Abstract
Background
Peri-implant diseases have been recognized as being among the ever-increasing complications related to dental implants. The aim of this study was to evaluate the adjunctive use of enamel matrix derivative (EMD) to mechanical debridement (MD) in patients with these conditions in terms of clinical parameters and cytokine levels of peri-implant crevicular fluid (PICF).
Methods
In the present double-blind clinical trial, 46 patients with peri-implant mucositis (PM) were randomly divided into control and test groups. Two different therapeutic protocols, consisting of non-surgical MD alone (control group) and MD with the application of EMD (test group), were considered for the two groups. Clinical parameters [bleeding on probing (BOP) and probing depth (PD)] and sampling from PICF were carried out before treatment and 3 months postoperatively. The levels of IL-6 and IL-17 cytokines in PICF were evaluated by enzyme-linked immunosorbent (ELISA).
Results
Three-month post-interventional assay revealed significant improvements in BOP and PD in the test group in comparison to the control group (P < 0.0001). Relative to control, IL-6 and IL-17 levels were reduced significantly (p < 0.05) in the test group compared to the control group.
Conclusion
Application of EMD can be considered an adjunct to MD in the non-surgical treatment of PM. However, complete recovery was not observed using either treatment approach showing that management of implant-associated disease is still a significant clinical problem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ever-increasing placement of endosseous dental implants has led to an inevitable increase in the total number of associated complications observed, that is, peri-implant diseases including peri-implant mucositis and peri-implantitis. Most unfortunately though, management of peri-implant diseases has shown itself to be a difficult problem with no reliable and predictable treatments for these problems described to date [1, 2]. Peri-implant diseases are infectious/inflammatory diseases that affect the soft as well as hard tissues around a functioning implant (peri-implant mucositis and/or peri-implantitis, respectively) [3]. Both conditions are common disorders with incidence rates of 18–56% with respect to implants and are also found in about 60–80% of patients with respect to peri-implant mucositis (PM) and 10–43% of implants in 19–56% of patients with respect to peri-implantitis (PI) [4, 5]. Microorganisms and the host immune response may play a crucial role in the etiopathological mechanisms of peri-implant diseases [6–10]. Major risk factors for PI include poor oral hygiene [11], a history of periodontitis [12], and smoking [13]. Systemic diseases such as diabetes mellitus, alcoholism, genetic factors, incomplete removal of luting material from the sulcus, insufficient keratinized gingiva (KG), implant proximity, implant surface roughness, and occlusal trauma are factors that may be involved in the etiology of peri-implant diseases although the presence of keratinized tissue is not quite as clear as a risk factor/indicator [2, 11, 14].
The symptoms associated with peri-implant lesions include bleeding on probing (BOP), pain on probing (in the experience of the authors, the pain on probing is often much more severe than pain on probing observed in similarly inflamed periodontal tissues) suppuration, increased pocket depth, and mucosal swelling. Failure to diagnose and treat PM in a timely fashion can lead to bone loss and eventually complete loss of osseointegration [2]. It is also not inappropriate to presume that inflammation and infection about implants might have similar systemic effects or risks as seen with regard to periodontitis or infection/inflammation about natural teeth. Therefore, early diagnosis and appropriate treatment are essential to stop and avert the progression of these lesions. Both non-surgical and surgical treatments have been attempted for management of peri-implant diseases, with the latter producing better results for cases with more than 2 mm of progressive bone loss [15, 16]. It has also been found that non-surgical treatment through mechanical debridement (MD) alone does not provide much benefit in the management of peri-implant diseases (e.g., without locally administered antimicrobial agents), and hence, in most cases, adjunctive treatment is recommended [17–19]. As mentioned then, adjunctive treatments that can be performed along with debridement can include the administration of local antibiotic (e.g., Arestin®, Atridox®), while it has also been suggested that photodynamic therapy and lasers might also have improve treatment outcomes of MD, although not as reliably as the use of locally administered antibiotic. In any case, however, the induction of complete recovery of peri-implant lesions following treatment has as yet not been achieved [20–29]. Therefore, other treatment strategies need to be studied to identify the most effective interventions for this category of disease (either singly or in combination).
Current approaches to clinical practice focus primarily on decreasing bacterial challenges rather than modulating the host response [30]. Given the role of cytokines in determining the inflammatory response and bone loss and possibly even in modulation of the disease-associated microbiome [31], studies are required to assess novel approaches to management of implant-associated diseases with the use of therapies that might modify host reactions as opposed to those that strictly alter the microbial elements. In order to do this, it is also important to assess the effects of any treatment on host-mediated production-destructive (and protective) cytokines around implants [32]. One therapeutic technique which has been employed successfully in the management of periodontal diseases and in particular bone loss associated with periodontitis involves the use of enamel matrix derivatives (EMDs) [33–35] Amelogenin and related proteins are probably the most important components of EMD [36]; however, there are several other components in EMD, some known and others as yet unidentified, that might also be important. In any case, EMD has been shown to result in significant clinical improvement in treatment of periodontal and also peri-implant diseases [37, 38].
Although EMD is thought to mediate many of its effects by modulation of host responses, including cellular differentiation and inflammation [39], it has also been demonstrated to have antibacterial properties that inhibit the growth of gram-negative bacteria such as Porphyromonas gingivalis, Aggregatibacter actinomycetemcomitans, and Prevotella intermedia [40, 41]. As indicated previously in relation to inflammation, EMD and in particular amelogenin also limit the release of cytokines induced by bacterial lipopolysaccharide [42] as well as epithelial cells and osteoclasts [43]. Amelogenin in EMD can stimulate mesenchymal cells (e.g., fibroblasts, osteoblasts, cementoblasts, and stem cells) (PMs) (e.g., TGF-β, VEGF, and PDGF) [44]. Consequently, it can enhance osteogenesis and inhibits osteoclastogenesis [45–48], the effects of which are very important in the regeneration of periodontal tissue [48]. Moreover, its effects on soft tissue wound healing [49, 50] might be critically important since the earlier establishment of a more “sterile” subsurface environment following surgery could provide just the right environment for enhancement of regeneration. Despite the efficacy of EMD in the treatment of periodontal disease, there are a limited number of clinical studies focusing on the use of EMD for surgical treatment of PI [51, 52]. In particular, to the best of our knowledge, the use of EMD as an adjunctive non-surgical treatment for PI has not yet been studied. Therefore, the aim of this randomized clinical trial was to compare the impact of non-surgical MD alone vs. adjunctive therapy using both MD and EMD on clinical and biological outcomes following management of patients who have dental implants affected either by severe PM and/or by mild PI.
Materials and methods
Study design
This is a prospective, double-blind randomized clinical trial that was approved by the Research Ethics Committee of Tabriz University of Medical Sciences (No. 9278) and registered with the local World Health Organization Registry Network (IRCT2013060113543N1).
Study participants
The patients were referred to the Tabriz University of Medical Sciences, Faculty of Dentistry, who had had at least one dental implant with mild PI or severe PM that had been in place for at least 1 year ranging from July until the end of November 2013.
Inclusion criteria
Patients were included if they meet the following eligibility criteria:
-
Adult patients (≥18 years of age) with at least one implant functioning for a minimum of 1 year
-
With PM, defined as presence of BOP, probing depth (PD) ≥4 mm, no recession of soft tissue, and bone loss not exceeding than the first-year annual amount (≤2 mm) revealed by long-cone parallel radiographic projection technique) [53]
Exclusion criteria
The subjects were excluded if they had the following conditions [19–21, 25–27, 29]:
-
If they were smokers or substance/alcohol abusers; were pregnant or lactating; were undergoing radiotherapy in the head and neck area; or had uncontrolled systemic disease or infectious diseases such as AIDS, hepatitis, and tuberculosis or if they needed antibiotic prophylaxis
-
If they had PD ≥6 mm at the baseline examination
-
Presence of untreated/active periodontal lesions. The patients with a history of non-surgical periodontal treatments were included.
-
If they had to use any medications affecting periodontal tissue conditions for a long time (e.g., phenytoin, cyclosporine, calcium channel blockers, bisphosphonates, non-steroidal anti-inflammatory drugs)
-
If they had to use systemic antibiotics during the past 3 months
-
If they had any treatment intervention for peri-implant diseases during the past 3 months
Study interventions
A total of 46 patients were selected based on the inclusion and exclusion criteria of the study and were given an informed consent form before they were enrolled. Prior to the study, plaque was disclosed using an erythrosine dye,Footnote 1 and full-mouth plaque scores were obtained. All patients received rubber cup prophylaxis with a low-abrasive paste and oral hygiene instructions. They were then assigned randomly to receive one of the following treatment protocols by a single clinician (AM), under local anesthesia.
Protocol 1 (control group)
Subgingival debridement using an ultrasonic deviceFootnote 2 with plastic tip and glycine-based powder air polishing.Footnote 3 Patients were given a commercial antimicrobial 0.12% chlorhexidine mouthrinseFootnote 4 prior to treatment to reduce the burden of bacteria in aerosols that are produced during use. The tip of the subgingival nozzle was directed at a 90° angle to the long axis of the implant and was activated for 5 s. The tip was positioned 3–4 mm from the implant and kept moving in circular or sweeping motion while the spray was being dispersed.
Protocol 2 (test group)
For the test group, protocol 1 was first performed, followed by the use of EMDFootnote 5 introduced subgingivally into the affected sites 2 weeks after MD. The 2-week healing interval was intended to allow the healing of the peri-implant soft tissue and the reduction of inflammation and bleeding so that EMD would not be washed out from the sulcus.
Emdogain application
After controlling any discharge and bleeding from the pockets around the implants, a 24% EDTA gel was applied for 2 min to decontaminate the implant surface, and then, normal saline was used to remove EDTA. After this process, EMD was placed in the pocket using an insulin syringe.
Post-operatively, the patients rinsed with chlorhexidine (0.12%)Footnote 6 for 2 weeks. In the first day, the patients were prescribed an anti-inflammatory drug (ibuprofen, 400 mg t.i.d.) for management of post-treatment pain. All patients had to report on the use of anti-inflammatory drug and to comment on any possible adverse events during the healing phase. At the end of study and in order to respect the ethical requirements of the trial, the patients were enrolled in a maintenance program with visits to the dental hygienist every third month. For those with less than acceptable clinical outcomes, supplementary surgical interventions were performed as indicated in an attempt to reduce further disease-related signs and symptoms.
Study outcomes
The primary outcome was the reduction of BOP in one positive site in the examined regions. Changes in pocket depth, pain on probing and suppuration, and the level of cytokines around the implant (IL-6 and IL-17) were considered as secondary outcomes.
Pre- and post-treatment assessments
Clinical parameters
The calibrated examiner (AK), blinded to the interventions and patients’ assignment, assessed the following variables before (baseline) and 3 months after treatment:
-
1.
BOP: bleeding in 15 s after gentle probing was recorded as the percentage of bleeding in six sites per examined implant (mid-buccal, mesio-buccal, disto-buccal, mid-lingual/palatal, mesio-lingual/palatal, and disto-lingual/palatal) within each patient.
-
2.
Pocket depth: using a periodontal probeFootnote 7 with light force (0.25 N), the maximum PD for each patient was recorded calculating the distance from the mucosal margin to the base of sulcus [54].
-
3.
Pain on probing (POP): presence (+) or absence (−) of pain upon probing.
-
4.
O’Leary’s plaque index: for the examined implant (local plaque index (LPI)) and for the whole mouth (whole-mouth plaque index (WMPI)), the number of plaque-containing surfaces was multiplied by 100 and divided by the total number of available tooth surfaces.
-
5.
Suppuration: presence (+) or absence (−) of spontaneous suppuration or after finger pressure.
Radiographic assessment
Each implant underwent intraoral periapical radiographyFootnote 8 using the long-cone parallel technique at baseline to determine the amount of bone loss using smooth components and threads of the implants as reference points.
Peri-implant crevicular fluid collection
All the patients underwent a sampling procedure at baseline and 3 months after treatment by a trained examiner (AK). Before collecting peri-implant crevicular fluid (PICF), supragingival plaque was removed using a plastic scaler. These areas were then isolated with cotton rolls and were dried gently using an air syringe for 10 s. Two stripsFootnote 9 were inserted gently into mesial and distal crevices of each implant until mild resistance was felt. They were then kept in place for 30 s. After removal, the paper strips were evaluated. If the strips were contaminated with blood or saliva, they were discarded and replaced by the other strips. The collected samples were placed in a single labeled Eppendorf tubeFootnote 10 containing 350 μL of phosphate-buffered salineFootnote 11 and a protease inhibitor cocktail.Footnote 12 They were then sent to the laboratory immediately after sealing.
Enzyme-linked immunosorbent assay
In the laboratory, the strips were removed from the tubes after 15 min of shaking at room temperature, and the tubes were centrifuged (10 min., 5000×g) in order to separate the cell contents and bacterial biofilms from the supernatant that would presumably contain the proteins of interest. Samples from all the patients were then stored at −70 °C for subsequent analysis. Enzyme-linked immunosorbent (ELISA) was used to quantify IL-6 and IL-17 in PICF sample. According to the manufacturer’s recommendationsFootnote 13, 100 μL of detection antibody was added to all the wells, except the blank wells, mixed gently, and incubated for 60 min at room temperature. After rinsing the plates, standards and PICF were added to the individual wells in triplicate. Following incubation, the plates were washed and incubated with 100 μL of conjugate (SA-HRP) for 60 min at room temperature. The plates were washed three times again, and 100 μL of TMB substrate was added and incubated for 15 min at room temperature in the dark. The reaction was stopped by the addition of 100 μL of inhibiting solution, and the color was measured in an automated microplate spectrophotometer at 450 nm. The quantitative values of cytokines were reported in microgram per milliliter. The ELISA assays were carried out by a blinded operator.
Statistical considerations
Based on a Schwarz et al. [27] study, with a standard deviation of 1.3 (Fisher’s test), significance level of 5% and power of 63%, at least 20 patients per group were needed to identify a positive site among six positive sites around the implant. The sample size was increased to 23 patients per group to account for dropouts. The participants were divided into two groups: control and test using a randomization software program (http://www.randomizer.org).
We used the patient as a “unit” for randomization since only one implant for each patient was included in the study. Therefore, each variable could be analyzed on the patient level. The Kolmogorov–Smirnov test was used to determine the normality of the data. In order to compare the study variables during various assessment intervals of the study (baseline and 3 months after intervention), Friedman’s test was used. The Mann-Whitney U test was used to compare the variables between the case and the control subjects. Two-sided values of p ≤ 0.05 were regarded as statistically significant differences.
Results
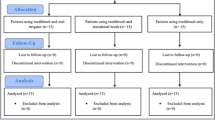
A total of 46 patients with severe PM and/or mild PI were selected. Five patients dropped out for reasons unrelated to the study (moved to other cities, pregnancy, and personal matters). Forty-one patients (21 in the control group and 20 in the test group) were available for the data analyses (Fig. 1). The baseline demographic characteristics of both groups are shown in Table 1. There were no statistically significant differences in the demographic characteristics of either group at baseline.
Clinical parameters
Healing at treatment sites was clinically uneventful; no diverse reactions were noted around the treated sites, nor did patients report any adverse events.
Probing depth
There were no significant reductions in PD following treatment in the control group even up to 3 months post-intervention. Alternatively, there was a mean reduction of PD in the experimental group following treatment with EMD in the range of 3 mm (P < 0.0001, Table 2).
Bleeding on probing
The average percentage of sites positive for BOP at the different study intervals for both groups is shown in Table 2. At baseline, the median (interquartile range) percentage of BOP sites for both the control and test groups was 75% (75–100). After 3 months, no significant changes in BOP were found in the control group compared to baseline, whereas a significant decrease that appeared to be quite substantial at 50% was observed in BOP in the test group vs. control (P < 0.0001).
Plaque index
At baseline, the average LPI and WMPI were 75 and 55% in the control group and 75 and 62.5% in the test group, respectively. LPI in both groups decreased significantly during the follow-up period (P < 0.0001, Table 2).
Pain on probing
Forty-seven percent of patients in the control group and 50% in the test group had POP at baseline. No changes were observed in the control group after 3 months compared to baseline, but EMD decreased markedly to only 10% in the test group (P = 0.002, Table 3).
Suppuration
Of the 41 patients evaluated, three subjects in the test group showed suppuration in the infected implants; EMD resulted in improvements in only one case (P = 0.368, Table 3).
Laboratory results
Interleukin-6
At baseline, the concentrations of IL-6 in PICF of the control and test groups were 54.40 and 33.20 μg/mL, respectively. There was no statistically significant difference between the groups (P = 0.205). Both treatment methods caused a marked decrease in IL-6 after 3 months; however, this change was significantly higher in the test group compared with the control group with a magnitude difference between the two groups of twofold (P = 0.008, Table 2).
Interleukin-17
The mean level of IL-17 was ∼30 μg/mL in both groups at baseline. No significant change was noticed compared to the baseline after 3 months in the control group, whereas a significant decrease in IL-17 was observed in the test group as shown in Table 2.
Discussion
The aim of this clinical trial was to compare the use of non-surgical MD with and without EMD on clinical and cytokine profiles of PM. We found that EMD combined with MD significantly reduced PD and BOP after 3 months, while no significant improvement was obtained in areas treated with MD alone. It is known that non-surgical MD alone has a limited effect in the treatment of PI, and additional interventions are needed in most cases. For example, Renvert et al. [19] showed that no significant PD change (0.2 mm) was found in mild cases of PI with non-surgical debridement (titanium hand instruments or ultrasonic device) after 6 months. Greater reduction in mean PD (0.6 mm) was obtained after 3 months in the mechanical treatment of mild to moderate PI by using an air-abrasive device (amino acid glycine powder) [55]. The reason why non-surgical mechanical treatment for PM and PI is not as effective as it is for gingivitis and periodontitis may be attributed to the histological and structural differences between periodontal and peri-implant tissues as well as the rough surface geometry of the implant [56]. It is also conceivable that gingivitis and periodontitis represent very different disease entities in comparison to peri-implant diseases [57], the latter perhaps being less associated with infection and more related to inflammatory processes and subsequent infection. Accordingly, anti-infective treatment protocols and use of local antibiotics, among the more popular adjunctive treatments, do not often lead to a complete remission of mucosal inflammation around implants [19–21, 26, 28, 29].
Several studies have shown that surgical treatment of periodontal disease using EMD leads to significant reductions in PD and gain in clinical attachment level (CAL) as compared to control [33–35, 58]. Moreover, non-surgical application of EMD along with SRP in the treatment of moderate to severe chronic periodontitis has been reported to demonstrate 30–44% reduction in BOP and a 2–2.8-mm reduction in mean PD after 3 months [59, 60]. In the present study, it was found that EMD treatment led to a remarkable reduction in PD and BOP compared to baseline, while no significant changes were found in the control group, even up to 3 months after treatment. We recruited patients with no soft tissue recession; therefore, the amount of PD and CAL was equal. Although we argue that inflammation is key, we must also recognize that infection (whether primary or secondary) must play an important role in the progression of PD and PI and this is in agreement then with findings reported in a systematic review showing that the additional use of local anti-infective therapy in combination with non-surgical debridement led to significant reductions in mean PD in cases of PI over a 4-month period [26].
Complete remission of mucosal inflammation around implants was achieved in 30% of the patients in the test group, similar to findings reported elsewhere [21] in which 15% of individuals with PI who received local delivery of minocycline microspheres and 30% who received photodynamic therapy had complete remission of mucosal inflammation around the implant. It is known that soft tissue healing in PI often occurs during the first 3 months after treatment [20]. In the current study, the majority of participants in the control and test groups (72 and 70%, respectively) had KG ≥2 mm. While the importance of KG for preserving the health of peri-implant tissues is controversial, in patients with suboptimal plaque control, the existence of KG around implants can maintain peri-implant health [61]. Besides, a number of reports highlight the importance of oral hygiene instruction and supportive periodontal therapy in strengthening the long-term results of PI treatment [22]. In the current study, the average of LPI and WMPI in the patients was diminished significantly, a change associated with decreases in inflammation severity and pocket depths. Furthermore, EMD significantly reduced POP, which can be associated with a further reduction of inflammation in the test group compared to the control group. In the present study, a 3-mm reduction in mean PD was achieved at 3 months following treatment only in the patients who were treated with EMD in adjunction with MD. It would appear then that the effect of MD + EMD in the treatment of mild to moderate PI is comparable to the use of topical MD + chlorhexidine [55], local delivery of minocycline microspheres [29], and photodynamic therapy [21].
To reiterate here though, although EMD does have some antimicrobial properties, these are not nearly as potent as those found with locally administered antimicrobials, and so, the impact of EMD on treatment outcome could be related more to its effects on local cellular function/host reaction than on the microbial elements alone, and this will be discussed in more detail in the following.
Consistent with previous studies indicating the relationship of pro-inflammatory cytokines with the PI lesions [9, 10, 62, 63], the cytokines, either in the control or test group, were diminished in the PICF of all the studied patients. IL-6 plays a role in both the innate and adaptive immune responses and leads to the activation of signals which stimulate Th17 cells to produce IL-17 leading to activation of osteoclasts and bone loss along with IL-1β and TNF-α. In turn, IL-17 causes activation of neutrophils (these cells play a critical role in bone loss and tissue destruction in periodontitis [64] and likely in PI and PM and osteoclasts as well as production of other destructive cytokines) (IL-1β, IL-6, IL-8) [10]. Given that in our study, the amount of IL-6 and IL-17 significantly decreased in the test group as compared to the control group, it can be postulated that EMD may play a role in controlling inflammation by reducing pro-inflammatory cytokines and regulating the immune system in a manner consistent with a host modulation phenomenon.
Given the presence of many classifications for peri-implant diseases by individuals [65] and/or organizations [66–68], the field of implantology requires a standardized means to communicate the level of disease severity and its impact on the prognosis for implants for each level of the disease. This being said, it is unlikely that the classification system used here had any impact on the interpretation of the treatment outcomes observed in this study.
Our study has some limitations. Due to the design parameters, only short-term outcomes could be assessed. Clearly, longer-term assessments are required, but in this regard, we can look at our findings as an initial proof of principle. It would also be of some interest to determine whether repeated interventions (instead of single) using EMD or only MD could have produced better outcomes. In this regard, we do not know what is the optimum dose regimen that should be used for EMD in treatment of these types of conditions and this too would have to be tested. Moreover, the use of EMD in combination other therapeutic approaches for the treatment of PM including but not limited to different techniques for cleansing of implant surfaces, the use of various anti-infective and/or host-modifying agents, and even the use of regenerative procedures require further investigation.
Conclusions
The results of this randomized clinical trial showed that non-surgical MD has limited effects in the treatment of PM. At this time, we postulate that the use of EMD in conjunction with MD should be considered when managing these conditions at least initially.
Recommendations
-
1.
Further clinical trials are necessary on the treatment modalities for peri-implantitis with longer follow-ups.
-
2.
Due to the chronic nature of this disease condition, repetition of treatment procedures might be necessary to prevent recurrence.
Notes
Top Dent Lifco Dental AB, Enko¨ping, Sweden
Piezon ® 250, EMS Electro Medical Systems SA, Nyon, Switzerland
Air-Flow Master ®, Perio Powder ®; EMS Electro Medical Systems SA, Nyon, Switzerland
Laboratorios KIN, S.A., ,Barcelona, Spain
Emdogain ® Straumann, Basel, Switzerland
Laboratorios KIN, S.A., ,Barcelona, Spain
12 UNC color coded probe, Hu-Friedy Mfg. Co., LLC.Chicago, IL
Insight Dental Films, Eastman Kodak Company, Rochester, NY, USA
Periopaper ® Oraflow, Plainview, NY, USA
Eppendorf ® AG, Hamburg, Germany
PBS, Invitrogen, USA
Sigma-Aldrich, Saint Louis, MO, USA
MABTECH AB, R&D Systems, Büro Deutschland, Germany
References
Aljateeli M, Fu JH, Wang HL (2012) Managing peri-implant bone loss: current understanding. Clin Implant Dent Relat Res 14(Suppl 1):e109–e118
Algraffee H, Borumandi F, Cascarini L (2012) Peri-implantitis. Br J Oral Maxillofac Surg 50(8):689–694
Lindhe J, Meyle J (2008) Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol 35(8 Suppl):282–285
Atieh MA et al (2013) The frequency of peri-implant diseases: a systematic review and meta-analysis. J Periodontol 84(11):1586–1598
Zitzmann NU, Berglundh T (2008) Definition and prevalence of peri-implant diseases. J Clin Periodontol 35(8 Suppl):286–291
Hultin M et al (2002) Microbiological findings and host response in patients with peri-implantitis. Clin Oral Implants Res 13(4):349–358
Van Dyke TE (2012) The impact of genotypes and immune reactivity on peri-implant inflammation: identification and therapeutic use of anti-inflammatory drugs and immunomodulators. Eur J Oral Implantol 5(Suppl):S51–S60
Irshad M et al (2013) Cytokine and matrix metalloproteinase expression in fibroblasts from peri-implantitis lesions in response to viable Porphyromonas gingivalis. J Periodontal Res 48(5):647–656
Javed F et al (2011) Proinflammatory cytokines in the crevicular fluid of patients with peri-implantitis. Cytokine 53(1):8–12
Severino VO, Napimoga MH, de Lima Pereira SA (2011) Expression of IL-6, IL-10, IL-17 and IL-8 in the peri-implant crevicular fluid of patients with peri-implantitis. Arch Oral Biol 56(8):823–828
Heitz-Mayfield LJ (2008) Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol 35(8 Suppl):292–304
Renvert S, Persson GR (2009) Periodontitis as a potential risk factor for peri-implantitis. J Clin Periodontol 36(Suppl 10):9–14
Strietzel FP et al (2007) Smoking interferes with the prognosis of dental implant treatment: a systematic review and meta-analysis. J Clin Periodontol 34(6):523–544
Kozlovsky A et al (2007) Impact of implant overloading on the peri-implant bone in inflamed and non-inflamed peri-implant mucosa. Clin Oral Implants Res 18(5):601–610
Nogueira-Filho G, Iacopino AM, Tenenbaum HC (2011) Prognosis in implant dentistry: a system for classifying the degree of peri-implant mucosal inflammation. J Can Dent Assoc 77:3
Lang NP et al (2004) Consensus statements and recommended clinical procedures regarding implant survival and complications. Int J Oral Maxillofac Implants 19(Suppl):150–154
Zeza B, Pilloni A (2012) Peri-implant mucositis treatments in humans: a systematic review. Ann Stomatol (Roma) 3(3–4):83–89
Renvert S, Roos-Jansaker AM, Claffey N (2008) Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol 35(8 Suppl):305–315
Renvert S et al (2009) Mechanical non-surgical treatment of peri-implantitis: a double-blind randomized longitudinal clinical study. I: clinical results. J Clin Periodontol 36(7):604–609
Renvert S et al (2008) Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri-implantitis: a randomized clinical trial. J Periodontol 79(5):836–844
Schar D et al (2013) Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: six-month outcomes of a prospective randomized clinical trial. Clin Oral Implants Res 24(1):104–110
Thierbach R, Eger T (2013) Clinical outcome of a nonsurgical and surgical treatment protocol in different types of peri-implantitis: a case series. Quintessence Int 44(2):137–148
Esposito M, Grusovin MG, Worthington HV (2012) Treatment of peri-implantitis: what interventions are effective? A Cochrane systematic review. Eur J Oral Implantol 5(Suppl):S21–S41
Esposito M et al (2008) The efficacy of interventions to treat peri-implantitis: a Cochrane systematic review of randomised controlled clinical trials. Eur J Oral Implantol 1(2):111–125
Deppe H et al (2013) Nonsurgical antimicrobial photodynamic therapy in moderate vs severe peri-implant defects: a clinical pilot study. Quintessence Int 44(8):609–618
van Winkelhoff AJ (2012) Antibiotics in the treatment of peri-implantitis. Eur J Oral Implantol 5(Suppl):S43–S50
Schwarz F et al (2005) Clinical evaluation of an Er:YAG laser for nonsurgical treatment of peri-implantitis: a pilot study. Clin Oral Implants Res 16(1):44–52
Kotsakis, G. A., et al. (2014) A systematic review and meta-analysis of the effect of various laser wavelengths in the treatment of peri-implantitis. J Periodontol
Salvi GE et al (2007) Adjunctive local antibiotic therapy in the treatment of peri-implantitis II: clinical and radiographic outcomes. Clin Oral Implants Res 18(3):281–285
Renvert S, Roos-Jansåker AM, Claffey N (2008) Non-surgical treatment of peri-implant mucositis and peri-implantitis: a literature review. J Clin Periodontol 35(s8):305–315
Petković A et al (2010) Proinflammatory cytokines (IL-1β and TNF-α) and chemokines (IL-8 and MIP-1α) as markers of peri-implant tissue condition. Int J Oral Maxillofac Surg 39(5):478–485
Heitz-Mayfield LJ (2008) Peri-implant diseases: diagnosis and risk indicators. J Clin Periodontol 35(s8):292–304
Esposito M et al (2009) Enamel matrix derivative (Emdogain) for periodontal tissue regeneration in intrabony defects. A Cochrane systematic review. Eur J Oral Implantol 2(4):247–266
Koop R, Merheb J, Quirynen M (2012) Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: a systematic review. J Periodontol 83(6):707–720
Li W, Xiao L, Hu J (2012) The use of enamel matrix derivative alone versus in combination with bone grafts to treat patients with periodontal intrabony defects: a meta-analysis. J Am Dent Assoc 143(9):e46–e56
Hammarström L, Heijl L, Gestrelius S (1997) Periodontal regeneration in a buccal dehiscence model in monkeys after application of enamel matrix proteins. J Clin Periodontol 24(9 Pt 2):669–677
Koop R, Merheb J, Quirynen M (2012) Periodontal regeneration with enamel matrix derivative in reconstructive periodontal therapy: a systematic review. J Periodontol 83(6):707–720
Sculean A et al (2003) Histologic evaluation of human intrabony defects following non-surgical periodontal therapy with and without application of an enamel matrix protein derivative. J Periodontol 74(2):153–160
Chano L et al (2003) Emdogain® regulation of cellular differentiation in wounded rat periodontium. J Periodontal Res 38(2):164–174
Spahr A et al (2002) Effect of the enamel matrix derivative Emdogain on the growth of periodontal pathogens in vitro. J Clin Periodontol 29(1):62–72
Walter C et al (2006) Moderate effect of enamel matrix derivative (Emdogain gel) on Porphyromonas gingivalis growth in vitro. Arch Oral Biol 51(3):171–176
Wada Y, Mizuno M, Tamura M (2009) Enamel matrix derivative neutralized the effect of lipopolysaccharide on osteoprotegerin and receptor activator of nuclear factor kappa B ligand expression of osteoblasts. Arch Oral Biol 54(4):306–312
Bosshardt DD (2008) Biological mediators and periodontal regeneration: a review of enamel matrix proteins at the cellular and molecular levels. J Clin Periodontol 35(s8):87–105
Jiang J et al (2001) Effects of enamel matrix derivative on gene expression of primary osteoblasts. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 91(1):95–100
Qu Z et al (2010) Effect of Emdogain on proliferation and migration of different periodontal tissue-associated cells. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 109(6):924–931
Weishaupt P et al (2008) Stimulation of osteoblasts with Emdogain increases the expression of specific mineralization markers. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106(2):304–308
Kemoun P et al (2011) The role of cell surface markers and enamel matrix derivatives on human periodontal ligament mesenchymal progenitor responses in vitro. Biomaterials 32(30):7375–7388
Lyngstadaas SP et al (2009) Enamel matrix proteins; old molecules for new applications. Orthod Craniofac Res 12(3):243–253
Wennstrom JL, Lindhe J (2002) Some effects of enamel matrix proteins on wound healing in the dento-gingival region. J Clin Periodontol 29(1):9–14
Khedmat S et al (2010) Effects of enamel matrix derivative on the viability, cytokine secretion, and phagocytic activity of human monocytes. J Endod 36(6):1000–1003
Sculean A et al (2004) Treatment of peri-implantitis with EDTA decontamination and application of an enamel matrix protein derivate—a report of 3 cases. PERIO (Periodontal Practice Today) 1:237–245
Froum SJ, Froum SH, Rosen PS (2012) Successful management of peri-implantitis with a regenerative approach: a consecutive series of 51 treated implants with 3-to 7.5-year follow-up. International Journal of Periodontics and Restorative Dentistry 32(1):11
Sanz M, Chapple IL (2012) Clinical research on peri-implant diseases: consensus report of Working Group 4. J Clin Periodontol 39(Suppl 12):202–206
Etter TH et al (2002) Healing after standardized clinical probing of the perlimplant soft tissue seal. Clin Oral Implants Res 13(6):571–580
Sahm N et al (2011) Non-surgical treatment of peri-implantitis using an air-abrasive device or mechanical debridement and local application of chlorhexidine: a prospective, randomized, controlled clinical study. J Clin Periodontol 38(9):872–878
Mellado-Valero A et al (2013) Decontamination of dental implant surface in peri-implantitis treatment: a literature review. Med Oral Patol Oral Cir Bucal 18(6):e869–e876
1996) Meffert, R., Periodontitis vs. peri-implantitis: the same disease? The same treatment? Critical Reviews in Oral Biology & Medicine 7(3):278–291
Froum SJ et al (2001) A comparative study utilizing open flap debridement with and without enamel matrix derivative in the treatment of periodontal intrabony defects: a 12-month re-entry study. J Periodontol 72(1):25–34
Gutierrez MA, Mellonig JT, Cochran DL (2003) Evaluation of enamel matrix derivative as an adjunct to non-surgical periodontal therapy. J Clin Periodontol 30(8):739–745
Mombelli A et al (2005) Enamel matrix proteins and systemic antibiotics as adjuncts to non-surgical periodontal treatment: clinical effects. J Clin Periodontol 32(3):225–230
Brito C et al (2014) Is keratinized mucosa indispensable to maintain peri-implant health? A systematic review of the literature. J Biomed Mater Res B Appl Biomater 102(3):643–650
Casado PL et al (2013) Peri-implant disease and chronic periodontitis: is interleukin-6 gene promoter polymorphism the common risk factor in a Brazilian population? Int J Oral Maxillofac Implants 28(1):35–43
Kadkhodazadeh M et al (2013) IL-17 gene polymorphism is associated with chronic periodontitis and peri-implantitis in Iranian patients: a cross-sectional study. Immunol Investig 42(2):156–163
Koh A et al (2007) Role of osteopontin in neutrophil function. Immunology 122(4):466–475
Froum SJ, Rosen PS (2012) A proposed classification for peri-implantitis. International Journal of Periodontics and Restorative Dentistry 32(5):533
Lindhe J, Meyle J (2008) Peri-implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol 35(s8):282–285
Rosen P et al (2013) Peri-implant mucositis and peri-implantitis: a current understanding of their diagnoses and clinical implications. J Periodontol 84(4):436–443
Lang NP, Berglundh T (2011) Periimplant diseases: where are we now?—Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol 38(s11):178–181
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Funding
The work was supported by the Dental and Periodontal Research Center, Tabriz University of Medical Sciences, Tabriz, Iran.
Ethical approval
The study was approved by the Research Ethics Committee of Tabriz University of Medical Sciences (No. 9278) and registered with the local World Health Organization Registry Network (IRCT2013060113543N1).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
ESM 1
(DOC 217 kb)
Rights and permissions
About this article
Cite this article
Kashefimehr, A., Pourabbas, R., Faramarzi, M. et al. Effects of enamel matrix derivative on non-surgical management of peri-implant mucositis: a double-blind randomized clinical trial. Clin Oral Invest 21, 2379–2388 (2017). https://doi.org/10.1007/s00784-016-2033-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-016-2033-7