling effects and would allow for a customized treatment approach that targeted key areas to ensure that treatment is beneficial.
In our quantitative analysis, it has not been possible to tease apart the varying wavelengths and parameters across the included studies and if they are differentially effective. However, we decided to exclude studies that utilized multiple laser application intervention to minimize heterogeneity.
Given that, this meta-analysis, to the authors’ knowledge, is the first to confine the investigation to near-infrared laser efficacy in treating dentine hypersensitivity. Existing literature on laser therapy for dentine hypersensitivity provided mixed opinions regarding their efficacy. Moreover, previous reviews emphasized on dominant placebo effect over long-term efficacy masking the beneficial effects of laser treatment. However, our study did not observe any such placebo effect at 1-month post-treatment.
Considering the large research base for laser therapy and inconclusive evidence implies a clear need for better quality research. Plausibly, this would have major implications for the clinical application of laser therapy and present the prospect of delivering efficient treatment to a large number of individuals.
The current quantitative analysis included a total of 6 studies. This is a relatively small number compared to previous analyses. The reason for the small number of studies could be explained in terms of the stringent inclusion and exclusion criteria. The current meta-analysis was confined to a narrow field of research into near-infrared lasers in dentine hypersensitivity which, relatively when compared to established treatments such as conventional desensitizing agents, is still very much in its infancy. As such, there may not yet be the volume of research available to conduct a large meta-analysis with the criteria utilized in this study. The current analysis included all studies which met the criteria and did not exclude studies with high risk because, given the limited number of studies, it was important to include as many studies as possible especially when the topic of research has scientific uncertainty [36]. Furthermore, research suggests that further investigation would be carried out within a particular field. The reduced power of this study may be an artefact of the limited studies which fit the set inclusion and exclusion criteria of this meta-analysis.
Conclusion
The findings from this meta-analysis indicate that near-infrared lasers are an efficacious treatment option in patients with dentine hypersensitivity compared to controls. Results at immediate post-intervention are maintained at 1-month follow-up indicating that effects are enduring. Findings did suggest that single application of laser treatment was significantly better at reducing VAS scores when compared to placebo/no treatment group at immediate and 1-month follow-up periods. Indeed, the results risk of bias analysis and significant statistical heterogeneity across the studies reduce the strength of evidence derived from our meta-analysis. This calls for more RCTs with improved methodology and standardized laser protocol in the future.
LLL low-level laser therapy, DP desensitizing paste, GaAlAs gallium-aluminium-arsenide diode laser, VAS visual analog scale
NM not mentioned, GaAlAs gallium-aluminium-arsenide diode laser
Data availability
Not applicable.
Code availability
Not applicable.
References
Curro FA (1990) Tooth hypersensitivity in the spectrum of pain. Dent Clin North Am 34:429–437
Canadian Advisory Board on Dentin Hypersensitivity (2003) Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc 69:221–226
Rees JS, Addy M (2004) A cross-sectional study of buccal cervical sensitivity in UK general dental practice and a summary review of prevalence studies. Int J Dent Hyg 2:64–69. https://doi.org/10.1111/j.1601-5029.2004.00068.x
Pashley DH, Tay F, Haywood VB, Collins MA, Drisko CL (2008) Dentin hypersensitivity: consensus - based recommendations for the diagnosis and management of dentin hypersensitivity. Compend Contin Educ Dent 8:1–37
West NX, Lussi A, Seong J, Hellwig E (2013) Dentin hypersensitivity: pain mechanisms and aetiology of exposed cervical dentin. Clin Oral Investig 17:S9–S19. https://doi.org/10.1007/s00784-012-0887-x
Magloire H, Maurin JC, Couble ML, Shibukawa Y, Tsumura M, Thivichon-Prince B, Bleicher F (2010) Topical review. Dental pain and odontoblasts: facts and hypotheses. J Orofac Pain 24:335–349
Panagakos F, Schiff T, Guignon A (2009) Dentin hypersensitivity: effective treatment with an in-office desensitizing paste containing 8% arginine and calcium carbonate. Am J Dent 22:3A-7A
Schmalz G, Hellwig F, Mausberg RF, Schneider H, Krause F, Haak R, Ziebolz D (2017) Dentin protection of different desensitizing varnishes during stress simulation: an in vitro study. Oper Dent 42:E35–E43. https://doi.org/10.2341/16-068-l
Lin PY, Cheng YW, Chu CY, Chien KL, Lin CP, Tu YK (2013) In-office treatment for dentin hypersensitivity: a systematic review and network meta-analysis. J Clin Periodontol 40:53–64. https://doi.org/10.1111/jcpe.12011
Asnaashari M, Moeini M (2013) Effectiveness of lasers in the treatment of dentin hypersensitivity. J Lasers Med Sci 4:1–7
Sgolastra F, Petrucci A, Severino M, Gatto R, Monaco A (2013) Lasers for the treatment of dentin hypersensitivity: a meta-analysis. J Dent Res 92:492–499. https://doi.org/10.1177/0022034513487212
Kong Y, Lei Y, Li S, Zhang Y, Han J, Hu M (2020) Network meta-analysis of the desensitizing effects of lasers in patients with dentine hypersensitivity. Clin Oral Investig 24:1917–1928. https://doi.org/10.1007/s00784-019-03051-3
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al (2011) The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Higgins JP, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558. https://doi.org/10.1002/sim.1186
Hunter J, Schmidt F (2000) Fixed effects vs. random effects meta-analysis models: implications for cumulative research knowledge. Int JSelAssess 8:275–292. https://doi.org/10.1111/1468-2389.00156
Petrie A, Bulman J, Osborn J (2003) Further statistics in dentistry Part 8: systematic reviews and meta-analyses. Br Dent J 194:73–78. https://doi.org/10.1038/sj.bdj.4809877
Duval S, Tweedie R (2000) Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 56:455–463. https://doi.org/10.1111/j.0006-341x.2000.00455.x
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097. https://doi.org/10.1371/journal.pmed1000097
Lier BB, Rösing CK, Aass AM, Gjermo P (2002) Treatment of dentin hypersensitivity by Nd:YAG laser. J Clin Periodontol 29:501–506. https://doi.org/10.1034/j.1600-051x.2002.290605.x
Birang R, Poursamimi J, Gutknecht N, Lampert F, Mir M (2007) Comparative evaluation of the effects of Nd:YAG and Er:YAG laser in dentin hypersensitivity treatment. Lasers Med Sci 22:21–24. https://doi.org/10.1007/s10103-006-0412-z
Maximiano V, Machado AC, Yoshida ML, Pannuti CM, Scaramucci T, Aranha AC (2018) Nd:YAG laser and calcium sodium phosphosilicate prophylaxis paste in the treatment of dentin hypersensitivity: a double-blind randomized clinical study. Clin Oral Investig 23:3331–3338. https://doi.org/10.1007/s00784-018-2759-5
Bal MV, Keskiner I, Sezer U, Acikel C, Saygun I (2015) Comparison of low level laser and arginine-calcium carbonate alone or combination in the treatment of dentin hypersensitivity: a randomized split-mouth clinical study. Photomed Laser Surg 33:200–205. https://doi.org/10.1089/pho.2014.3873
Yilmaz H, Kurtulmus-Yilmaz S, Cengiz E, Bayindir H, Aykac Y (2011) Clinical evaluation of Er, Cr: YSGG and GaAlAs laser therapy for treating dentine hypersensitivity: a randomized controlled clinical trial. J Dentistry 39:249–254. https://doi.org/10.1016/j.jdent.2011.01.003
Yilmaz HG, Kurtulmus-Yilmaz S, Cengiz E (2011) Long-term effect of diode laser irradiation compared to sodium fluoride varnish in the treatment of dentine hypersensitivity in periodontal main- tenance patients: a randomized controlled clinical study. Photomed Laser Surg 29:721–725. https://doi.org/10.1089/pho.2010.2974
Marto CM, Baptista Paula A, Nunes T, Pimenta M, Abrantes AM, Pires AS et al (2019) Evaluation of the efficacy of dentin hypersensitivity treatments-a systematic review and follow-up analysis. J Oral Rehabil 46(10):952–990. https://doi.org/10.1111/joor.12842
Gillam DG (2015) Management of dentin hypersensitivity. Curr Oral Health Rep 2:87–94. https://doi.org/10.1007/s40496-015-0047-x
Karcioglu O, Topacoglu H, Dikme O, Dikme O (2018) A systematic review of the pain scales in adults: which to use? Am J Emerg Med 36(4):707–714. https://doi.org/10.1016/j.ajem.2018.01.008
Idon PI, Sotunde OA, Ogundare TO (2009) Beyond the relief of pain: dentin hypersensitivity and oral health-related quality of life. Front Dent 16(5):325–334. https://doi.org/10.18502/fid.v16i5.2272
Hu ML, Zheng G, Han JM, Yang M, Zhang YD, Lin H (2019) Effect of lasers on dentine hypersensitivity: evidence from a meta-analysis. J Evid Based Dent Pract 19:115–130. https://doi.org/10.1016/j.jebdp.2018.12.004
Davari A, Ataei E, Assarzadeh H (2013) Dentin hypersensitivity: etiology, diagnosis and treatment; a literature review. J Dent (Shiraz) 149(3):136–145
Lan WH, Lee BS, Liu HC, Lin CP (2004) Morphologic study of Nd:YAG laser usage in treatment of dentinal hypersensitivity. J Endod 30:131–134. https://doi.org/10.1097/00004770-200403000-00001
Aranha AC, Pimenta LA, Marchi GM (2009) Clinical evaluation of desensitizing treatments for cervical dentin hypersensitivity. Braz Oral Res 23:333–339. https://doi.org/10.1590/s1806-83242009000300018
Yilmaz HG, Cengiz E, Kurtulmus-Yilmaz S, Leblebicioglu B (2011) Effectiveness of Er, Cr:YSGG laser on dentine hypersensitivity: a controlled clinical trial. J Clin Periodontol 38:341–346. https://doi.org/10.1111/j.1600-051x.2010.01694.x
Parker S, Cronshaw M, Anagnostaki E, Mylona V, Lynch E, Grootveld M (2020) Current concepts of laser tissue interaction. Dent J (Basel) 8(3):61. https://doi.org/10.3390/dj8030061
Kreisler M, Al-Haj H, D’Hoedt B (2002) Intrapulpal temperature changes during root surface irradiation with an 809-nm GaAlAs laser. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 93(6):730–735. https://doi.org/10.1067/moe.2002.124766
Turner RM, Bird SM, Higgins JP (2013) The impact of study size on meta - analyses: examination of underpowered studies in cochrane reviews. PLoS ONE 8:e59202. https://doi.org/10.1371/journal.pone.0059202
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bellal, S., Feghali, R.E., Mehta, A. et al. Efficacy of near infrared dental lasers on dentinal hypersensitivity: a meta-analysis of randomized controlled clinical trials . Lasers Med Sci 37, 733–744 (2022). https://doi.org/10.1007/s10103-021-03391-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10103-021-03391-1