Abstract
Objective
To evaluate the effects of adjunctive delivery of a sodium hypochlorite gel in the treatment of peri-implant mucositis (PM).
Materials and methods
Forty-six subjects with 68 implants diagnosed with PM were randomly assigned to two treatment groups. Prior to mechanical debridement, a sodium hypochlorite gel was delivered to the implants of the test group while implants of the control group received a placebo gel. Application of both test and placebo gels was repeated 5 times at baseline. The primary outcome variable was the change in pocket probing depth (PPD) between baseline and 6 months.
Results
After 6 months, the mean PPD decreased statistically significantly from 3.93 ± 1.09 mm to 3.04 ± 0.46 mm in the test (p = 0.0001) and from 3.68 ± 0.85 mm to 3.07 ± 0.58 mm in the control (p = 0.0001) group, respectively. No statistically significant difference (p = 0.53) was observed with respect to PPD changes from baseline to 6 months between test (0.88 ± 1.04 mm) and control group (0.61 ± 0.75 mm), respectively. The number of implants with bleeding on probing (BoP) decreased statistically significantly from 33 to 18 in the test group (p = 0.0001) and from 34 to 23 in the control group (p = 0.0001) after 6 months.
Conclusions
In conclusion and within the limits of the present study, changes in PPD from baseline to 6 months were not statistically significantly different between groups. Complete resolution of mucosal inflammation was not achieved with either of the therapies.
Clinical relevance
The present outcomes have showed that a complete resolution of peri-implant mucositis is not possible to obtain by means mechanical debridement with or without a sodium hypochlorite gel application.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Peri-implant mucositis has been defined as an inflammatory lesion in the soft tissues surrounding an endosseous implant without loss of supporting peri-implant bone [1]. Clinical signs of inflammation may include bleeding on probing, erythema, swelling, and suppuration. A cause-effect relationship between experimental accumulation of bacterial biofilms around titanium dental implants and the development of experimental peri-implant mucositis has been demonstrated in humans [2,3,4,5]. Various anti-infective protocols similar to those applied for the treatment of gingivitis have been adopted to treat peri-implant mucositis [6,7,8,9,10,11,12]. The outcomes of the study by Heitz-Mayfield et al. 2011 indicated that non-surgical debridement and self-performed plaque control with and without adjunctive application of chlorhexidine gel were effective in the treatment of peri-implant mucositis. That study, however, also documented that this anti-infective protocol did not yield complete resolution of mucosal inflammation. In fact, only 38% of the implants were without any sites bleeding on probing after 3 months. In addition, implants with supramucosal restoration margins showed greater improvement following treatment of peri-implant mucositis compared with those with submucosal restoration margins [13].
Collectively, outcomes of clinical studies failed to report complete resolution of inflammation in the management of peri-implant mucositis. Therefore, as untreated peri-implant mucositis may in some patients progress to peri-implantitis, complete resolution of inflammation should be considered the prerequisite for the prevention of peri-implantitis [14].
A novel formulation of a sodium hypochlorite (NaOCl) gel was recently introduced as an adjunct for mechanical biofilm removal. Outcomes of an in vitro study indicated that the new NaOCl gel possesses antimicrobial activities, in particular against Gram-negative species associated with periodontitis [15]. Despite the fact that the NaOCl gel failed to eliminate a multi-species biofilm, a strong effect on the reduction of biofilm vitality and alteration in the composition of biofilm matrix was found pointing to its high potential as an adjunct in mechanical therapy of periodontal diseases. However, at the present time, data on the clinical application of this novel NaOCl gel formulation for the treatment of biofilm-associated peri-implant infections are still scarce, and thus, its potential clinical benefits unknown [16].
Therefore, the aim of this randomized controlled clinical trial was to compare the effects of non-surgical mechanical debridement with or without adjunctive delivery of a sodium hypochlorite gel in the treatment of peri-implant mucositis up to 6 months.
Materials and methods
Patient selection
All patients included in the study were enrolled and treated in the Department of Periodontology, University of Naples Federico II, Italy, and in the Department of Periodontology, Victor Babes University, Timisoara, Romania. The study protocol was submitted to and approved by the ethical committee of the Victor Babes University, Timisoara, Romania, (Nr. 06/18.05.2015) for both centers.
In addition, the study protocol was registered with the German Clinical Trials Register (registration number: DRKS00006977).
Written informed consent was obtained and the study was conducted according to the principles of the declaration of Helsinki on experimentation involving human subjects. Subjects were included based on the following criteria:
Age ≥ 18 years
Non-smokers and smokers ≤ 10 cigarettes/day
Subjects with gingivitis or treated periodontal conditions (i.e., absence of residual PPD > 5 mm)
Peri-implant mucositis defined as ≥ 1 implant site with presence of BoP and absence of radiographic bone loss compared with a previous radiograph [17] (Figs. 1 and 2)
Implant-supported fixed dental prostheses
Subjects were excluded based on the following criteria:
Presence of medical conditions contraindicating treatment of peri-implant mucositis
Regular use of anti-inflammatory drugs or antibiotics within 3 months prior to study enrollment
Pregnant or lactating females
Removable implant-supported dental prostheses
Prothesis reconstruction with visible gap between abutment and suprastructure
Peri-implantitis, defined as inflammation in the peri-implant mucosa with concomitant loss of supporting bone [18]
Null hypothesis
No statistically significant differences are observed with respect to the clinical parameters pocket probing depth (PPD), bleeding on probing (BoP), and modified plaque index (mPlI) between the two treatment modalities (i.e., adjunctive delivery of an amino acid buffered sodium hypochlorite gel vs. placebo gel).
Primary and secondary outcome variables
The primary outcome variable was the change in PPD. Secondary outcome variables included the changes in the clinical parameters BoP, mPlI, full-mouth plaque score (FMPS), and full-mouth bleeding score (FMBS).
Sample size calculation
Assuming a 0.9-mm PPD difference between study groups and a standard deviation in PPD of 1.0 mm in each group, 20 experimental subjects and 20 control subjects would be needed to reject the null hypothesis with p = 0.05 and a statistical power of 80% [10].
Experimental design
A double-arm, randomized, controlled clinical trial (RCT) of 6 months duration was designed. The results of the present trial are reported according to the CONSORT guidelines (http://www.consort-statement.org/).
Randomization
The subjects were randomly assigned to one of the two experimental procedures. The allocation was carried out using a commercially available computer software package (NCSS-PASS, Number Cruncher Statistical Systems, Kaysville, UT, USA). Treatment allocation was performed immediately before delivery of the treatment by opening an opaque envelope containing the information.
Masking and calibration
All treatment procedures were provided by two periodontists (VIS and SIS). Both periodontists were masked with respect to the experimental procedures. All parameters were recorded at baseline and after 1, 3, and 6 months by two calibrated examiners (AB and DR). The two examiners were calibrated based on repeated assessments of the clinical parameters of 7 subjects not enrolled in the study (Interclass Correlation Coefficient 0.92). A triple-blind study design was implemented, i.e., all subjects, the two treatment providers, and the two examiners were masked with respect to the experimental procedures.
Clinical assessment and procedures
The following clinical parameters were assessed:
Presence of plaque according to the plaque index (PlI) [19] and to the modified index for oral implants (mPI) [20].
Presence of peri-implant mucosal inflammation assessed with the bleeding on probing index [21].
Pocket probing depths (PPD) assessed as the distance from the peri-implant mucosal margin to the bottom of the sulcus.
Full-mouth plaque score (FMPS) representing the percentage of sites covered with plaque in the entire dentition [22].
Full-mouth bleeding score (FMBS) representing the percentage of sites with bleeding on probing in the entire dentition [23].
All clinical parameters were recorded at 4 sites/implant (i.e., mesial, distal, oral, buccal) by means of a manual periodontal probe (PerioWise color coded probe, Premier, Plymouth Meeting, PA, USA) applying a probing force of approximately 0.2 N.
Experimental procedures
All subjects were randomized following baseline measurements. Prior to peri-implant mucositis treatment, full-mouth supragingival scaling was delivered to all subjects. Prior to mechanical debridement of the study implants with the use of an ultrasonic scaler with a plastic tip (Kavo SONICflex®, Biberach, Germany), an amino acid buffered sodium hypochlorite gel (Perisolv®, Regedent, Zürich, Switzerland) was delivered for 30 s to the implants of the test group (Figs. 3 and 4) while implants of the control group received a topical application of a placebo gel for 30 s. In both groups, no rinsing was performed after gel application before the ultrasonic debridement.
The placebo gel (carboxymethyl cellulose solution with pH 7.27, carrier of amino acids and water with pH neutral) displayed the same consistency and color as Perisolv®.
According to the manufacturer’s instructions, application of both the test and placebo gels was repeated 5 times around the study implants at baseline. At the end of the treatment session, a full-mouth prophylaxis procedure with a rubber cup and a polishing paste was performed.
Clinical follow-up
All subjects were instructed to brush the study implants at least twice daily as part of their routine self-performed plaque control. A 0.12% chlorhexidine gel (Curasept ADS®, Curaden AG, Kriens, Switzerland) was prescribed twice daily for 2 weeks following treatment. Clinical parameters were recorded at 1, 3, and 6 months following treatment (Fig. 5). Presence/absence of adverse effects related to the experimental procedures was recorded.
Data analysis
Data from all implants diagnosed with peri-implant mucositis in the same subject were pooled, averaged, and included in the analysis. Therefore, the patient was considered as the statistical unit. However, an adjunctive implant-based analysis was also performed. Mean values and standard deviations (SD) were calculated for age, PPD, FMPS, and FMBS. Differences in mean age were calculated with the Student’s t test while the Fisher’s exact test was used to calculate differences in gender, smoking habits, and number of periodontally compromised subjects. The chi-square test was applied to calculate differences in implant position.
For the parameters mPlI and BoP scores, the number of implants was reported.
In order to estimate the center effect, a generalized linear model for repeated measures was applied.
Intra-group comparisons for PPD, mPlI, and BoP at baseline, 1, 3, and 6 months were assessed by means of Friedman’s test for repeated measures. Mann-Whitney’s test was used to assess inter-group comparisons at baseline, 1, 3, and 6 months.
Differences in number of implants with absence of BoP were assessed by means of the chi-square test.
A p value < 0.05 was considered statistically significant.
Results
Demographic characteristics
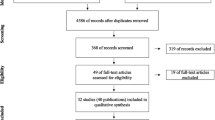
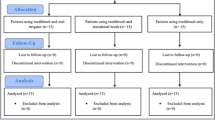
The flow chart of the study is presented in Fig. 6. A total of 48 subjects with 70 implants were enrolled. Following assessment of eligibility, 2 subjects with 1 implant each declined participation to the study. Therefore, 46 subjects with 68 implants were randomized. Experimental therapy was delivered to 23 subjects with 34 implants (test group) while 23 subjects with 34 implants (control group) received the placebo therapy. One subject with 1 implant in the test group was lost to follow-up because of pregnancy. Twenty-two subjects with 33 implants in the test group and 23 subjects with 34 implants in the control group were available for the statistical evaluation.
The demographic characteristics of the subject’s sample at baseline are presented in Table 1. No statistically significant differences (p > 0.05) were observed with respect to mean age, gender, number of cigarette smokers, and number of subjects with treated periodontal conditions between test and control groups.
No adverse effects related to the experimental procedures were recorded.
Implant location
Table 2 presents the distribution of implants between the test and control groups with respect to anterior and posterior areas of mandible and maxilla. A similar percentage of implants had been placed in posterior (i.e., 82%), as well as in anterior (i.e., 18%), areas of mandible and maxilla in both groups. No statistically significant differences (p > 0.05) were observed with respect to implant locations between test and control groups, respectively.
Full-mouth plaque score and full-mouth bleeding score
Full-mouth plaque score (FMPS) and full-mouth bleeding score (FMBS) at baseline and at the 6-month follow-up are summarized in Table 3. Six months following therapy, mean FMPS scores revealed statistically significant changes compared with baseline in both groups. The mean FMPS decreased from 42.4 ± 8.3% to 21.1 ± 6.4% in the test (p = 0.001) and from 43.5 ± 8.7% to 21.8 ± 4.2% in the control groups (p = 0.001), respectively. A statistically significant decrease in mean FMBS was recorded from 32.7 ± 7.1% to 17.3 ± 6.2% in the test (p = 0.001) and from 33.3 ± 5.0% to 18.2 ± 5.0% in the control (p = 0.001) groups, respectively. No statistically significant differences (p > 0.05) were observed with respect to FMPS and FMBS between groups at baseline and at the 6-month follow-up.
Pocket probing depth
Table 4 presents changes in pocket probing depth (PPD) from baseline to 1, 3, and 6 months following therapy in the test and control groups, respectively. After 6 months, the mean PPD decreased statistically significantly (p = 0.0001) from 3.93 ± 1.09 mm to 3.04 ± 0.46 mm in the test and from 3.68 ± 0.85 mm to 3.07 ± 0.58 mm in the control groups, respectively. No statistically significant differences (p > 0.05) were observed with respect to PPD between groups at baseline, 1-, 3-, and at the 6-month follow-up.
Moreover, no statistically significant difference (p = 0.53) was observed with respect to PPD change from baseline to 6 months between test (0.88 ± 1.04 mm) and control (0.61 ± 0.75 mm) groups, respectively.
Modified plaque index
Table 5 presents changes in the number of implant with plaque and BoP-positive from baseline to 1, 3, and 6 months following therapy in the test and control groups, respectively. After 6 months, the number of implant with plaque decreased statistically significantly (p = 0.002) from 29 to 19 in the test and from 31 to 22 in the control groups (p = 0.0001), respectively. No statistically significant differences (p > 0.05) were observed with respect to the number of implant with plaque between groups at baseline, 1, 3, and 6 months following therapy.
BoP-positive implants
As shown in Table 5, 6 months following therapy, the number of BoP-positive implants decreased statistically significantly (p = 0.0001) from 33 to 18 in the test and from 34 to 23 in the control groups (p = 0.0001), respectively. No statistically significant difference (p = 0.271) between the number of BoP-positive implants in the test and control groups was observed from baseline to 6 months.
Center effect for the clinical parameters PPD, BoP, and mPlI
Table 6 summarizes the center effect for the clinical parameters PPD, BoP, and mPlI. An estimate of 0.300 ± 0.338 was recorded for PPD value, while estimates of − 0.228 ± 0.171 and 0.271 ± 0.174 were found for BoP and mPlI respectively, since no treatment by center interaction was observed.
BoP-negative implants 6 months following therapy
The number and percentage of implants free of any BoP-positive sites is summarized in Table 7. No statistically significant difference (p = 0.32) was observed when comparing the number of BoP-negative implants between the test group (n = 15) and the control group (n = 11) 6 months following therapy.
Discussion
The aim of the present randomized controlled trial was to evaluate the clinical effects in the treatment of peri-implant mucositis with either adjunctive delivery of a sodium hypochlorite gel or a placebo gel up to 6 months. The results indicated no statistically significant differences between the two groups with respect to pocket probing depth changes and number of BoP-negative implants at 6 months. The 6-month outcomes of that study indicated that mechanical debridement with adjunctive delivery of Perisolv was equally effective as non-surgical mechanical debridement alone.
Although clinical and biochemical outcomes have showed that healing of experimental mucositis in human model occurs within 3 weeks [4], in the present study, findings at 3 and 6 months were also reported in order to monitor the maintenance of the clinical results. In any case, no statistical significant differences between 1, 3, and 6 months were noted.
A sample of 45 patients were treated in the present study. The sample size was determined assuming a 0.9-mm PPD difference between study groups according to previous study conduced by Hallstrom and co-workers [10]. A difference of 0.9 mm can be expected because in a randomized controlled clinical trial conducted by Heitz-Mayfield and co-workers implants with peri-implant mucositis treated with non-surgical therapy and chlorhexidine gel application respect to non-surgical therapy and placebo application showed a difference of 1.1 mm in PPD change [11].
Despite the fact that a statistically significant reduction in BoP was achieved in the present study, only 45% of the implants in the test group and 32% of the implants in the control group yielded complete resolution of BoP at 6 months.
Hence, as all clinical parameters of the present study improved over 6 months in both the test and the placebo groups, the null hypothesis was not rejected.
Previous clinical studies evaluating the treatment of peri-implant mucositis with either mechanical debridement alone [7, 12] or with adjuncts including essential oils [24], chlorhexidine rinsing [9, 25], chlorhexidine rinsing combined with chlorhexidine gel [6], use of 0.3% triclosan toothpaste [8], chlorhexidine gel [13], systemic antimicrobials [10], glycine powder air-polishing [26], and probiotics [27, 28] reported improvements in clinical parameters such as reductions in PPD and BoP. However, adjunctive deliveries such as chlorhexidine [6], systemic azythromycin [10], or glycine powder air-polishing [26] failed to yield additional clinical benefits in the treatment of peri-implant mucositis compared with mechanical debridement alone.
Furthermore, data on the potential adjunctive clinical benefits following delivery of probiotic supplements for the management of peri-implant mucostis are also controversial [27, 28]. Variations in study designs and eligibility criteria may partly explain differences in treatment outcomes.
Hence, based on the treatment outcomes reported above, in addition to individually taylored oral hygiene instructions, professionally delivered mechanical removal of hard and soft tissue deposits should be considered the standard of care in the management of peri-implant mucositis.
The clinical effects of adjunctive delivery of a sodium hypochlorite gel (i.e., Perisolv®) were also investigated for the non-surgical management of peri-implantitis in a randomized controlled trial [16]. The 3-month outcomes of that study indicated that mechanical debridement with adjunctive delivery of Perisolv® was equally effective as non-surgical mechanical debridement alone in the reduction of mucosal inflammation [16].
Peri-implant mucositis lesions may be present for extended periods of time without progression to peri-implantitis. The conversion of peri-implant mucositis lesions to peri-implantitis in humans is difficult to study in an experimental design for obvious ethical reasons. The importance of treating peri-implant mucositis as a preventive measure for the onset of peri-implantitis was highlighted in a retrospective study [14].
Over an observation period of 5 years, half of the subjects received supportive care and treatment of peri-implant mucositis while the other half did not. At the end of the observation period, 18% of the subjects enrolled in supportive care progressed to peri-implantitis while the incidence of peri-implantitis amounted to 43.9% in subjects without supportive care [14]. At the 5-year examination, 30.5% of subjects in the group with supportive care showed healthy peri-implant soft tissue conditions while in the group without supportive care none of the subjects showed peri-implant soft tissue health [14]. Thus, management of peri-implant mucositis should be considered a prerequisite for the prevention of peri-implantitis [29].
In conclusion and within the limits of the present study, changes in PPD from baseline to 6 months were not statistically significantly different between groups.
Complete resolution of mucosal inflammation was not achieved with either of the therapies.
References
Heitz-Mayfield LJ, Salvi GE (2018) Peri-implant mucositis. J Clin Periodontol 45(suppl 20):s237–s245. https://doi.org/10.1111/jcpe.12953
Pontoriero R, Tonelli MP, Carnevale G, Mombelli A, Nyman SR, Lang NP (1994) Experimentally induced peri-implant mucositis. A clinical study in humans. Clin Oral Implants Res 5:254–259
Zitzmann NU, Berglundh T, Marinello C, Lindhe J (2001) Experimental peri-implant mucositis in man. J Clin Periodontol 28:517–523. https://doi.org/10.1034/j.1600-051x.2001.028006517.x
Salvi GE, Aglietta M, Eick S, Sculean A, Lang NP, Ramseier CA (2012) Reversibility of experimental peri-implant mucositis compared with experimental gingivitis in humans. Clin Oral Implants Res 23:182–190. https://doi.org/10.1111/j.1600-0501.2011.02220.x
Meyer S, Giannopoulou C, Courvoisier D, Schimmel M, Müller F, Mombelli A (2017) Experimental mucositis and experimental gingivitis in persons aged 70 or over. Clinical and biological responses. Clin Oral Implants Res 28:1005–1012. https://doi.org/10.1111/clr.12912
Porras R, Anderson GB, Caffesse R, Narendran S, Trejo PM (2002) Clinical response to 2 different therapeutic regimens to treat peri-implant mucositis. J Periodontol 73:1118–1125
Maximo MB, de Mendonca AC, Renata Santos V, Figuereido LC, Feres M, Duarte PM (2009) Short-term clinical and microbiological evaluations of peri-implant diseases before and after mechanical anti-infective therapies. Clinl Oral Implants Res 20:99–108. https://doi.org/10.1111/j.1600-0501.2008.01618.x
Ramberg P, Lindhe J, Botticelli D, Botticelli A (2009) The effect of a triclosan dentifrice on mucositis in subjects with dental implants: a six-month clinical study. J Clin Dent 20:103–107
Thöne-Mühling M, Swierkot K, Nonnenmacher C, Mutters R, Flores-de-Jacoby L, Mengel R (2010) Comparison of two full-mouth approaches in the treatment of peri-implant mucositis: a pilot study. Clin Oral Implants Res 21:504–512. https://doi.org/10.1111/j.1600-0501.2009.01861.x
Hallström H, Persson GR, Lindgren S, Olofsson M, Renvert S (2012) Systemic antibiotics and debridement of peri-implant mucositis. A randomized clinical trial. J Clin Periodontol 39:574–581. https://doi.org/10.1111/j.1600-051X.2012.01884.x
De Siena F, Francetti L, Corbella S, Taschieri S, Del Fabbro M (2013) Topical application of 1% chlorhexidine gel versus 0.2% mouthwash in the treatment of peri-implant mucositis. An observational study. Int J Dent Hyg 11:41–47. https://doi.org/10.1111/idh.12002
Riben-Grundström C, Norderyd O, Andrè U, Renvert S (2015) Treatment of peri-implant mucositis using a glycine powder air-polishing or ultrasonic device: a randomized clinical trial. J Clin Periodontol 42:462–469. https://doi.org/10.1111/jcpe.12395
Heitz-Mayfield LJ, Salvi GE, Botticelli D, Mombelli A, Faddy M, Lang NP, Implant Complication Research Group (2011) Anti-infective treatment of peri-implant mucositis: a randomized controlled clinical trial. Clin Oral Implants Res 22:237–241. https://doi.org/10.1111/j.1600-0501.2010.02078.x
Costa FO, Takenaka-Martines S, Cota LO, Ferreira SD, Silva GL, Costa JE (2012) Peri-implant disease in subjects with and without preventive maintenance: a 5-year follow-up. J Clin Periodontol 39:173–181. https://doi.org/10.1111/j.1600-051x.2011.01819.x
Jurczyk K, Nietzsche S, Ender C, Sculean A, Eick S (2016) In-vitro activity of sodium-hypochlorite gel on bacteria associated with periodontitis. Clin Oral Investig 20:2165–2173. https://doi.org/10.1007/s00784-016-1711-9
Roos-Jansåker AM, Almhöjd US, Jansson H (2017) Treatment of peri-implantitis: clinical outcome of chloramine as an adjunctive to non-surgical therapy: a randomized clinical trial. Clin Oral Implants Res 28:43–48. https://doi.org/10.1111/clr.12612
Zitzmann NU, Berglundh T (2008) Definition and prevalence of peri-implant diseases. J Clin Periodontol 35:286–291. https://doi.org/10.1111/j.1600-051X.2008.01274.x
Lindhe J, Meyle J (2008) Peri-implant diseases: consensus report of the Sixth European Workshop on Periodontology. J Clin Periodontol 35:282–285. https://doi.org/10.1111/j.1600-051X.2008.01283.x
Silness J, Löe H (1964) Periodontal disease in pregnancy. II. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand 22:121–135
Mombelli A, van Oosten MAC, Schürch E, Lang NP (1987) The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol Immunol 2:145–151
Lang NP, Joss A, Orsanic T, Gusberti FA, Siegrist BE (1986) Bleeding on probing. A predictor for the progression of periodontal disease? J Clin Periodontol 13:590–596
O’Leary TJ, Drake RB, Naylor JE (1972) The plaque control record. J Periodontol 43:38
Claffey N, Nylund K, Kiger R, Garrett S, Egelberg J (1990) Diagnostic predictability of scores of plaque, bleeding, suppuration and probing depth for probing attachment loss. 3 1/2 years of observation following initial periodontal therapy. J Clin Periodontol 17:108–114
Ciancio SG, Lauciello F, Shibly O, Vitello M, Mather M (1995) The effect of an antiseptic mouthrinse on implant mainteinance: plaque and peri-implant gingival tissue. J Periodontol 66:962–965
Felo A, Shibly O, Ciancio SG, Lauciello FR, Ho A (1997) Effect of subgingival chlorhexidine irrigation on peri-implant maintenance. Am J Dent 10:107–110
Ji YJ, Tang ZH, Wang R, Cao J, Jin LJ (2014) Effect of glycine powder air-polishing as an adjunct in the treatment of peri-implant mucositis: a pilot clinical trial. Clin Oral Implants Res 25:683–689
Hallström H, Lindgren S, Widen C, Renvert S, Twetman S (2016) Probiotic supplements and debridement of peri-implant mucositis: a randomized controlled trial. Acta Odontol Scand 74:60–66. https://doi.org/10.3109/00016357.2015.1040065
Galofré M, Palao D, Vicario M, Nart J, Violant D (2018) Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: a triple-blind randomized clinical trial. J Periodontal Res 53:378–390. https://doi.org/10.1111/jre.12523
Salvi GE, Zitzmann NU (2014) The effects of anti-infective preventive measures on the occurrence of biologic implant complications and implant loss: a systematic review. Int J Oral Maxillofac Implants 29(suppl):292–307. https://doi.org/10.11607/jomi.2014suppl.g5.1.Review
Acknowledgments
The experimental materials used in the present study were provided by Regedent AG, Zürich, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Iorio-Siciliano, V., Blasi, A., Stratul, SI. et al. Anti-infective therapy of peri-implant mucositis with adjunctive delivery of a sodium hypochlorite gel: a 6-month randomized triple-blind controlled clinical trial. Clin Oral Invest 24, 1971–1979 (2020). https://doi.org/10.1007/s00784-019-03060-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03060-2