Abstract
Members of the ephrin and Eph family are local mediators of cell function through largely contact-dependent processes in development and in maturity. Production of ephrinB2 mRNA and protein are increased by PTH and PTHrP in osteoblasts. Both a synthetic peptide antagonist of ephrinB2/EphB4 receptor interaction and recombinant soluble extracellular domain of EphB4 (sEphB4), which is an antagonist of both forward and reverse EphB4 signaling, were able to inhibit mineralization and the expression of several osteoblast genes involved late in osteoblast differentiation. The findings are consistent with ephrinB2/EphB4 signaling within the osteoblast lineage having a paracrine role in osteoblast differentiation, in addition to the proposed role of osteoclast-derived ephrinB2 in coupling of bone formation to resorption. This local regulation might contribute to control of osteoblast differentiation and bone formation at remodeling sites, and perhaps also in modeling.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction – Bone Remodeling
The maintenance of adequate trabecular and cortical bone requires that bone formation and resorption should be balanced, such that a high or low level of resorption is usually associated with a similar change in the level of bone formation. Bone resorption and formation take place asynchronously throughout the skeleton in both trabecular and cortical bone, at many sites known as bone metabolic units (BMUs). The theory that resorption is followed by an equal amount of formation in the BMU came to be known as “coupling.” There are a number of situations in which this equal balance does not hold. During aging there is a negative balance at individual BMUs [14] with gradual diminution in the amount of bone, whereas during growth it is proposed that there is a positive balance, with the amount of bone replaced at individual BMUs exceeding that which was lost [23]. In the states of post-menopausal osteoporosis or of ovariectomy in animal models, coupling is perturbed to the extent that the bone resorbed within each BMU is inadequately replaced by formation; the net result being bone loss. The tightly regulated processes of bone formation and resorption are essential in bone remodeling, for the achievement and maintenance of skeletal strength and form. Circulating hormones are important controlling factors, but the key influences are locally generated cytokines, which influence bone cell function and communication in complex ways, and often are themselves regulated in turn by the hormones.
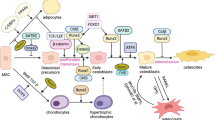
Bone remodeling is essential in order to repair microdamage to bone and to respond to pressure changes [22]. The bone-remodeling sequence begins with signals generated from cells in the osteoblast lineage, osteocytes and bone lining cells, aimed at generating active osteoclasts to resorb old or damaged bone [2, 20]. This is followed by the reversal phase on completion of bone resorption, with the death and departure of multinucleate osteoclasts, when the resorbed surface is cleared by mononuclear cells, probably both macrophage and mesenchymal in origin [6]. In the bone-formation phase, the resorbed bone is replaced by the actions of osteoprogenitor cells, which differentiate into osteoblasts. Uncalcified matrix (osteoid) is deposited for subsequent mineralization. The lacuna is gradually filled with new bone. These processes clearly require controlled production of molecules by the participating cells and their communication with neighbours.
2 Communication from the Osteoblast to the Hemopoietic Lineage
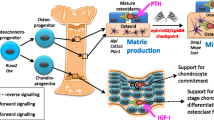
The crucial local factors that control osteoclast formation were discovered in the late 1990s. Osteoblasts express a membrane protein, Receptor Activator of NF-κB ligand (RANKL) regulated by osteotropic hormones, including parathyroid hormone and calcitriol, as well as cytokines such as interleukin-6 [27]. RANKL plays an essential role in osteoclast differentiation, activation, and survival. Proximity between osteoblastic lineage and hemopoietic cells is required for RANKL to bind to its respective receptor (RANK), which is expressed by monocyte/macrophage lineage cells, thereby stimulating some of these to form osteoclasts. The binding of RANKL to its receptor in mononuclear hematopoietic precursors initiates the processes that ultimately lead to the formation of multinucleate osteoclasts. Osteoprotegerin (OPG) acts as a decoy receptor for RANKL to suppress osteoclast formation. Studies in genetically altered mice have clearly established the essential physiological roles of RANKL and OPG in controlling osteoclast formation and activity, and greatly enhanced our understanding of this stage of the bone-remodeling process – the early recruitment of osteoclast precursors, and the important role played in this by cells of the osteoblast lineage.
3 Communication from Osteoclasts to the Osteoblasts in Order to Contribute to Bone Formation
Much less is known of communication in the reverse direction, even though it has long been recognized that rates of bone resorption are generally matched by those of bone formation. A local “coupling factor” linking bone resorption to subsequent formation was proposed as the key regulator of the remodeling process [17]. The concept developed that coupling might be achieved by the activities of one or more growth factors released from bone matrix during resorption, with most credence given to IGF I and II, and TGFβ [3, 21]. This model of coupling in the BMU by growth factor release from the matrix raises a number of questions that relate to the time course and the distance between the resorption and formation processes and whether activation can be controlled with sufficient precision: (i) which cells produce the growth factors and under what circumstances; (ii) do they stimulate bone formation in vivo; (iii) which can be released from the matrix in active form and in a spatially and temporally controlled manner; (iv) is there evidence for an increase in the abundance of these substances at sites of bone remodeling; and, (v) are there regulated mechanisms by which they are activated?
On the other hand, it is possible that coupling of bone formation might be achieved through activities generated from active osteoclasts. Evidence consistent with this came from Nakamura et al. [19], who used osteoprotegerin (OPG)–/– mice, which are severely osteoporotic because of excessive osteoclast formation, to show that these mice have greatly increased bone formation resulting from a local active factor. They suggested that this factor is more likely derived from cells than released from matrix [27]. Other evidence came from studies in mice, in which each of the two gp130-dependent signaling pathways was specifically attenuated. Inactivation of the SHP2/ras/MAPK signaling pathway (gp130Y757F/Y757F mice) yielded mice with greater osteoclast numbers and bone resorption, as well as greater bone formation than wild type mice. This increased bone remodeling resulted in less bone because the increase in resorption was relatively greater than that in formation. When gp130Y757F/Y757F mice were crossed with IL-6 null mice they had similarly high osteoclast numbers and increased bone resorption; however, these mice showed no corresponding increase in bone formation and thus had extremely low bone mass. This indicated that stimulation of bone formation coupled to the high level of bone resorption in gp130Y757F/Y757F mice is of cellular rather than resorbed matrix origin, and is an IL-6-dependent process, though it does not necessarily show that it is mediated by IL-6 itself [26].
The first specific mechanism proposed as an osteoclast – derived mediator of coupling was ephrinB2, with the finding that osteoclast-derived ephrinB2 acts through a contact-dependent mechanism on EphB4, its receptor in the osteoblast, to promote osteoblast differentiation and bone formation [30]. This was of interest because ephrin/Eph family members have been recognized for some time as local mediators of cell function through largely contact-dependent processes in development and in maturity [5, 10, 24]. They mediate cell attraction and adhesion, but often also provide signals that separate the cells, and have demonstrated roles in tissue remodeling, including cell migration, vascular development, axon guidance and synapse plasticity. Based on the ephrin ligand structure, the ephrins are in two classes, with the ephrin A class glycophosphatidylinositol (GPI)-tethered to the membrane and the B class consisting of type II membrane proteins [10, 24]. Although first considered to bind with class-specificity, i.e. ephrinA to EphA and ephrinB to EphB, there are exceptions, with EphA4 being activated through ephrinB1 [11] and ephrinA5 binding to and signaling through EphB2 [9, 18]. A particular feature of ephrin/Eph biology is their capacity for bi-directional signaling, in that when an ephrin acts upon its Eph receptor tyrosine kinase, the latter can signal in the reverse direction, acting through the ligand by promoting rapid phosphorylation on highly conserved tyrosine residues within the cytoplasmic tail [15].
Transgenic mice constructed to overexpress EphB4 in the osteoblast lineage showed increased bone formation parameters, and treatment of osteoblastic cells in vitro with ephrinB2-Fc fusion protein resulted in increased expression of genes associated with osteoblast differentiation [30]. By transfecting osteoblasts with ephrinB2 mutant forms it was concluded also that the action of ephrinB2 did not require its participation in intracellular signaling in the osteoblasts, but rather that the ephrinB2 extracellular domain stimulated the EphB4 receptors. Of great interest also in this work was the evidence that through reverse signaling, osteoblast-derived EphB4 could act upon ephrinB2 in osteoclasts to suppress osteoclast differentiation by inhibiting the cFos/NFATc1 cascade that is essential for osteoclast differentiation.
The concept of ephrin-Eph involvement in the coupling process is an intriguing one. The fact that these interactions seem to require cell contact between the osteoclast and a differentiating osteoblast makes it likely that it could be just one of many contributory factors to coupling. The findings to be discussed below extend the involvement of ephrinB2 to indicate that it might also be involved in remodeling in ways that determine the amount of bone formed in the BMU.
4 How Do Cells of the Osteoblast Lineage Know How Much Bone to Make in a BMU?
Among the many unanswered questions concerning bone remodeling is why osteoclasts stop resorbing after excavating a certain amount of bone, and either die or move on. So, it would also be intriguing to understand how the osteoblast lineage cells, differentiating to form bone within the BMU, do so virtually precisely to replace the amount of bone that has been lost. An interesting insight into this comes from the work of Boyde and colleagues [7] who showed in vitro that, if they provided rat calvarial cells to bone slices with crevices and grooves excavated on them, the production of bone was limited to the space available. Their findings suggested that the topography of the bone affected the timing, siting and extent of new bone formation, and that in vivo this would take place in the resorbed spaces vacated by osteoclasts. Both the proposed growth factor involvement and the work of Gray et al. imply that once the formation process is established, the participating cells themselves are able to sense spatial limits, and most likely do so by chemical communication within that population of cells. Growth factors and cytokines produced by those cells are candidate mediators, as are gap junction intercellular communicators. Relevant to the latter, deletion of connexin43 has been shown to result in impaired response of bone formation to loading and to treatment with PTH [4]. Regulation from outside the BMU population could be provided by osteocyte-derived sclerostin, which could communicate with those cells to limit bone formation [25, 28].
Another possible regulatory influence arises from our finding that production of ephrinB2 by osteoblasts is substantially enhanced by PTH and PTHrP [1]. In using gene profiling to study the actions of PTH(1-34) and PTHrP(1-141) on differentiating osteoblasts, we found that among the ephrin and Eph molecules represented on the array, ephrinB2 mRNA and protein production was substantially enhanced by treatment with either PTH or PTHrP (Fig. 1). This effect was confirmed in UMR106 rat osteogenic sarcoma cells (Fig. 1) and in mouse calvarial osteoblasts [1]. The response was a rapid one, evident within 1 h, and the increased ephrinB2 protein production was maintained for greater than 24 h (Fig. 1). Furthermore treatment of either 3-week old rats or ovariectomized 6-month old rats with a single subcutaneous injection of PTH resulted in a tenfold increase in ephrinB2 mRNA in metaphyseal bone [1]. Localization of ephrinB2 by immunohistology in young rat bone showed it in clusters of osteoblasts, predominantly in association with mature rather than woven bone [1], consistent with ephrinB2 involvement in remodeling.
Since clustered ephrinB2 treatment of mouse calvarial osteoblasts has been shown to enhance expression of genes associated with osteoblast differentiation, and mice overexpressing EphB4 have increased bone formation parameters, we used inhibitors of ephrinB2-EphB4 receptor interaction in in vitro experiments to determine whether, within a population of osteoblastic cells, they influenced mineralization of differentiating osteoblasts or expression of differentiation-related genes. Two classes of receptor antagonist were used. The first was the peptide, TNYLFSPNGPIARAW, discovered through phage display and shown to be a specific antagonist of ephrinB2 interaction with EphB4 [13]. The second was the recombinant extracellular domain of EphB4 (sEphB4), shown to inhibit both forward and reverse signaling between ephrinB2 and EphB4 [12]. Each of these receptor antagonists was able to inhibit mineralization of the mouse stromal cells, Kusa 4b10, in a dose-dependent manner (Fig. 2A). Furthermore, in Kusa 4b10 cells at a late stage of differentiation, sEphB4 inhibited the expression of mRNA for a number of genes associated with osteoblast differentiation (Fig. 2B), similar to our previous findings with the peptide receptor antagonist, TNYL [1]. Additionally, in mouse calvarial osteoblasts differentiated over 7 days in conditions that result in several hundredfold increase in expression of osteocyte markers, both sEphB4 and TNYL over 24 h decreased expression of mRNA for osteocalcin, sclerostin, DMP-1 and MEPE (Fig. 2C). These findings are consistent with a role for ephrinB2 interaction with EphB4 within the osteoblast lineage, quite distinct from the proposed role of osteoclast-derived ephrinB2. The communication process is illustrated in Fig. 3, depicting ephrin-Eph interaction playing a role in communication within the osteoblast population in the bone formation phase of bone remodeling.
(A) Kusa 4b10 cells under mineralizing conditions treated with increasing concentrations of sEphB4 and mineralization measured at 13 and 14 days; (B) Kusa 4b10 cells, mRNA for several genes after 24-h treatment with sEphB4 (5 μg/ml) or control; (C) mouse calvarial osteoblasts after differentiation for 7 days, and 24-h treatment with either peptide antagonist (TNYL) or sEphB4
With locally produced PTHrP in bone as the likely ligand for the PTH receptor in the osteoblast lineage [16], we propose that production of ephrinB2 in osteoblasts is enhanced by activation of the PTH receptor, probably through the paracrine action of PTHrP. A contact-dependent process operating within a group of maturing osteoblasts in the BMU might present ephrinB2 with easier access to its receptor than relying on osteoblast-osteoclast juxtaposition. Nevertheless, ephrinB2/EphB4 forward and reverse signaling is an attractive means of regulating the volumes of bone resorbed and formed.
These considerations of ephrin-Eph interactions in bone are thus far limited to observations made with bone cells. The abundance of important ephrin signaling processes in the vasculature [29] make it imperative to determine whether that source also applies in the process of bone remodeling. In proposing the model of intercellular communication within the osteoblast lineage that we report here, it will be essential to evaluate the relative importance of forward and reverse signaling. For example, ephrinB2 forward signaling and EphB4 reverse signaling were found to affect cell adhesion and migration differentially between arterial and venous endothelial cells [8]. Although our data in osteoblasts taken together taken with that of Zhao et al., in osteoclast-osteoblast communication might favor actions through ephrinB2 forward signaling, the possibility of significant effects through EphB4 reverse signaling has not been excluded. Resolution of this and related questions will require further in vivo and ex vivo studies in genetically manipulated mice.
5 Summary
During bone remodeling there are contributions from many important pathways of intercellular communication among the osteoblast lineage, osteoclasts and cells of the immune system. Although coupling of formation to resorption in bone remodeling is often ascribed to a hypothetical “coupling factor,” the overall mechanism seems too complex to be explained by any single factor, and it is likely that there are several contributors. One of the important stages in remodeling is the filling of the excavated space in the BMU, where differentiation of osteoblast precursors must be controlled and the amount of bone replaced must be limited. The finding of ephrinB2 regulation within this lineage by a local modulator of bone remodeling, PTHrP, introduces this pathway as another that might influence the process of bone renewal.
References
Allan, E.H., Hausler, K.D., Wei, T., Gooi, J.H., Quinn, J.M., Crimeen-Irwin, B., Pompolo, S., Sims, N.A., Gillespie, M.T, Onyia, J.E., & Martin, T.J. (2008). EphrinB2 Regulation by Parathyroid Hormone (PTH) and PTHrP Revealed by Molecular Profiling in Differentiating Osteoblasts. J Bone Miner Res, 23(8), 1170–1181.
Bonewald, L.F. (2007). Osteocyte messages from a bony tomb. Cell Metab, 5(6), 410–411.
Centrella, M., McCarthy, T.L., & Canalis, E. (1991). Transforming growth factor-beta and remodeling of bone. J Bone Joint Surg Am, 73(9), 1418–1428.
Civitelli, R. (2008). Cell-cell communication in the osteoblast/osteocyte lineage. Arch Biochem Biophys, 473(2), 188–192.
Compagni, A., Logan, M., Klein, R., & Adams, R.H. (2003). Control of skeletal patterning by ephrinB1-EphB interactions. Dev Cell, 5(2), 217–230.
Everts, V., Delaisse, J.M., Korper, W., Jansen, D.C., Tigchelaar-Gutter, W., Saftig, P., & Beertsen, W. (2002). The bone lining cell: its role in cleaning Howship’s lacunae and initiating bone formation. J Bone Miner Res, 17(1), 77–90.
Gray, C., Boyde, A., & Jones, S.J. (1996). Topographically induced bone formation in vitro: implications for bone implants and bone grafts. Bone, 18(2), 115–123.
Hamada, K., Oike, Y., Ito, Y., Maekawa, H., Miyata, K., Shimomura, T., & Suda, T. (2003). Distinct roles of ephrin-B2 forward and EphB4 reverse signaling in endothelial cells. Arterioscler Thromb Vasc Biol, 23(2), 190–197.
Himanen, J.P., Chumley, M.J., Lackmann, M., Li, C., Barton, W.A., Jeffrey, P.D., Vearing, C., Geleick, D., Feldheim, D.A., Boyd, A.W., Henkemeyer, M., & Nikolov, D.B. (2004). Repelling class discrimination: ephrin-A5 binds to and activates EphB2 receptor signaling. Nat Neurosci, 7(5):501–509.
Himanen, J.P., & Nikolov, D.B. (2003). Eph receptors and ephrins. Int J Biochem Cell Biol, 35(2), 130–134.
Holmberg, J., Armulik, A., Senti, K.A., Edoff, K., Spalding, K., Momma, S., Cassidy, R., Flanagan, J.G., & Frisen, J. (2005). Ephrin-A2 reverse signaling negatively regulates neural progenitor proliferation and neurogenesis. Genes Dev, 19(4), 462–471.
Kertesz, N., Krasnoperov, V., Reddy, R., Leshanski, L., Kumar, S.R., Zozulya, S., & Gill, P.S. (2006). The soluble extracellular domain of EphB4 (sEphB4) antagonizes EphB4-EphrinB2 interaction, modulates angiogenesis, and inhibits tumor growth. Blood, 107(6), 2330–2338.
Koolpe, M., Burgess, R., Dail, M., & Pasquale, E.B. (2005). EphB receptor-binding peptides identified by phage display enable design of an antagonist with ephrin-like affinity. J Biol Chem, 280(17), 17301–17311.
Lips, P., Courpron, P., & Meunier, P.J. (1978). Mean wall thickness of trabecular bone packets in the human iliac crest: changes with age. Calcif Tissue Res, 26(1), 13–17.
Lu Q, Sun E.E., Klein R.S., Flanagan J.G. (2001). Ephrin-B reverse signaling is mediated by a novel PDZ-RGS protein and selectively inhibits G protein-coupled chemoattraction. Cell, 105(1), 69–79.
Miao, D., He, B., Jiang, Y., Kobayashi, T., Soroceanu, M.A., Zhao, J., Su, H., Tong, X., Amizuka, N., Gupta, A., Genant, H.K., Kronenberg, H.M., Goltzman, D., & Karaplis, A.C. (2005). Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1-34. J Clin Invest, 115(9), 2402–2411.
Mohan, S., & Baylink, D.J. (1991). Bone growth factors. Clin Orthop Relat Res, 263, 30–48.
Murai, K.K., & Pasquale, E.B. (2003). 'Eph’ective signaling: forward, reverse and crosstalk. J Cell Sci, 116(Pt 14), 2823–2832.
Nakamura, M., Udagawa, N., Matsuura, S., Mogi, M., Nakamura, H., Horiuchi, H., Saito, N., Hiraoka, B.Y., Kobayashi, Y., Takaoka, K., Ozawa, H., Miyazawa, H., & Takahashi, N. (2003). Osteoprotegerin regulates bone formation through a coupling mechanism with bone resorption. Endocrinology, 144(12), 5441–5449.
Noble, B.S., Peet, N., Stevens, H.Y., Brabbs, A., Mosley, J.R., Reilly, G.C., Reeve, J., Skerry, T.M., & Lanyon, L.E. (2003). Mechanical loading: biphasic osteocyte survival and targeting of osteoclasts for bone destruction in rat cortical bone. Am J Physiol Cell Physiol, 284(4), C934–C943.
Oreffo, R.O., Mundy, G.R., Seyedin, S.M., & Bonewald, L.F. (1989). Activation of the bone-derived latent TGF beta complex by isolated osteoclasts. Biochem Biophys Res Commun, 158(3), 817–823.
Parfitt, A.M. (1996). Skeletal heterogeneity and the purposes of bone remodeling: implications for the understanding of osteoporosis. In: Marcus R, Feldman D, Kelsey J (eds.) Osteoporosis. Academic Press, San Diego, CA, pp. 315–339.
Parfitt, A.M. (2002). Targeted and nontargeted bone remodeling: relationship to basic multicellular unit origination and progression. Bone, 30(1), 5–7.
Pasquale, E.B. (2005). Eph receptor signaling [stet] casts a wide net on cell behaviour. Nat Rev Mol Cell Biol, 66, 462–475.
Robling, A.G., Bellido, T., & Turner, C.H. (2006). Mechanical stimulation in vivo reduces osteocyte expression of sclerostin. J Musculoskelet Neuronal Interact, 6(4), 354.
Sims, N.A., Jenkins, B.J., Quinn, J.M., Nakamura, A., Glatt, M., Gillespie, M.T., Ernst, M., & Martin, T.J. (2004). Glycoprotein 130 regulates bone turnover and bone size by distinct downstream signaling pathways. J Clin Invest, 113(3), 379–389.
Suda, T., Takahashi, N., Udagawa, N., Jimi, E., Gillespie, M.T., & Martin, T.J. (1999). Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev, 20(3), 345–357.
van Bezooijen, R.L., ten Dijke, P., Papapoulos, S.E., & Lowik, C.W. (2005). SOST/sclerostin, an osteocyte-derived negative regulator of bone formation. Cytokine Growth Factor Rev, 16(3), 319–327.
Yancopoulos, G.D., Davis, S., Gale, N.W., Rudge, J.S., Wiegand, S.J., & Holash, J. (2000). Vascular-specific growth factors and blood vessel formation. Nature, 407(6801), 242–248.
Zhao, C., Irie, N., Takada, Y., Shimoda, K., Miyamoto, T., Nishiwaki, T., Suda, T., & Matsuo, K. (2006). Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell Metab, 4(2), 111–121.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2009 Springer Science+Business Media, LLC
About this paper
Cite this paper
Martin, T. et al. (2009). Communication Between EphrinB2 and EphB4 Within the Osteoblast Lineage. In: Choi, Y. (eds) Osteoimmunology. Advances in Experimental Medicine and Biology, vol 658. Springer, Boston, MA. https://doi.org/10.1007/978-1-4419-1050-9_6
Download citation
DOI: https://doi.org/10.1007/978-1-4419-1050-9_6
Published:
Publisher Name: Springer, Boston, MA
Print ISBN: 978-1-4419-1049-3
Online ISBN: 978-1-4419-1050-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)