Abstract
Objectives
This study aimed to investigate the possible crosstalk between LPS/toll-like receptor 4 (TLR4) and ephrinB2 signaling in mediating osteogenic differentiation of PDLSCs.
Materials and methods
Human periodontal ligament stem cells (hPDLSCs) were harvested and treated with different concentrations of LPS under osteogenic induction. qPCR, alkaline phosphatase (ALP) staining, and Alizarin Red S staining were performed to assess osteogenic gene expression, ALP activity, and mineralized nodule formation. EphrinB2 mRNA and protein expressions after LPS treatment were also determined. To explore the role of ephrinB2 in LPS-impaired osteogenic differentiation of hPDLSCs, hPDLSCs were stimulated with ephrinB2-Fc or transfected with ephrinB2 lentivirus, and then, the osteogenic differentiation capacity was evaluated.
Results
LPS inhibited osteogenic differentiation of hPDLSCs and downregulated ephrinB2 expression in hPDLSCs during osteogenic differentiation. Blockage of TLR4 partially reversed LPS-induced decrease in ephrinB2 expression. EphrinB2-Fc promoted mineralized nodule formation and increased the expression of ALP, osteocalcin (OCN), and bone morphogenetic protein 2 (BMP2) in hPDLSCs. EphrinB2-overexpressing hPDLSCs treated with LPS expressed higher ALP and BMP2 mRNA and higher ALP activity and showed more mineralized nodule formation, when compared with wide-type hPDLSCs treated with LPS.
Conclusions
Our data suggested that LPS decreased the osteogenic differentiation capacity of hPDLSCs partially through downregulation of ephrinB2 expression via LPS/TLR4 signaling. Upregulation of ephrinB2 partially reversed the impaired osteogenic potential of hPDLSCs induced by LPS.
Clinical relevance
Our results provided a new insight of mechanism underling LPS-mediated osteogenic differentiation inhibition of PDLSCs and clarified a potential target for the management of periodontitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Periodontal pathogenic bacteria (mainly Gram-negative anaerobes) trigger an inflammatory reaction in the tooth-supporting tissues, causing periodontal and bone damage and subsequent tooth loss [1, 2]. Lipopolysaccharide (LPS), the major virulent factor of Gram-negative bacteria, plays a key role in a series of inflammatory responses and progressive periodontal tissue disruption [3,4,5,6]. However, the underlying mechanisms of LPS-host interaction have not been fully elucidated.
Periodontal ligament stem cells (PDLSCs), residing within the periodontal ligament tissues, are responsible for periodontal ligament and alveolar bone homeostasis [7,8,9]. Importantly, PDLSCs possess osteogenic potential to repair and regenerate the damaged periodontal bone tissues. It has been demonstrated that PDLSCs can migrate to the site of periodontal lesions and mediate periodontal regeneration [10,11,12]. In addition, PDLSCs have been shown to generate cementum/PDL-like tissues after transplantation in immunodeficient mice [13] and have been utilized to reconstruct the destroyed periodontal bone tissues in vivo [14, 15]. Nevertheless, an inflammatory environment can impair osteogenic differentiation of PDLSCs. The osteogenic differentiation capacity of PDLSCs isolated from periodontitis-affected periodontal ligament tissues was significantly lower than that of PDLSCs isolated from healthy periodontal ligament tissues [16]. A variety of inflammatory factors, like tumor necrosis factor-alpha (TNF-α) [17] and interleukin-1 (IL-1) [18], have inhibitory effects on osteogenic differentiation of PDLSCs. As one of the major mediators of inflammation, LPS has been reported to inhibit the osteogenic potential of PDLSCs [19,20,21,22], although there are some controversies [23, 24].
Interaction between ephrinB2 expressed on osteoclasts and EphB4 expressed on osteoblasts regulates osteoclast-osteoblast communication, thereby maintaining bone homeostasis [25]. In addition to being expressed on osteoclasts, ephrinB2 is also expressed on osteoblasts or bone mesenchymal stem cells (BMSCs), and it acts in a paracrine or autocrine manner on EphB4 or EphB2 in itself to enhance the osteogenic potential [26,27,28,29,30]. In addition to bone cells, periodontal ligament fibroblasts also co-express ephrin and Eph molecules [31, 32]. Our group recently revealed that ephrinB2 overexpression promoted mineralized nodule formation and upregulated osteogenic gene expression in PDLSCs [33]. However, the role of ephrinB2 in osteogenic differentiation of PDLSCs under an inflammatory environment has not yet been investigated.
In this study, we aimed to investigate the effects of LPS on osteogenic potential of PDLSCs and to further explore the role of ephrinB2 in this process. The results indicated that LPS inhibited osteogenic capacity of PDLSCs partially through toll-like receptor 4 (TLR4)-mediated downregulation of ephrinB2, and the impaired osteogenic potential of PDLSCs induced by LPS could be partially reversed by ephrinB2 overexpression in PDLSCs. Our study provided a potential target for the management of periodontitis.
Materials and methods
Isolation and characterization of human periodontal ligament stem cells
All experiments were performed after receiving approval from the Ethics Committee of Xuzhou Medical University (20161108). hPDLSCs were isolated from freshly extracted third molars, which were collected from 10 healthy adults (18–25 years old) with their consent. Periodontal ligament tissues were scraped from the middle-third root surfaces and digested with 3 mg/mL collagenase type I (Gibco-Life Technologies, Grand Island, NY, USA) and 4 mg/mL dispase (Gibco-Life Technologies, Grand Island, NY, USA) for 1 h at 37 °C. Then, the cells and remaining tissues were cultured in a growth medium, containing α-minimum essential medium (α-MEM; Gibco-Thermo Fisher Scientific, Beijing, China), 10% fetal bovine serum (FBS; Gibco, South America), 100 U/mL penicillin, and 100 μg/mL streptomycin (Vicmed, Xuzhou, China). The medium was changed every 3 days, and subculture was performed when cells reached 70–80% confluence. To maintain stable cell behavior and avoid cell senescence, cells of passages 2–5 were used in this study.
Multiple differentiation potential of hPDLSCs was tested. For osteogenic differentiation, cells were induced in α-MEM, 10% FBS, 10 mmol/L β-glycerophosphate, 50 μg/mL l-ascorbic acid phosphate, and 10 nmol/L dexamethasone; and Alizarin Red S staining was performed 4 weeks later. For adipogenic differentiation, cells were cultured in α-MEM containing 10% FBS, 1 μmol/L dexamethasone, 1 μg/mL insulin, and 0.5 mmol/L 3-isobutyl-1-methylxanthine for 4 weeks; and then, Red Oil O staining was performed. For neurogenic differentiation, cells were induced in Neurobasal A medium (Gibco-Thermo Fisher Scientific, Grand Island, NY, USA) supplemented with 40 ng/mL basic fibroblast growth factor (bFGF; PeproTech, Rocky Hill, NJ, USA) and 20 ng/mL epidermal growth factor (EGF; PeproTech, Rocky Hill, NJ, USA), and β III-tubulin expression was analyzed 4 weeks later.
Expression of mesenchymal stem cell markers was measured by flow cytometry. Briefly, single-cell suspensions (2–5 × 105 cells/tube) were incubated with fluorescent-conjugated anti-human antibodies or their respective isotype controls for 45 min. The following antibodies were used as follows: CD90 PerCP, CD73 FITC, CD45 APC, CD105 APC (BD Biosciences, San Jose, CA, USA), and STRO-1 PE (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Analysis was performed on a flow cytometer (FACS Canto II, BD Biosciences, San Jose, CA, USA).
In vitro osteogenic assay
To explore the effect of LPS on osteogenic differentiation of hPDLSCs, they were seeded in 24-well plates (1 × 104/well) or 6-well plates (5 × 104/well) and cultured in an osteogenic medium supplemented with LPS (O55:B5, Sigma-Aldrich) at concentrations of 0, 0.1, 1, and 10 μg/mL. The medium was replaced every 3 days. On days 14 and 18, calcium nodules were assessed by Alizarin Red S staining. On day 10, alkaline phosphatase (ALP) staining and ALP activity assay were performed. On day 14, osteogenic markers were tested by quantitative polymerase chain reaction (qPCR). To explore the role of ephrinB2 in osteogenic differentiation of hPDLSCs, 0.5 or 1 μg/mL ephrinB2-Fc (R&D Systems, Wiesbaden, Germany) was added into the osteogenic medium. IgG-Fc (1 μg/mL) (R&D Systems, Wiesbaden, Germany) was used as the negative control. Alizarin Red S staining and qPCR were performed to assess the osteogenic differentiation ability.
Alizarin Red S staining and ALP staining
For Alizarin Red S staining, cells were fixed with 4% paraformaldehyde and stained with 2% Alizarin Red S solution (pH 4.2) for 30 min. Staining intensity was quantified with Image J software (Rawak Software, Germany). For ALP staining, cells were fixed with 4% paraformaldehyde and stained with nitro-blue tetrazolium/5-bromo-4-chloro-3′-indolylphosphate (NBT/BCIP) substrate solution (Beyotime, Shanghai, China) for 1 h. Additionally, ALP activity in whole-cell lysates was quantified by the Alkaline Phosphatase Detection Kit (Jiancheng Bioengineering, Nanjing, China) following the manufacturer’s protocol.
Anti-TLR4 antibody or resatorvid treatment
The role of TLR4 in LPS-induced decrease in ephrinB2 expression was investigated by using anti-TLR4 antibody (0.5 μg/mL; Proteintech, Wuhan, Hubei, China) or resatorvid (1 μM, MedChemExpress, Shanghai, China) to block or antagonize TLR4. hPDLSCs were seeded in 6-well plates at 2 × 105/well and cultured in an osteogenic medium with or without 0.1 μg/mL LPS. For the anti-TLR4 antibody group, cells were pretreated with 0.5 μg/mL anti-TLR4 antibody for 4 h and subjected to an osteogenic medium containing 0.1 μg/mL LPS and 0.5 μg/mL anti-TLR4 antibody. For the resatorvid group, 1 μM resatorvid was added at the time of changing to osteogenic medium containing LPS. Western blotting analysis of ephrinB2 level was performed 3 days later.
Cell transfection
EfnB2 lentiviral particles (LPP-M0409-Lv233-400) and corresponding EGFP lentiviral particles (LPP-EGFP-Lv233-100) were purchased from GeneCopoeia (Rockville, Maryland, USA). hPDLSCs were seeded in six-well plates (2.5 × 105/well) and expanded in a growth medium until they reached 70% confluence. Then, the medium was changed to α-MEM containing 5% FBS, 4 μg/mL polybrene (Vicmed, Xuzhou, China), and 40 μL ephrinB2 lentiviral particles for 12 h. hPDLSCs infected with corresponding control lentiviral particles were treated as control. After infection with lentivirus, successfully transduced hPDLSCs were selected by 1.5 μg/mL puromycin (Vicmed, Xuzhou, China). qPCR and Western blotting analysis were performed to confirm ephrinB2 overexpression in ephrinB2-transfected hPDLSCs.
qPCR
Total RNA was extracted with TRIzol Reagent (Invitrogen, CA, USA), and it was purified and reverse transcribed to cDNA using HiScript Q RT SuperMix for qPCR (Vazyme, Nanjing, China) according to the manufacturer’s instructions. qPCR analysis was performed with UltraSYBR Mixture (Cwbio, Beijing, China) using an ABI7500 sequence detection system (Applied Biosystems, Darmstadt, Germany). The primers used in this study are listed as follows: ALP: forward, 5′-CCTCGTTGACACCTGGAAGAG-3′, and reverse, 5′-TTCCGTGCGGTTCCAGA-3′; osteocalcin (OCN): forward, 5′-CTACCTGTATCAATGGCTGGG-3′, and reverse, 5′-GGATTGAGCTCACACACCT-3′; runt-related transcription factor 2 (RUNX2): forward, 5′-TCTTAGAACAAATTCTGCCCTTT-3′, and reverse, 5′-TGCTTTGGTCTTGAAATCACA-3′; bone morphogenetic protein 2 (BMP2): forward, 5′-TTCCACCATGAAGAATCTTTGGA-3′, and reverse, 5′-CCTGAAGCTCTGCTGAGGTGAT-3′; ephrinB2: forward, 5′-TATGCAGAACTGCGATTTCCAA-3′, and reverse, 5′-TGGGTATAGTACCAGTCCTTGTC-3′; β-actin: forward, 5′-ACGTTGCTATCCAGGCTGTG-3′, and reverse, 5′-GGCCATCTCTTGCTCGAAGT-3′.
Western blotting analysis
Total proteins were extracted with RIPA buffer (Beyotime, Shanghai, China) and 1 mM PMSF (Beyotime, Shanghai, China). Protein concentration was determined using the BCA Protein Assay Kit (Beyotime, Shanghai, China). Forty micrograms of protein per sample was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to nitrocellulose membranes (Pall Corporation, Pensacola, Florida, USA), blocked with 5% skim milk (Vicmed, Xuzhou, Jiangsu, China), and incubated with primary antibodies for ephrinB2 (1:2000, Abcam, Cambridge, UK), phospho-ephrinB2 (Tyr324/329, 1:500, Cell Signaling Technology, Danvers, MA, USA), EphB4 (1:200; Santa Cruz Biotechnology, Dallas, TX, USA), phospho-EphB4 (1:1000; Signalway Antibody, College Park, MD, USA), or β-actin (1:3000, Beyotime, Shanghai, China) overnight at 4 °C. Then, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Proteintech, Wuhan, Hubei, China) for 2 h at room temperature. Protein bands were visualized by the chemiluminescence kit (NCM Biotech, Suzhou, Jiangsu, China) and Tanon 4500 Immunodetection System (Tanon, Shanghai, China). The relative band intensity was analyzed with ImageJ software (Rawak Software, Germany).
Statistical analysis
All experiments were repeated three times. Data were presented as mean ± SD. SPSS 19.0 software (IBM Corp, Armonk, NY, USA) was used to perform statistical analysis. One-way ANOVA followed by Bonferroni’s post hoc test was used to determine significant differences among more than two groups. Two-tailed Student’s t test was used to determine significant differences between two groups. The difference was considered significant when p was less than 0.05.
Results
Characterization of hPDLSCs
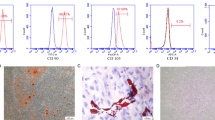
Mineralized nodules, lipid droplets, and β III-tubulin expression were observed in hPDLSCs after osteogenic, adipogenic, and neurogenic induction, respectively. Flow cytometry confirmed that hPDLSCs were positive for CD73, CD90, and CD105 and negative for CD45. Also, 4.49% of hPDLSCs were positive for STRO-1 (Fig. 1).
a Osteogenic, adipogenic, and neurogenic differentiation of hPDLSCs detected by Alizarin Red S staining, Red Oil O staining, and β III-tubulin expression, respectively. Left scale bar = 200 μm, middle scale bar = 20 μm, right scale bar = 50 μm. b Mesenchymal stem cell marker expression detected by flow cytometry
LPS-impaired osteogenic capacity of hPDLSCs
To assess the influence of LPS on osteogenic differentiation of hPDLSCs, they were treated with LPS at different concentrations (0–10 μg/mL) under osteogenic induction. ALP and Alizarin Red S staining revealed that LPS treatment significantly downregulated ALP expression on day 10 and inhibited calcium deposit formation on days 14 and 18. Furthermore, 0.1 μg/mL LPS inhibited mRNA expressions of ALP, OCN, RUNX2, and BMP2 on day 14. LPS at 0.1 μg/mL also inhibited RUNX2 protein expression, as indicated by Western blotting analysis. To rule out the possibility that LPS impaired the mineralization level via attenuating cell proliferation, proliferation of hPDLSCs under different concentrations of LPS was measured, and no significant difference was found (Fig. 2).
a, b hPDLSCs under osteogenic induction were treated with different concentrations of LPS. Then, ALP staining, Alizarin Red S staining, and mRNA of ALP, OCN, RUNX2, and BMP2 were detected. c RUNX2 protein expression in hPDLSCs treated with or without 0.1 μg/mL LPS was measured on days 1, 3, 5, 7, and 10 of osteogenic induction. d Proliferation of hPDLSCs treated with different concentrations of LPS. Data are shown as mean ± SD. *p < 0.05 (n = 3); **p < 0.01 (n = 3)
LPS downregulated ephrinB2 expression in hPDLSCs during osteogenic differentiation
EphrinB2 plays a vital role in bone formation and homeostasis. To determine whether LPS treatment influenced ephrinB2 expression in hPDLSCs during osteogenic differentiation, ephrinB2 mRNA level in osteogenic hPDLSCs treated with different concentrations of LPS was measured. It was noted that 0.1 μg/mL LPS significantly downregulated ephrinB2 mRNA level on days 3, 7, and 14 of osteogenic induction. Western blotting analysis revealed that ephrinB2 protein expression was inhibited by 0.1 μg/mL LPS on days 3 and 5 of osteogenic induction, which was in accordance with ephrinB2 mRNA expression. Phosphorylated ephrinB2 was also downregulated by LPS treatment. However, EphB4 and its phosphorylated form were not affected by LPS (Fig. 3).
a EphrinB2 mRNA expression in hPDLSCs treated with different concentrations of LPS. Data are shown as mean ± SD. **p < 0.01 (n = 3) vs. 0 μg/mL LPS group. b–f EphrinB2, p-ephrinB2, EphB4, and p-EphB4 protein expression in hPDLSCs treated with 0.1 μg/mL LPS. Data are shown as mean ± SD. *p < 0.05 (n = 3). g, h Western blotting analysis of the effect of blocking TLR4 on LPS-induced decrease in ephrinB2 expression. Data are shown as mean ± SD. *p < 0.05 (n = 3); **p < 0.01 (n = 3)
TLR4 is the main receptor that recognizes LPS, and the role of LPS and TLR4 in osteogenic differentiation of BMSCs and PDLSCs has been reported. To ascertain the involvement of TLR4 in LPS attenuating ephrinB2 expression in hPDLSCs, anti-TLR4 antibody and TLR4 inhibitor (resatorvid) were used. Western blotting analysis showed that LPS-induced downregulation of ephrinB2 was partially reversed by anti-TLR4 antibody or resatorvid treatment (Fig. 3).
EphrinB2-Fc stimulated osteogenic differentiation of hPDLSCs
To explore the effect of ephrinB2 on osteogenic differentiation of hPDLSCs, recombinant ephrinB2-Fc was added to the osteogenic induction medium. Alizarin Red S staining showed more mineralized nodule formation in ephrinB2-Fc treatment groups, when compared with no ephrinB2-Fc treatment group. ALP transcription increased after 0.5 and 1 μg/mL ephrinB2-Fc treatment on day 7, and OCN and BMP2 transcription increased after 0.5 μg/mL ephrinB2-Fc treatment on day 7 (Fig. 4).
EphrinB2 overexpression partially reversed LPS-impaired osteogenic potential of hPDLSCs
To further determine whether ephrinB2 plays a role in the impaired osteogenic potential of hPDLSCs induced by LPS, hPDLSCs were transfected with ephrinB2 lentivirus. Successful transfection was demonstrated by green fluorescence expression and ephrinB2 mRNA and protein overexpression. qPCR, ALP, and Alizarin Red S staining revealed that ephrinB2-overexpressing hPDLSCs treated with LPS expressed higher ALP and BMP2 mRNA levels and higher ALP activity and showed more calcium nodule deposition, when compared with wide-type hPDLSCs treated with LPS (Fig. 5). These results indicated that ephrinB2 overexpression partially reversed LPS-impaired osteogenic capacity of hPDLSCs.
a Green fluorescence was observed in lentivirus-infected hPDLSCs. Scale bar = 200 μm. b, c EphrinB2 mRNA and protein were overexpressed in ephrinB2-transfected hPDLSCs. d–f After ephrinB2 was overexpressed, mRNA of ALP, OCN, RUNX2, and BMP2, ALP staining, and Alizarin Red S staining of osteogenic hPDLSCs treated with 0.1 μg/mL LPS were assessed. Data are shown as mean ± SD. *p < 0.05 (n = 3); **p < 0.01 (n = 3)
Discussion
PDLSCs maintain the homeostasis of the periodontium structure and physiological functions. At the same time, these cells have been shown to be promising sources of stem cells for periodontal tissue regeneration [7, 34]. It has been reported that the osteogenic differentiation property of PDLSCs is attenuated under an inflammatory microenvironment [16]. LPS, a major pro-inflammatory factor in periodontitis, exerts an inhibitory effect on osteogenic differentiation of PDLSCs [19, 21, 22]. Therefore, it is crucial to investigate the underlying mechanism of LPS affecting osteogenic potential of PDLSCs for the treatment of periodontitis. In this study, we found that LPS inhibited osteogenic differentiation of PDLSCs partially through downregulation of ephrinB2 via LPS/TLR4 signaling.
The initiation of periodontitis and the subsequent events of destruction of the alveolar bone and periodontal connective tissue by bacteria and LPS are well documented. Microbial components, especially LPS, result in secretion and accumulation of pro-inflammatory cytokines, including IL-1, TNF-α, prostaglandin E2 (PGE2), and interleukin-6 (IL-6) in periodontal tissues, which induce synthesis of excess receptor activator of nuclear factor-κB ligand (RANKL) and alter the ratio of RANKL/osteoprotegerin (OPG). RANKL is the key osteoclast differentiation factor, leading to osteoclast mature and bone resorption, while OPG is a soluble receptor of RANKL that counterbalances the function of RANKL. The excess of RANKL induced by LPS disrupts bone homeostasis, contributing to eventual bone loss and periodontal tissue breakdown [6, 35,36,37]. However, the exact effect of LPS on osteogenic differentiation of PDLSCs remains inconclusive. In the present study, we found that different concentrations of LPS (0.1, 1, and 10 μg/mL) impaired osteogenic differentiation of PDLSCs, and 0.1 μg/mL LPS had the most significant inhibitory effect, as demonstrated by decrease in both mineralized nodule formation and osteogenic marker expression. In addition, LPS had no influence on proliferation of PDLSCs, which was consistent with previous findings [21]. As many previous studies did [20, 23, 38], we also used Escherichia coli (E. coli) LPS in this study, because of its representativeness, easy accessibility and similar effect on osteogenic differentiation of PDLSCs with Porphyromonas gingivalis (P.g.) LPS [22, 23], the main pathological factor for periodontitis. Even though, we cannot exclude the possibility that our research findings could be different if P.g. LPS would be used. So further studies focused on P.g. LPS is needed.
EphrinB2 is considered a key molecule in regulating bone homeostasis in both normal conditions [25] and diverse pathologic conditions, such as hyperglycemia-induced bone deterioration [39], vitamin D intoxication [40], and cancer-induced bone disease [41]. Additionally, mechanical force [31, 32] and a range of hormones [26, 42] could regulate ephrinB2 expression, thereby influencing bone homeostasis. However, it has not been investigated whether ephrinB2 is involved in LPS-induced inhibition of osteogenic differentiation of PDLSCs. First, we tested ephrinB2 expression in osteogenic PDLSCs after LPS treatment. Our study revealed that different concentrations of LPS attenuated mRNA expression of ephrinB2, and 0.1 μg/mL LPS most significantly inhibited ephrinB2 mRNA expression. Downregulation of ephrinB2 and p-ephrinB2 protein by 0.1 μg/mL LPS was also observed. Similarly, it has been reported that P.g. LPS inhibited the expression of ephrinB2 in an osteoblast-osteoclast co-culture system [43]. EphB4 is a receptor for ephrinB2, and ephrinB2-EphB4 interaction in osteoblasts specifically stimulated their differentiation [25, 32]. Thus, expressions of EphB4 and p-EphB4 after LPS treatment were also tested. However, it was found that LPS had no influence on EphB4 and p-EphB4 expressions, which suggested that EphB4 was not receptor for ephrinB2 in LPS-impaired osteogenic differentiation of hPDLSCs. Besides EphB4, EphB1 and EphB2 have also been reported as likely candidate receptors for ephrinB2 in bone formation [30], and they may be the possible receptor for ephrinB2 in this process, which need further research.
Toll-like receptors (TLRs) are essential for the recognition of microbial pathogenic molecules, such as LPS, flagellin, and nucleic acids [44]. PDLSCs express a variety of TLRs [20], and among them, TLR4 was shown to recognize LPS, stimulate the production of cytokines and other pro-inflammatory mediators, and mediate LPS-induced attenuation of osteogenic differentiation [20, 45]. To investigate whether TLR4 is the key receptor for LPS-induced downregulation of ephrinB2 expression, anti-TLR4 antibody or resatorvid, a specific inhibitor of TLR4, was applied. Blockage of TLR4 partially reversed the LPS-induced ephrinB2 downregulation in PDLSCs, which suggested that TLR4 partially mediated LPS-induced ephrinB2 downregulation. Similarly, TNF-α, a pro-inflammatory factor produced by TLR4 activation, had an inhibitory effect on EphB4 expression in osteoblasts [46]. The expression of ephrin ligand in osteoclast precursors or periodontal ligament fibroblasts could be regulated by NF-κB signaling [47] or ERK1/2 MAPK signaling [48], respectively. At the same time, triggering of the TLR4 pathway leads to activation of NF-κB, IRF-3, and MAPK pathways and subsequent regulation of immune and inflammatory genes [49]. Thus, activation of the TLR4 receptor might decrease ephrinB2 expression via downstream NF-κB or MAPK pathways, which need further research.
Previous studies demonstrated that ephrinB2-Fc could enhance the osteogenesis of osteoblasts [25, 30], BMSCs [28, 29], and dental pulp stem cells (DPSCs) [50]. EphrinB2 overexpression promoted calcium deposition and osteogenic gene expression in PDLSCs [33]. Similarly, our results also revealed that ephrinB2-Fc treatment promoted calcium nodule formation and increased ALP, OCN, and BMP2 transcription in PDLSCs. Strikingly, ephrinB2 upregulation in PDLSCs partially reversed the impaired osteogenic differentiation of PDLSCs induced by LPS. These results suggested that ephrinB2 partially mediated the inhibitory effect of LPS on osteogenic differentiation of PDLSCs.
In conclusion, LPS inhibited osteogenic potential of hPDLSCs partially via downregulation of ephrinB2 expression. EphrinB2 upregulation or ephrinB2-Fc treatment could partially reverse the impaired osteogenic differentiation of PDLSCs induced by LPS.
References
Kinane DF (2001) Causation and pathogenesis of periodontal disease. Periodontol 2000(25):8–20
Feng Z (2000) Weinberg a (2006) role of bacteria in health and disease of periodontal tissues. Periodontol 40:50–76
Jones KJ, Ekhlassi S, Montufar-Solis D, Klein JR, Schaefer JS (2010) Differential cytokine patterns in mouse macrophages and gingival fibroblasts after stimulation with porphyromonas gingivalis or Escherichia coli lipopolysaccharide. J Periodontol 81:1850–1857
Morandini AC, Sipert CR, Gasparoto TH, Greghi SL, Passanezi E, Rezende ML, Sant’ana AP, Campanelli AP, Garlet GP, Santos CF (2010) Differential production of macrophage inflammatory protein-1alpha, stromal-derived factor-1, and IL-6 by human cultured periodontal ligament and gingival fibroblasts challenged with lipopolysaccharide from P. gingivalis. J Periodontol 81:310–317
Beutler B, Rietschel ET (2003) Innate immune sensing and its roots: the story of endotoxin. Nat Rev Immunol 3:169–176
Darveau RP (2010) Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 8:481–490
Zhu W, Liang M (2015) Periodontal ligament stem cells: current status, concerns, and future prospects. Stem Cells Int 2015:972313
Liu YC, Lerner UH, Teng YT (2010) Cytokine responses against periodontal infection: protective and destructive roles. Periodontol 2000 52:163–206
Wada N, Maeda H, Tanabe K, Tsuda E, Yano K, Nakamuta H, Akamine A (2001) Periodontal ligament cells secrete the factor that inhibits osteoclastic differentiation and function: the factor is osteoprotegerin/osteoclastogenesis inhibitory factor. J Periodontal Res 36:56–63
Liu Y, Zheng Y, Ding G, Fang D, Zhang C, Bartold PM, Gronthos S, Shi S, Wang S (2008) Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 26:1065–1073
Kunze M, Huber A, Krajewski A, Lowden E, Schuhmann N, Buening H, Hallek M, Noack M, Perabo L (2009) Efficient gene transfer to periodontal ligament cells and human gingival fibroblasts by adeno-associated virus vectors. J Dent 37:502–508
Huang GT, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88:792–806
Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, Young M, Robey PG, Wang CY, Shi S (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364:149–155
Han J, Menicanin D, Marino V, Ge S, Mrozik K, Gronthos S, Bartold PM (2014) Assessment of the regenerative potential of allogeneic periodontal ligament stem cells in a rodent periodontal defect model. J Periodontal Res 49:333–345
Yu N, Oortgiesen DA, Bronckers AL, Yang F, Walboomers XF, Jansen JA (2013) Enhanced periodontal tissue regeneration by periodontal cell implantation. J Clin Periodontol 40:698–706
Liu Y, Liu W, Hu C, Xue Z, Wang G, Ding B, Luo H, Tang L, Kong X, Chen X, Liu N, Ding Y, Jin Y (2011) MiR-17 modulates osteogenic differentiation through a coherent feed-forward loop in mesenchymal stem cells isolated from periodontal ligaments of patients with periodontitis. Stem Cells 29:1804–1816
Yang N, Li Y, Wang G, Ding Y, Jin Y, Xu Y (2017) Tumor necrosis factor-alpha suppresses adipogenic and osteogenic differentiation of human periodontal ligament stem cell by inhibiting miR-21/Spry1 functional axis. Differentiation 97:33–43
Mao CY, Wang YG, Zhang X, Zheng XY, Tang TT, Lu EY (2016) Double-edged-sword effect of IL-1beta on the osteogenesis of periodontal ligament stem cells via crosstalk between the NF-kappaB, MAPK and BMP/Smad signaling pathways. Cell Death Dis 7:e2296
Liu H, Zheng J, Zheng T, Wang P (2019) Exendin-4 regulates Wnt and NF-kappaB signaling in lipopolysaccharide-induced human periodontal ligament stem cells to promote osteogenic differentiation. Int Immunopharmacol 75:105801
Li C, Li B, Dong Z, Gao L, He X, Liao L, Hu C, Wang Q, Jin Y (2014) Lipopolysaccharide differentially affects the osteogenic differentiation of periodontal ligament stem cells and bone marrow mesenchymal stem cells through toll-like receptor 4 mediated nuclear factor kappaB pathway. Stem Cell Res Ther 5:67
Kukolj T, Trivanović D, Djordjević IO, Mojsilović S, Krstić J, Obradović H, Janković S, Santibanez JF, Jauković A, Bugarski D (2018) Lipopolysaccharide can modify differentiation and immunomodulatory potential of periodontal ligament stem cells via ERK1,2 signaling. J Cell Physiol 233:447–462
Kato H, Taguchi Y, Tominaga K, Umeda M, Tanaka A (2014) Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch Oral Biol 59:167–175
Albiero ML, Amorim BR, Martins L, Casati MZ, Sallum EA, Nociti FH Jr, Silvério KG (2015) Exposure of periodontal ligament progenitor cells to lipopolysaccharide from Escherichia coli changes osteoblast differentiation pattern. J Appl Oral Sci 23:145–152
Albiero ML, Amorim BR, Casati MZ, Sallum EA, Nociti FHJ, Silverio KG (2017) Osteogenic potential of periodontal ligament stem cells are unaffected after exposure to lipopolysaccharides. Braz Oral Res 31:e17
Zhao C, Irie N, Takada Y, Shimoda K, Miyamoto T, Nishiwaki T, Suda T, Matsuo K (2006) Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab 4:111–121
Allan EH, Häusler KD, Wei T, Gooi JH, Quinn JM, Crimeen-Irwin B, Pompolo S, Sims NA, Gillespie MT, Onyia JE, Martin TJ. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res 23:1170–1181
Martin TJ, Allan EH, Ho PW, Gooi JH, Quinn JM, Gillespie MT, Krasnoperov V, Sims NA (2010) Communication between ephrinB2 and EphB4 within the osteoblast lineage. Adv Exp Med Biol 658:51–60
Toda H, Yamamoto M, Uyama H, Tabata Y (2017) Effect of hydrogel elasticity and ephrinB2-immobilized manner on Runx2 expression of human mesenchymal stem cells. Acta Biomater 58:312–322
Zhang F, Zhang Z, Sun D, Dong S, Xu J, Dai F (2015) EphB4 promotes osteogenesis of CTLA4-modified bone marrow-derived mesenchymal stem cells through cross talk with Wnt pathway in xenotransplantation. Tissue Eng Part A 21:2404–2416
Benson MD, Opperman LA, Westerlund J, Fernandez CR, San Miguel S, Henkemeyer M, Chenaux G (2012) Ephrin-B stimulation of calvarial bone formation. Dev Dyn 241:1901–1910
Hou J, Chen Y, Meng X, Shi C, Li C, Sun H (2014) Compressive force regulates ephrinB2 and EphB4 in osteoblasts and osteoclasts contributing to alveolar bone resorption during experimental tooth movement. Korean J Orthod 44:320–329
Diercke K, Kohl A, Lux CJ, Erber R (2011) Strain-dependent up-regulation of ephrin-B2 protein in periodontal ligament fibroblasts contributes to osteogenesis during tooth movement. J Biol Chem 286:37651–37664
Zhu SY, Wang PL, Liao CS, Yang YQ, Yuan CY, Wang S, Dissanayaka WL, Heng BC, Zhang CF (2017) Transgenic expression of ephrinB2 in periodontal ligament stem cells (PDLSCs) modulates osteogenic differentiation via signaling crosstalk between ephrinB2 and EphB4 in PDLSCs and between PDLSCs and pre-osteoblasts within co-culture. J Periodontal Res 52:562–573
Bassir SH, Wisitrasameewong W, Raanan J, Ghaffarigarakani S, Chung J, Freire M, Andrada LC, Intini G (2016) Potential for stem cell-based periodontal therapy. J Cell Physiol 231:50–61
Wilson M (1995) Biological activities of lipopolysaccharides from oral bacteria and their relevance to the pathogenesis of chronic periodontitis. Sci Prog 78:19–34
Hienz SAPS, Ivanovski S (2015) Mechanisms of bone resorption in periodontitis. J Immunol Res 2015:615486
Page RC (1991) The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontal Res 26:230–242
He W, Wang Z, Luo Z, Yu Q, Jiang Y, Zhang Y, Zhou Z, Smith AJ, Cooper PR (2015) LPS promote the odontoblastic differentiation of human dental pulp stem cells via MAPK, but not NF-KB signaling pathway. J Cell Physiol 230:554–561
Wu M, Ai W, Chen L, Zhao S, Liu E (2016) Bradykinin receptors and EphB2/EphrinB2 pathway in response to high glucose-induced osteoblast dysfunction and hyperglycemia-induced bone deterioration in mice. Int J Mol Med 37:565–574
Sun J, Sun B, Wang W, Han X, Liu H, Du J, Feng W, Liu B, Amizuka N, Li M (2016) Histochemical examination of the effects of high-dose 1,25(OH)2D3 on bone remodeling in young growing rats. J Mol Histol 47:389–399
Pennisi A, Ling W, Li X, Khan S, Shaughnessy JD Jr, Barlogie B, Yaccoby S (2009) The ephrinB2/EphB4 axis is dysregulated in osteoprogenitors from myeloma patients and its activation affects myeloma bone disease and tumor growth. Blood 114:1803–1812
Li C, Shi C, Kim J, Chen Y, Ni S, Jiang L, Zheng C, Li D, Hou J, Taichman RS, Sun H (2015) Erythropoietin promotes bone formation through EphrinB2/EphB4 signaling. J Dent Res 94:455–463
Zhang Y, Wang XC, Bao XF, Hu M, Yu WX (2014) Effects of Porphyromonas gingivalis lipopolysaccharide on osteoblast-osteoclast bidirectional EphB4-EphrinB2 signaling. Exp Ther Med 7:80–84
Beutler B (2004) Inferences, questions and possibilities in toll-like receptor signaling. Nature 430:257–263
He W, Wang Z, Luo Z, Yu Q, Jiang Y, Zhang Y, Zhou Z, Smith AJ, Cooper PR.LPS (2015) Promote the odontoblastic differentiation of human dental pulp stem cells via MAPK signaling pathway. J Cell Physiol 230:554–561
Wang LM, Zhang J, Sun QF, Yang CZ, Yang PS (2018) Tumor necrosis factor-alpha inhibits osteogenic differentiation of pre-osteoblasts by downregulation of EphB4 signaling via activated nuclear factor-kappaB signaling pathway. J Periodontal Res 53:66–72
Irie N, Takada Y, Watanabe Y, Matsuzaki Y, Naruse C, Asano M, Iwakura Y, Suda T, Matsuo K (2009) Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem 284:14637–14644
Sen S, Zingler S, Lux CJ, Erber R (2015) Compression induces Ephrin-A2 in PDL fibroblasts via c-fos. J Dent Res 94:464–472
Horng T, Medzhitov R (2001) TIRAP: an adapter molecule in the toll signaling pathway. Nat Immunol 2:835–841
Heng BC, Wang S, Gong T, Xu J, Yuan C, Zhang C (2018) EphrinB2 signaling enhances osteogenic/odontogenic differentiation of human dental pulp stem cells. Arch Oral Biol 87:62–71
Funding
This work was supported by the National Nature Science Foundation of China (NSFC) (No. 81700954).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All experiments were performed after receiving approval from the Ethics Committee of Xuzhou Medical University (20161108).
Informed consent
hPDLSCs were isolated from 10 healthy adults with their consent.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, W., Yuan, C., Geng, T. et al. Lipopolysaccharide inhibits osteogenic differentiation of periodontal ligament stem cells partially through toll-like receptor 4-mediated ephrinB2 downregulation. Clin Oral Invest 24, 3407–3416 (2020). https://doi.org/10.1007/s00784-020-03211-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-020-03211-w