Abstract
Objectives
The aim of this in vitro study was to evaluate the role of highly fluoridated dentifrice on remineralization characteristics of lowly and highly pre-demineralized enamel artificial caries lesions.
Methods
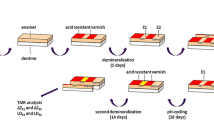
Bovine enamel specimens were prepared (pH 4.95; 21 days) and discriminated in either lowly [L] or highly [H] pre-demineralized artificial caries lesions. Specimens with a mean ΔZbaseline,L (95% CI) of 5120 (4995; 5245) vol.% × μm and a mean ΔZbaseline,H of 8187 (8036; 8339) vol.% × μm were selected and randomly allocated to 12 groups (n = 20). Treatments during pH-cycling (28 days; 6 × 60 min demineralization/day) were brushing 2×/day with fluoride-free (0 ppm F− [L0/H0]), 1100 ppm F− [L1100/H1100], 2800 ppm F− [L2800/H2800], 5000 ppm F− [L5000/H5000], 5000 ppm F− + glycerin [L5000 + glycerin/H5000 + glycerin], and 5000 ppm F− + TCP [L5000 + TCP/H5000 + TCP] containing dentifrices. Dentifrice slurries were prepared with deionized water (1:3wt/wt). After cycling specimens presenting lesion surface loss were discarded and for the remaining 202 specimens, transversal microradiographic (TMR) analyses (ΔZpH-cycle/LDpH-cycle) were performed again. Changes in mineral loss (ΔΔZ = ΔZbaseline − ΔZpH-cycle) and lesion depth (ΔLD = LDbaseline − LDpH-cycle) were calculated.
Results
Significant differences for ΔΔZ could be found between L0, L1100, and L5000 as well as H0, H1100, and H2800/H5000 (p ≤ 0.01; ANCOVA). Except for 0 ppm F−, higher ΔΔZ could be found in highly compared with lowly demineralized specimens (p ≤ 0.004; ANCOVA). After pH-cycling, a second lesion front could only be observed in H5000 and H5000 + TCP. The correlation between ΔΔZ and F− was moderate for lowly and highly demineralized lesions (rL = 0.591; pL < 0.001; rH = 0.746; pH < 0.001), indicating a fluoride dose response for both.
Conclusion
For both baseline substrate conditions, a dose response for fluoride could be revealed.
Clinical significance
Remineralization characteristics of enamel directly depended on baseline mineral loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The efficiency of dentifrices in order to inhibit caries lesion formation is supported by more than half a century of research [1, 2]. For coronal caries, a preventive effect of 23% could be found for dentifrices containing 1100–1250 ppm F− compared with non-fluoride dentifrices [2]. For dentifrices containing 2400–2800 ppm F−, the preventive effect increased to 36% [2]. Interestingly, in both reviews [1, 2], the preventive effect of dentifrices containing 5000 ppm F− has not been analyzed. However, for dentifrices containing 5000 ppm F−, it could recently be demonstrated that root caries incidence is significantly reduced and that significantly more root caries lesions are inactivated when compared to dentifrices containing 1100–1450 ppm F− [3]. Furthermore, in vivo studies indicated a dose-response relationship between the preventive effect and fluoride concentration ([F−]) [2, 4]. Thus, when analyzing fluoride dentifrices, in vitro or in situ respective models should be capable to demonstrate a (significant) fluoride dose response similar to the anticipated clinical response [4,5,6,7].
Although several previous pH-cycling models have shown a dose response for fluoride concentrations in the range 0–1250 ppm F− [8], only two models could demonstrate a fluoride dose response for fluoride concentrations up to 2800 ppm F− [9, 10] or 5000 ppm F− [11]. In the most recent study [9], three different methods of pre-demineralization were compared, and correlations between four different outcomes (lesion surface microhardness, mineral loss, lesion depth, and maximum mineral density of the lesion surface zone) were analyzed. The results of transversal microradiographic (TMR) data indicated a fluoride dose response for bovine and human enamel specimens. Under net-remineralizing conditions, the lowest mineral gain was observed for 0 ppm F− whereas the highest mineral gain was observed for 2800 ppm F−. However, no correlations with fluoride concentrations have been published. The second study revealed a fluoride dose response for dentifrices containing up to 3000 ppm F− by comparing numerous different experiments with similar settings [10]. However, models slightly differed in study length, de- and remineralizing solutions, and the baseline mineral loss of the specimens. The third study analyzed dentifrices containing 0, 500, 1500, and 5000 ppm F− [11]. When analyzing Ca2+ uptake and loss, a significantly increased remineralization and decreased demineralization could be observed in the first 3 days for 5000 ppm F− compared with those for 1500 ppm F−. However, when analyzing the mineral content by using TMR, no significant differences could be found between 5000 ppm F− and 1500 ppm F− (as NaF). In summary, all studies recommended that there is a need for further studies including lesions of various degrees of severity (baseline substrate conditions) before conclusions on optimal fluoride efficacy can be drawn.
In recent years, several different highly fluoridated dentifrices with different fluoride compounds (e.g., amine fluoride [AmF] instead of NaF [11]) or with additional active components (e.g., functionalized tricalcium phosphate (TCP)) have been introduced. Indeed, both studies indicated that 5000 ppm F− as AmF or 5000 ppm F− plus TCP, respectively, might promote remineralization significantly more effectively than 5000 ppm F− as NaF.
Thus, the aim of the present study was, firstly, to reveal a dose response for fluoride dentifrices for lowly as well as highly demineralized enamel artificial caries lesions, secondly, to investigate the relation between baseline TMR data and re- and demineralization characteristics of enamel, and thirdly, to compare the caries preventive effect of different dentifrices containing 5000 ppm F−. The null hypotheses were that, firstly, a significant correlation between mineral loss and fluoride concentration could be observed between lowly and highly demineralized lesions and that, secondly, no significant difference in the remineralizing effect of 5000 ppm F− plus TCP and solely 5000 ppm F− could be observed.
Materials and methods
Specimen preparation
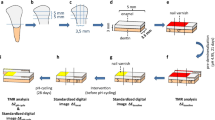
Bovine incisors were obtained from freshly slaughtered cattle (negative BSE test) and stored in 0.08% thymol. Teeth were cleaned and 450 enamel blocks (5 mm × 3.5 mm × 3 mm) were prepared (Exakt 300; Exakt Apparatebau, Norderstedt, Germany) [12, 13]. The enamel blocks were embedded in epoxy resin (Technovit 4071; Heraeus Kulzer, Hanau, Germany), ground flat, and polished (4000 grit; silicon carbide, Phoenix Alpha, Wirtz-Buehler, Düsseldorf, Germany; Mikroschleifsystem Exakt, Exakt Apparatebau, Norderstedt, Germany).
Lesion formation
Two-thirds of the surface of each specimen was covered with nail varnish in order to assure enough mechanical and acid resistance for the untreated control area. To create artificial enamel caries lesions in uncovered areas, specimens were stored in a demineralization solution for 21 days (2.5 ml solution/mm2 enamel surface) [14]. The solution contained 50 mM acetic acid, 3 mM CaCl2.H2O, 3 mM KH2PO4, 6 μM methylhydroxydiphosphonate, and traces of thymol (pH 4.95; 37 °C). During that period, pH was monitored daily and, if necessary, adjusted with small amounts of either 10% HCl or 10 M KOH to maintain a constant pH value. This way, 120 lowly demineralized lesions [S] with a mean (95% confidence interval [CI]) baseline mineral loss (ΔZbaseline,L) of 5120 (4995; 5245) vol.% × μm and a mean baseline lesion depth (LDbaseline,L) of 138 (134; 141) μm were chosen from the 450 specimens originally prepared. Furthermore, 120 highly demineralized lesions [A] with a mean (95% CI) baseline mineral loss (ΔZbaseline,H) of 8187 (8036; 8339) vol.% × μm and a mean baseline lesion depth (LDbaseline,H) of 185 (180; 189) μm were chosen.
pH-cycling condition
A computer-controlled pH-cycling and brushing machine [15] was used to simulate oral pH-fluctuation patterns and daily oral care. The pH-cycling (28 days) involved six demineralization periods of 1 h each (total 6 h/day) and six remineralization periods of at least 2 h during the day and a longer overnight period (total 18 h/day). The remineralizing solutions contained 1.5 mM CaCl2, 0.9 mM KH2PO4, and 20 mM N-2-hydroxyethylpiperazine-N’2-ethanesulfonic acid (HEPES) as buffer at pH 7.0 (37 °C). The demineralization solution contained 0.6 μM methylhydroxydiphosphonate, 3 mM CaCl2, 3 mM KH2PO4, and 50 mM acetic acid adjusted to pH 4.87 (37 °C) [14]. The pH-cycling solutions were refreshed with every cycle (6×/day). The amounts of each solution were large enough to prevent the solutions from becoming saturated with or depleted of mineral ions (0.7 ml solution/mm2 enamel surface).
Surface treatment and dentifrice slurries
The lowly and highly demineralized caries-like enamel lesions were randomly divided into 6 groups each, resulting in 12 subgroups. Before the first and last remineralizing phase of each day, the specimens were brushed for 10 s (Oral-B Indicator; Procter & Gamble, Schwalbach am Taunus, Germany) with the respective dentifrice slurry: fluoride-free [L0/H0], 1100 ppm F− [L1100/H1100], 2800 ppm F− [L2800/H2800], 5000 ppm F− [L5000/H5000]), 5000 ppm F− plus glycerin [L5000 + glycerin/H5000 + glycerin], and 5000 ppm F− plus tricalcium phosphate [L5000 + TCP/H5000 + TCP] containing dentifrices (Table 1).
Dentifrice slurry remained on the specimens for another 110 s. Subsequently, specimens were perfused with distilled water to remove the slurry. In total, brushing procedure for each specimen lasted 120 s, hence simulating the recommended brushing and application time of 2 min [16]. The machine was adjusted to a constant brushing frequency of 60 strokes/min and a constant brushing load of 1.5 N [17].
Dentifrice slurries (except for L5000 + glycerin and H5000 + glycerin) were prepared with deionized water in a ratio of 3:1 parts by weight and refreshed every 2 days. During this period, all slurries were stable. Only slurries of L5000 and H5000 dispersed into an aqueous and a dentifrice phase. To stabilize the emulsion of L5000 and H5000 glycerin, a humectant and emulsifier being found in several dentifrices were added. Thus, dentifrice slurries of L5000 + glycerin and H5000 + glycerin were prepared with deionized water (two parts), glycerin (one part), and dentifrice (one part) in a ratio 2:1:1.
Determination of free fluoride in the slurries
For the fluoride-containing dentifrices, total soluble fluoride concentrations in the slurries were determined as described previously [18].
Transversal microradiography analysis
After pH-cycling from each specimen, a slice of approximately 300 μm thickness (Exakt GmbH, Norderstedt, Germany) was obtained and subsequently ground flat and polished to a thickness of 100 μm (± 10 μm) using waterproof silicon carbide papers (FEPA grit sizes: 800, 1200, 2400, 4000; Struers). Parallelism of the specimens was tested with a digital micrometer with a precision of 0.001 mm (Mitutoyo, Japan). Contact microradiographs of the enamel specimens were obtained with a nickel-filtered copper (CuKa) X-ray source (PW 1730; Philips, Kassel, Germany) operating at 20 kV and 20 mA. The radiation source-to-film distance was 28 cm. The exposure time was 10 s, and a high-resolution film (Motion picture fine grain positive film 71337″; FUJIFILM Corporation Japan) was1 used and developed under standardized conditions according to the manufacturer’s recommendations.
Microradiographs were digitalized by an image-analyzing system (Diskus software version 4.80; Königswinter, Germany) that is interfaced to a universal microscope (Leica DMRX; Germany) and a personal computer. A TMR software (version 5.25 by Joop de Vries, Groningen, Netherlands) was used to calculate the mineral loss (ΔZbaseline/ΔZpH-cycle) and lesion depth (LDbaseline/LDpH-cycle) before and after the pH-cycling [19]. Furthermore, graphics of mean mineral density profiles were prepared for all groups with the TMR/WIM Calculation Program (v5.25; University of Groningen, the Netherlands) [20].
Calculation of integrated mineral loss and lesion depth
Mineral content was calculated by the TMR software based on the specimen’s gray levels as described previously [12]. In short, the average mineral content of sound enamel was assumed to be 87 vol.% and the mineral density of sound enamel to be 2.88 g/cm3 as measured by previous studies [21]. The lesion depth was calculated using a threshold of 95% of the mineral content of sound enamel (i.e., 82.7%). Thus, integrated mineral loss (ΔZ) and lesion depth (LD) could be calculated [22]. Changes in mineral loss (ΔΔZ = ΔZbaseline − ΔZpH-cycle) and lesion depth (ΔLD = LDbaseline − LDpH-cycle) were then calculated [20, 23].
Statistical analysis
Data were analyzed using SPSS statistical software (SPSS 22.0; SPSS, Munich, Germany). Variables were tested for normal distribution (Shapiro-Wilk test). Changes in mineral loss and lesion depth before and after pH-cycling (ΔZbaseline vs. ΔZpH-cycle and LDbaseline vs. LDpH-cycle) were analyzed using two-tailed paired t test. Analysis of covariance (ANCOVA) for lowly and highly demineralized lesions was used to detect differences in the changes in mineral loss (∆∆Z) and lesion depth (ΔLD) between interventions. More technically, the ANCOVA statistical model may be described as a general linear mixed model with TMR data (ΔZ, LD) and treatment as fixed effects. Correlation between [F−] and ΔΔZ as well as between [F−] and ΔLD were assessed using the Spearman’s rank correlation coefficient. For this, only groups L0, L1100, L2800, and L5000 and H0, H1100, H2800, and H5000 were used. Spearman’s rank correlation coefficient was also used to analyze correlation between baseline substrate conditions (ΔZbaseline) and change in mineral loss (ΔΔZ). All tests were performed at a 5% level of significance.
Power calculations
The number of specimens per group was calculated on the basis of pre-studies (non-published data). The α-error was set at 5%. Considering the differences between the 0 and 1100 ppm fluoride dentifrice, the statistical power calculated for ∆∆Z was 85% (mean difference of 450 (SD 600)) and for ∆LD was 89% (mean difference of 4 (SD 5)). Dropout rate was assumed not to exceed 20%. Approximately 20 specimens should have been enrolled into the study for analyses of at least 16 specimens per groups. Since the retro-perspective power analysis with 13 specimens has still provided a power of at least 88% for ∆∆ZS (mean difference of 1026 (SD 801) and 82% for ∆∆ZA (mean difference of 1423 (SD 1201), no additional specimens were included later.
Results
Mineral loss and lesion depth
After pre-demineralization, there was a significant difference in mineral loss (p < 0.001; ANCOVA) and lesion depth (p < 0.001; ANCOVA) between lowly and highly demineralized specimens. Furthermore, specimens within the subgroup of the respective baseline substrate condition (lowly/highly) did not differ significantly in mineral loss (p = 1.000; ANCOVA) and lesion depth (p = 1.000; ANCOVA) (Table 2). Mean (95% confidence interval) baseline mineral loss was 5120 (4995; 5245) vol.% × μm for lowly demineralized lesions and 8187 (8036; 8339) vol.% × μm for highly demineralized lesions. Due to losses during preparation, final TMR analysis was performed with 13–19 specimens per subgroup (Table 2). After pH-cycling, all subgroups showed signs of remineralization indicated by significantly lower ∆Z and LD values than before pH-cycling (p ≤ 0.012; two-tailed paired t test), except for LD of L0, H0, and L5000 + TCP (p ≥ 0.368; two-tailed paired t test) (Table 2).
Highly demineralized lesions showed a significantly higher increase in mineral content (ΔΔZ) (p ≤ 0.004; ANCOVA) and lesion depth (ΔLD) (p ≤ 0.047; ANCOVA) than the respective lowly demineralized lesions, expect for 0 ppm F−. For both baseline substrate conditions, a significantly higher increase in mineral content (∆∆Z) could be observed for all fluoride groups (except for TCP-containing 5000 ppm F−) compared with 0 ppm F− (p ≤ 0.01; ANCOVA, Fig. 1a, b). The application of 5000 ppm F− induced a significantly higher gain in mineral content compared with that of 1100 ppm F− (p ≤ 0.007; ANCOVA). Furthermore, only for highly demineralized lesions, the application of 2800 ppm F− induced a significantly higher gain in mineral content compared with that of 1100 ppm F− (p = 0.001; ANCOVA).
Means with confidence intervals (95%) of the changes in mineral losses (∆∆Z (a, b)) and lesion depths (∆LD (c, d)). In each of the four diagrams, lowly demineralized (L) lesions can be seen at the left side and highly demineralized (H) ones on the right side. Different letters indicate significant differences between treatments among lowly (large caps) and highly demineralized (small caps) specimens (p < 0.05; ANCOVA)
Correlation analyses
According to the Spearman’s rank correlation coefficient, a moderate and significant correlation could be found between F− concentrations (0, 1100, 2800, 5000 ppm F−) and ΔΔZ for lowly demineralized lesions (rL,ΔΔZ = 0.591; pL,ΔΔZ < 0.001) and a strong and significant correlation for highly demineralized lesions (rH,ΔΔZ = 0.746; pH,ΔΔZ < 0.001). The respective values for the correlations between ΔLD and F− concentrations were rL,ΔLD = 0.348 and pL,ΔLD = 0.005 and rH,ΔLD = 0.592 and pH,ΔLD < 0.001.
Correlations between baseline substrate conditions (ΔZbaseline and LDbaseline) and change in mineral loss (ΔΔZ) or change in lesion depth (ΔLD), respectively, are depicted in Table 3. With increasing fluoride concentrations, the significant correlation between ΔZbaseline and ΔΔZ increased from moderate to very strong.
Mineral density of the lesion surface zone
All 202 specimens revealed subsurface lesions without abrasive surface losses (Fig. 2). During pH-cycling, the maximum (SZmax) and minimum (SZmin) mineral density of the lesion surface zone increased in all groups indicating the incorporation of minerals. After pH-cycling, a second layer of demineralized tissue could be observed in specimens of H5000, H5000 + gylcerin, L5000 + TCP, and H5000 + TCP (Fig. 2).
Mean mineral density profiles of lowly (L) and highly demineralized (H) lesions were assessed after pre-demineralization (baseline) and after pH-cycling (pH-cycle) using the TMR/WIM calculation program. During pH-cycling, the maximum (SZmax) and minimum (SZmin) mineral density of the lesion surface zone increased in all groups indicating the incorporation of minerals
Fluoride analysis
The free fluoride content (SD) and the percentage of free fluoride in relation to given fluoride content (SD) are given in Table 1. In fluoride-free dentifrice, a negligible amount of free fluoride was measured [12.3 (3.8) ppm F−].
Discussion
The present in vitro study compared the caries preventive effect of dentifrices differing in fluoride concentration for lowly and highly demineralized caries-like enamel lesions. For both baseline substrate conditions, a significant correlation between mineral loss (∆∆Z) and fluoride concentration in the dentifrices could be observed. However, both study null-hypotheses had to be rejected since the correlations varied between moderate for lowly and strong for highly demineralized specimens. Furthermore, for specimens treated with TCP-containing 5000 ppm F−, a significantly lower mineral gain was observed compared with 5000 ppm F−.
In the present remineralizing pH-cycling study, a fluoride dose response could be observed for highly as well as lowly demineralized lesions. For both substrate conditions, significant differences in ∆∆Z could be observed between 0, 1100, and 5000 ppm F−. This was also reflected by the significant correlation between F− concentration and ∆∆Z. On the one hand, this is in agreement with the re- and demineralization characteristics being observed in vivo [2, 24] and in situ [19, 20, 25]. On the other hand, this seems to be in contrast to a similar in vitro study [11]. In the previous study, no significant difference in the change of mineral loss between 5000 ppm F− (as NaF) and 1500 ppm F− (as NaF) was observed although a significantly increased remineralization and a decreased demineralization were observed between both groups when analyzing Ca2+ uptake and loss [11]. It might be speculated that the anti-caries effect of 1100 ppm F− is (slightly) lower compared with the effect of 1500 ppm F−, resulting in a slightly higher difference when compared with 5000 ppm F−. Another possible reason for the different effect observed for the dentifrices in the previous study may be related to the baseline mineral loss, which was (presumably) not well-balanced. Due to the lower baseline mineral loss in group 5000 ppm F− compared with that in group 1500 ppm F−, the potential for remineralization was lower for specimens of group 5000 ppm F− compared with specimens of group 1500 ppm F− [11]. Thus, that may have also contributed for the absence of significant difference between the two fluoride concentrations.
In previous in situ studies, an increasing potential for remineralization with increasing ∆Zbaseline and LDbaseline under remineralizing conditions [25] and a decreasing potential for demineralization with increasing ∆Zbaseline and LDbaseline under demineralizing conditions [20] has been observed. Indeed, under the present remineralizing conditions, highly demineralized lesions were significantly more prone to remineralization than lowly demineralized ones. This is also reflected in the significant correlations between ∆Zbaseline and ∆∆Z; with increasing fluoride concentration, these correlations increased from moderate (0 ppm F−) to very strong (5000 ppm F−). Since the design of a pH-cycling study and the environment created by the model presumably have an overriding impact on its response (net demineralization or net remineralization) [8, 20, 25], it might be speculated that the dose-response effect observed for the various fluoride dentifrices in the present study might be the opposite under demineralizing conditions.
Specimens of H5000 and H5000 + TCP presented a secondary lesion body (lamination) after pH-cycling. Lamination can be observed when fluorides are incorporated into the surface. The incorporation seems to induce larger and less soluble crystallites [26]. Furthermore, fluorohydroxyapatites buffer the solution to a lesser extent than hydroxyapatite [26]. Thus, when acids diffuse through the relatively large pores of the lesion, they are not neutralized [10, 27]. In consequence, the intact tissue beyond the original lesion is dissolved resulting in a second lesion body [10, 27]. In several previous pH-cycling studies, lamination characteristics varied widely. In one pH-cycling model, an inverse correlation between fluoride concentration and severity of the lamination was observed [10, 11]. Contrastingly, in other models, lamination was only observed for dentifrices containing 2800 ppm F− [9] or 5000 ppm F− (present data) but not for lower fluoride concentrations. Since different pre-demineralization agents were used in the mentioned studies, it might be speculated that the use of 8% methyl cellulose gel [10, 11], soluble carbopol [9], and soluble acetic acid (present study) resulted in pores with different sizes and different diffusion properties (for both fluoride and acids). In consequence, different lamination characteristics during pH-cycling were observed. This raises the question, which pre-demineralization protocol most closely mimics clinical conditions. In our view, this is still unclear.
In recent years, TCP as additional active component has been used to further increase the remineralizing effect of varnishes and dentifrices. TCP is supposed to act as Ca2+ and PO43− reservoir, enhancing fluoride’s efficacy without compromising the fluoride bioavailability [28]. During storage, the inactivation of the ions is prevented by carboxylic acid (fumaric acid), which is created in the manufacturing process of ball milling β-TCP [29]. If the fumaric acid gets into contact with an aqueous environment (e.g., saliva), the β-TCP–fumaric acid interface breaks. In consequence, Ca2+ and PO43− are released [30]. For varnishes containing NaF plus TCP, the remineralizing effect for enamel (and dentin) varied between significantly inferior [31], as good as [30, 32] and significantly superior [33] when compared with NaF (without TCP). Regarding the anti-caries effect of dentifrices containing 5000 ppm F− plus TCP, only surrogate outcomes as photomicrographs [34] have been used in vitro. However, in situ, a significantly higher mineral gain could be observed for dentifrices containing 5000 ppm F− plus TCP when compared with dentifrices containing solely 5000 ppm F− [35]. In contrast to our hypothesis, a significantly lower mineral gain was observed for L5000 + TCP when compared with that for L5000. Interestingly, in the previous and in the present study, different pre-demineralization agents were used. Thus, it might be speculated that the use of an acidified gel system [35] and soluble acetic acid (present study) resulted in pores with different sizes and different diffusion properties (as discussed above). In consequence, different de- and remineralization characteristics during the in vitro and the in situ period might be observed.
Although the present model revealed a dose-response characteristic for fluoride dentifrice, dentifrices not only differed in fluoride (and TCP) content but also in other inactive ingredients. It might, thus, be speculated that the observed dose-response characteristic for fluoride was influenced by other ingredients (e.g., antimicrobial or abrasive ingredients). However, all 202 specimens revealed subsurface lesions without abrasive surface losses, and no antimicrobial interferences are expected in a chemical caries model. Nonetheless, it would be interesting to test dentifrices differing only in their fluoride (and TCP) content.
The model used in this study mimics the dynamics of enamel caries formation. However, pH-cycling models have several limitations [8]: (1) Demineralizing challenges were followed by remineralizing challenges. These changes were much faster than those expected to occur in in vivo conditions; (2) The brushing procedure did not adequately simulate topical use and clearance of products from the oral cavity; (3) The complex intraoral condition formed by the bacterial biofilm, saliva, and eating behavior could not be simulated; (4) The surface area/solution ratio and saliva/plaque fluid composition being found in vivo were not simulated. Therefore, the present findings should further be investigated in clinical studies.
Within the limitation of an in vitro study, it can be concluded that the present pH-cycling model was capable to reveal a dose-response characteristic for fluoride dentifrice to increase further remineralization for different baseline substrate conditions. Moreover, it could be shown that even under in vitro conditions, remineralization characteristics directly depend on specimens’ baseline substrate conditions. Furthermore, the dentifrice containing 5000 ppm F− revealed the highest anti-caries effect, whereas the dentifrices containing 5000 ppm F− plus TCP did not promote remineralization significantly more than dentifrices containing 1100 ppm F−.
References
Marinho VC, Higgins JP, Sheiham A and Logan S (2003) Fluoride toothpastes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev:CD002278. https://doi.org/10.1002/14651858.CD002278
Walsh T, Worthington HV, Glenny AM, Appelbe P, Marinho VC and Shi X (2010) Fluoride toothpastes of different concentrations for preventing dental caries in children and adolescents. Cochrane Database Syst Rev:CD007868. https://doi.org/10.1002/14651858.CD007868.pub2
Wierichs RJ, Meyer-Lueckel H (2015) Systematic review on noninvasive treatment of root caries lesions. J Dent Res 94(2):261–271. https://doi.org/10.1177/0022034514557330
Zero DT (1995) In situ caries models. Adv Dent Res 9(3):214–230; discussion 231–234. https://doi.org/10.1177/08959374950090030501
Featherstone JD (1992) Consensus conference on intra-oral models: evaluation techniques. J Dent Res 71 Spec No:955–956
Manning RH and Edgar WM (1992) Intra-oral models for studying de- and remineralization in man: methodology and measurement. J Dent Res 71 Spec No:895–900
Wefel JS (1990) Effects of fluoride on caries development and progression using intra-oral models. J Dent Res 69 Spec No:626–633; discussion 634–636. https://doi.org/10.1177/00220345900690S122
Buzalaf MA, Hannas AR, Magalhaes AC, Rios D, Honorio HM, Delbem AC (2010) pH-cycling models for in vitro evaluation of the efficacy of fluoridated dentifrices for caries control: strengths and limitations. J Appl Oral Sci 18(4):316–334. https://doi.org/10.1590/S1678-77572010000400002
Lippert F, Juthani K (2015) Fluoride dose-response of human and bovine enamel artificial caries lesions under pH-cycling conditions. Clin Oral Investig 19(8):1947–1954. https://doi.org/10.1007/s00784-015-1436-1
ten Cate JM, Exterkate RA, Buijs MJ (2006) The relative efficacy of fluoride toothpastes assessed with pH cycling. Caries Res 40(2):136–141. https://doi.org/10.1159/000091060
ten Cate JM, Buijs MJ, Miller CC, Exterkate RA (2008) Elevated fluoride products enhance remineralization of advanced enamel lesions. J Dent Res 87(10):943–947. https://doi.org/10.1177/154405910808701019
Esteves-Oliveira M, Santos NM, Meyer-Lueckel H, Wierichs RJ, Rodrigues JA (2017) Caries-preventive effect of anti-erosive and nano-hydroxyapatite-containing toothpastes in vitro. Clin Oral Investig 21(1):291–300. https://doi.org/10.1007/s00784-016-1789-0
Meyer-Lueckel H, Wierichs RJ, Schellwien T, Paris S (2015) Remineralizing efficacy of a CPP-ACP cream on enamel caries lesions in situ. Caries Res 49(1):56–62. https://doi.org/10.1159/000363073
Buskes JA, Christoffersen J, Arends J (1985) Lesion formation and lesion remineralization in enamel under constant composition conditions. A new technique with applications. Caries Res 19(6):490–496. https://doi.org/10.1159/000260887
Wierichs RJ, Kogel J, Lausch J, Esteves-Oliveira M, Meyer-Lueckel H (2017) Effects of self-assembling peptide P11-4, fluorides, and caries infiltration on artificial enamel caries lesions in vitro. Caries Res 51(5):451–459. https://doi.org/10.1159/000477215
Ganss C, Schlueter N, Preiss S, Klimek J (2009) Tooth brushing habits in uninstructed adults--frequency, technique, duration and force. Clin Oral Investig 13(2):203–208. https://doi.org/10.1007/s00784-008-0230-8
Aykut-Yetkiner A, Attin T, Wiegand A (2014) Prevention of dentine erosion by brushing with anti-erosive toothpastes. J Dent 42:856–861. https://doi.org/10.1016/j.jdent.2014.03.011
Meyer-Lueckel H, Wierichs RJ, Gninka B, Heldmann P, Dorfer CE, Paris S (2015) The effect of various model parameters on enamel caries lesions in a dose-response model in situ. J Dent 43(10):1261–1267. https://doi.org/10.1016/j.jdent.2015.08.003
Lippert F, Churchley D, Lynch RJ (2015) Effect of lesion baseline severity and mineral distribution on remineralization and progression of human and bovine dentin caries lesions. Caries Res 49(5):467–476. https://doi.org/10.1159/000431039
Wierichs RJ, Lausch J, Meyer-Lueckel H, Esteves-Oliveira M (2016) Re- and demineralization characteristics of enamel depending on baseline mineral loss and lesion depth in situ. Caries Res 50(2):141–150. https://doi.org/10.1159/000444537
Angmar B, Carlstrom D, Glas JE (1963) Studies on the ultrastructure of dental enamel. IV. The mineralization of normal human enamel. J Ultrastruct Res 8:12–23
Arends J, Dijkman T, Christoffersen J (1987) Average mineral loss in dental enamel during demineralization. Caries Res 21(3):249–254. https://doi.org/10.1159/000261028
Meyer-Lueckel H, Schulte-Monting J, Kielbassa AM (2002) The effect of commercially available saliva substitutes on predemineralized bovine dentin in vitro. Oral Dis 8(4):192–198. https://doi.org/10.1034/j.1601-0825.2002.01762.x
Biesbrock AR, Gerlach RW, Bollmer BW, Faller RV, Jacobs SA, Bartizek RD (2001) Relative anti-caries efficacy of 1100, 1700, 2200, and 2800 ppm fluoride ion in a sodium fluoride dentifrice over 1 year. Community Dent Oral Epidemiol 29(5):382–389. https://doi.org/10.1111/j.1600-0528.2001.290508.x
Strang R, Damato FA, Creanor SL, Stephen KW (1987) The effect of baseline lesion mineral loss on in situ remineralization. J Dent Res 66(11):1644–1646. https://doi.org/10.1177/00220345870660110801
Brown WE, Gregory TM, Chow LC (1977) Effects of fluoride on enamel solubility and cariostasis. Caries Res 11(Suppl 1):118–141. https://doi.org/10.1159/000260298
Tschoppe P, Meyer-Lueckel H (2011) Mineral distribution of artificial dentinal caries lesions after treatment with fluoride agents in combination with saliva substitutes. Arch Oral Biol 56(8):775–784. https://doi.org/10.1016/j.archoralbio.2011.01.002
Karlinsey RL, Mackey AC, Walker TJ, Frederick KE, Blanken DD, Flaig SM, Walker ER (2011) In vitro remineralization of human and bovine white-spot enamel lesions by NaF dentifrices: a pilot study. J Dent Oral Hyg 3(2):22–29
Karlinsey RL, Mackey AC, Walker ER, Frederick KE (2010) Preparation, characterization and in vitro efficacy of an acid-modified beta-TCP material for dental hard-tissue remineralization. Acta Biomater 6(3):969–978. https://doi.org/10.1016/j.actbio.2009.08.034
Mohd Said SN, Ekambaram M, Yiu CK (2017) Effect of different fluoride varnishes on remineralization of artificial enamel carious lesions. Int J Paediatr Dent 27:163–173. https://doi.org/10.1111/ipd.12243
Tuloglu N, Bayrak S, Tunc ES, Ozer F (2016) Effect of fluoride varnish with added casein phosphopeptide-amorphous calcium phosphate on the acid resistance of the primary enamel. BMC Oral Health 16(1):103. https://doi.org/10.1186/s12903-016-0299-4
Wierichs RJ, Stausberg S, Lausch J, Meyer-Lueckel H, Esteves-Oliveira M (2018) Caries-preventive effect of NaF, NaF+TCP, NaF+CPP-ACP and SDF varnishes on sound dentin and artificial dentin caries in vitro. Caries Res 52. https://doi.org/10.1159/000484483
Alamoudi SA, Pani SC, Alomari M (2013) The effect of the addition of tricalcium phosphate to 5% sodium fluoride varnishes on the microhardness of enamel of primary teeth. Int J Dent 2013(486358):1–5. https://doi.org/10.1155/2013/486358
Goettsche ZS, Ettinger RL, Wefel JS, Hogan MM, Harless JD, Qian F (2014) In vitro assessment of 3 dentifrices containing fluoride in preventing demineralization of overdenture abutments and root surfaces. J Prosthet Dent 112(5):1257–1264. https://doi.org/10.1016/j.prosdent.2014.01.031
Amaechi BT, Ramalingam K, Mensinkai PK, Chedjieu I (2012) In situ remineralization of early caries by a new high-fluoride dentifrice. Gen Dent 60:e186–e192
Acknowledgments
This study was conducted as part of the doctoral thesis of S.W.. We would like to thank Rainer Dautzenberg, Axel Honné, and the whole team of the scientific construction laboratory of the RWTH Aachen University for developing, constructing, and assembling the computer-controlled pH-cycling and brushing machine. Furthermore, we would like to thank Klaus Mussler for their excellent collaboration and technical assistance.
Funding
This study was funded by the authors and their institution.
Author information
Authors and Affiliations
Contributions
R.J.W., J.L., S.W., and M.E.-O. designed and planned the study; S.W. prepared the samples and performed the measurements; R.J.W. and S.W. performed the statistical analysis; R.J.W. and M.E.-O. wrote the manuscript; S.W. and H.M.-L. commented on, and all authors revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interests.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Wierichs, R.J., Westphal, S., Lausch, J. et al. Influence of highly concentrated fluoride dentifrices on remineralization characteristics of enamel in vitro. Clin Oral Invest 22, 2325–2334 (2018). https://doi.org/10.1007/s00784-018-2333-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-018-2333-1