Abstract
Objectives
The aim of this in vitro study was to compare the caries-preventive effect of various high fluoride- and calcium-containing caries-preventive agents (> 22.000 ppm F− [ppm]) in adjunct to use of regular (1450 ppm) or high (5000 ppm) fluoride toothpaste on sound as well as demineralized enamel.
Materials and methods
Bovine enamel specimens (n = 276; 5 mm × 3.5 mm × 3 mm) having one sound surface [ST] and one artificial caries lesion [DT] were randomly allocated to 12 groups. Interventions before pH-cycling were no intervention ([SC1/SC5]), application of varnishes/solutions containing NaF (22,600 ppm; Duraphat [NaF1/NaF5]); NaF + tricalcium phosphate (22,600 ppm; Clinpro White Varnish [TCP1/TCP5]); NaF + CPP-ACP (22,600 ppm; MI Varnish [CPP1/CPP5]); silver diammine fluoride (35,400 ppm; Cariestop 30%[SDF1/SDF5]); and NaF + calcium fluoride (45,200 ppm; Biophat[CaF1/CaF5]). During pH-cycling (28 days, 6 × 120 min demineralization/day) half of the specimens in each group were brushed (10 s; 2 × /day) with either 1,450 (NaF; named, e.g., TCP1) or 5,000 ppm (NaF; e.g., TCP5) dentifrice slurry. Differences in integrated mineral loss (∆∆Z) and lesion depth (∆LD) were calculated between values after initial demineralization and after pH-cycling using transversal microradiography.
Results
After pH-cycling, SC1/SC5 showed significantly increased ∆ZDT/LDDT values, indicating further demineralization (p < 0.05; paired t-test). Decreased ∆ZDT values, indicating non-significant remineralization, could only be observed in CaF1/CaF5 (p > 0.05; paired t-test). Additional use of all varnishes/solutions significantly decreased ∆∆ZDT/∆∆ZST and ∆LDDT/∆LDST compared to SC1/SC5 (p < 0.05;ANCOVA). Between 1450 and 5000 ppm dentifrices, a significant difference in ∆∆ZDT/∆∆ZST and ∆LDDT/∆LDST could only be observed for SC1/SC5 (p < 0.05; ANCOVA).
Conclusion
Under the conditions chosen, all fluoride varnishes/solutions significantly reduced demineralization. Furthermore, a significant dose–response characteristic for fluoride varnishes could be revealed. However, no additional benefit could be observed, when varnishes were combined with high fluoride instead of regular fluoride dentifrices.
Clinical relevance
For children and adolescents with high caries risks varnishes containing more than 22,600 ppm should be further investigated, as they offered higher caries-preventive effects in vitro. Furthermore, there seems to be no difference in the demineralization-inhibitory capacity of fluoride varnishes when used in combination with either standard or highly fluoridated dentifrices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A variety of clinical studies could demonstrate the benefits, safety, and cost-effectiveness of various means of fluoride delivery [1]. Besides dentifrices, varnishes and solutions with high fluoride content (> 22.000 ppm F−) are widely used, especially for high-risk patients [2]. Due to the adherence to the tooth surface for longer periods, they are supposed to act as slow-releasing fluoride reservoirs [2]. One of the most widely used varnishes contains 5% sodium fluoride (NaF) [3]. However, in recent years, the anti-caries effects of various other formulas have been utilized. For instance, calcium/phosphates have been added to NaF-containing varnishes. This way, fluoride efficacy is supposed to be enhanced without compromising fluoride bioavailability [4]. During storage, the interaction of Ca2+ and fluoride is protected by a fumaric barrier. If the fumaric barrier gets into contact with saliva, it breaks and Ca2+ and F− are released [5]. Proteins derived from milk have also been added to NaF-varnish. After incorporation of these protein complexes (e.g., casein phosphopeptide-stabilized amorphous calcium phosphate complexes (CPP-ACP)), they are supposed to buffer the biofilm pH. Simultaneously, Ca2+ and PO4− are supposed to dissociate [6]. Thus, a reservoir for storing bioavailable Ca2+ and PO4− is formed by adding CPP-ACP. Moreover, different fluoride compounds have either additionally been added to NaF (e.g., calcium fluoride (CaF)) to increase the fluoride deposition [7] and to induce a quicker and deeper initial interaction or have been used instead of NaF (e.g., silver diamine fluoride (SDF); [8]). A considerable antibacterial effect could be observed for SDF [9]. Even under bacteria-free conditions, it prevented further demineralization in enamel [10, 11] and dentin [8, 12], indicating a (high) anti-caries effect.
The remineralizing effects of varnishes/solutions containing SDF [10, 11], NaF plus TCP [5], or NaF plus CPP-ACP [5, 13] have been analyzed on enamel in several in vitro studies. Interestingly, net-demineralizing conditions were only simulated once when investigating varnishes [13]. However, in this study, firstly, a less sensitive optical coherence tomography method was used to measure mineral los and, secondly, no significant differences between the negative (no varnish) and standard control (NaF varnish) could be observed. Nonetheless, intermittent demineralizing conditions are common in the oral environment. Moreover, although the anti-caries effect of the formulas was analyzed on initially demineralized as well as sound (enamel) surfaces, none of them analyzed the effect on different baseline substrate conditions simultaneously.
Thus, the aim of the present study was to compare the demineralizing inhibitory effect of various high fluoride–containing caries-preventive agents (> 22.000 ppm F) in addition to the use of either regular (1450 ppm) or high (5000 ppm) fluoride dentifrices on sound enamel surfaces as well as on artificial enamel caries–like lesions under net-demineralizing conditions using a bacteria-free pH-cycling model. We hypothesized that no significant differences in mineral loss would be observed between the fluoride varnishes/solutions but for all compared with a no-varnish control (standard control). Furthermore, we hypothesized that a significant correlation between mineral loss and fluoride concentration of the varnishes would be observed for initially demineralized lesions.
Materials and methods
Specimen preparation
The study protocol conformed to the principles outlined in the German Ethics Committee’s statement for the use of human body material in medical research [16].
Bovine incisors were obtained from freshly slaughtered cattle (negative BSE test) and stored in 0.08% thymol. Teeth were cleaned and 300 enamel blocks (5 mm × 3.5 mm × 3 mm) were prepared (Exakt 300; Exakt Apparatebau, Norderstedt, Germany) (Fig. 1). The enamel blocks were embedded in epoxy resin (Technovit 4071; Heraeus Kulzer, Hanau, Germany), ground flat and polished (grit sizes: 800, 1200, 2400, 4000; silicon carbide, Phoenix Alpha, Wirtz-Buehler, Düsseldorf, Germany; Mikroschleifsystem Exakt, Exakt Apparatebau, Norderstedt, Germany) [17].
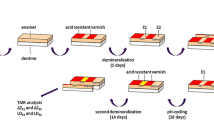
Specimen preparation. a Frontal view of bovine incisor and b–c lines for cutting perpendicular and parallel to the long axis of the tooth crown; d prepared specimens (5 mm × 3 mm × 3.5 mm); e specimen covered with acid resistant nail varnish (sound control area (S) (red); sound treatment area (ST)); f initially demineralized specimen (demineralized treatment area (DT)) and thin section after initial demineralization for transversal microradiographic analysis; g specimen covered with nail varnish (yellow); h specimen after the application of the agents; i preparation of the thin sections after pH-cycling for transversal microradiographic analysis
Lesion formation
Two-thirds of the surface of each specimen was covered with nail varnish in order to assure enough mechanical and acid-resistance for the sound control (S) and sound treatment (ST) areas (Fig. 1). Since S was used as a control to register a possible damage (e.g., destruction, erosion, abrasive surface loss) of the specimen during the in vitro study, nail varnish was not removed at any time. Specimens were stored in a demineralization solution for 21 days (2.5 ml solution/mm2 enamel surface) to create artificial enamel caries lesions in uncovered areas (demineralized treatment [DT]). The solution contained 50 mM acetic acid, 3 mM CaCl2xH2O, 3 mM KH2PO4, 6 µM methylhydroxydiphosphonate, and traces of thymol (pH 4.95; 37ºC) [18]. During that period, the pH was monitored daily and, if necessary, adjusted with small amounts of either 10% HCl or 10 mM KOH to maintain a constant pH value. After demineralization, artificial caries lesions with intact surfaces were observed in all specimens. In order to calculate baseline mineral loss and lesion depth, thin slices of 100 µm (± 10 µm) of each partially demineralized enamel specimen were cut perpendicularly to the surface, as described below. Subsequently, photographic images of the specimens were taken and 286 specimens were chosen from the 300 specimens originally prepared [19].
Power calculation
The number of specimens per group was calculated based on previous studies (non-published data). The α-error was set at 5%. Considering the differences (SD) between the SC1 and Sc5 (∆∆ZDT: mean difference of 350 (360) vol% × µm; ∆∆ZST: mean difference of 1878 (310) vol% × µm), the statistical power calculated for ∆∆ZDT was 85% and for ∆∆ZST 100%. Dropout rate was assumed not to exceed 20%. Approximately 23 specimens should have been enrolled into the study for analyses of at least 18 specimens per group. Since the retro-perspective power analysis for the smallest difference (difference between SC1 and Sc5) with 16 specimens has still provided a power of at least 100% for ∆∆ZDT (mean difference: 904 (406) vol% × µm), 100% for ∆∆ZST (mean difference: 751 (178) vol% × µm), 90% for ∆LDDT (mean difference: 20 (17) µm), and 100% for ∆LDST (mean difference: 35 (10) µm); no additional specimens were included in the study.
Treatments
Specimens were randomly allocated to twelve experimental groups (n = 286). Interventions before pH-cycling were as follows:
-
No intervention (standard control [SC])
-
Application of a varnishes/solutions containing sodium fluoride [NaF]
-
Sodium fluoride plus tricalcium phosphate [TCP]
-
Sodium fluoride plus casein phosphopeptide-stabilized amorphous calcium phosphate complexes (CPP-ACP) [CPP]
-
Silver diammine fluoride [SDF]
-
Sodium and calcium fluoride [CaF] (Table 1)
This first level of intervention (application of a varnish) is indicated by the capital letters. The second level of intervention (brushing with a dentifrice) is indicated with lower script letters. Meaning the groups combined with brushing with a 1450 ppm F− fluoride dentifrice got the reference number 1 (e.g., NaF1) and the ones combined with 5000 ppm F− got the reference number 5 (e.g., NaF5) as lower script letters.
Before application, specimens’ surfaces were cleaned using a Pro-Cup (Pro-Cup Nr. 990/30, Hawe Neos Dental, Bioggio, Switzerland) for 20 s, rinsed using distilled water for 30 s, and dried [12]. Subsequently, the respective varnish/solution was applied according to the manufacturer’s instructions with the exception of a prolonged application time (5 min instead of 3 min as partially instructed) [12]. Thereafter, specimens were immersed into distilled water for 2 h, before pH-cycling started (first phase: 2 h demineralization). Thus, the minimally recommended treatment period was simulated since the varnishes/solutions remained undisturbed on the specimens for 4 h before the first brushing procedure. As done previously (e.g., [12, 13]), varnishes/solutions have not actively been remove before pH-cycling and simulated toothbrushing. Since in some groups small amounts of the varnishes could be observed even after the pH-cycling period, further information on the influence of not removing the varnishes/solutions can be found in the discussion.
pH-cycling conditions
A computer-controlled pH-cycling and brushing machine [19] was used to simulate oral pH-fluctuation patterns and daily oral care. The pH-cycling lasted 28 days and conditions were chosen with a daily schedule of 6 cycles. Specimens were consecutively subjected to a demineralizing (60 min), a rinsing (30 s), a remineralizing (120 min), and again a rinsing (30 s) phase. During a 6-h “night” period, the specimens were subjected to a remineralizing solution. The remineralization solutions contained 1.5 mM CaCl2, 0.9 mM KH2PO4, and 20 mM Hepes, pH 7.0. The demineralization solution contained 0.6 µM methylhydroxydiphosphonate, 3 mM CaCl2, 3 mM KH2PO4, and 50 mM acetic acid adjusted to pH 4.87 [18]. The pH-cycling solutions were refreshed with every cycle. The amounts of each solution were large enough to prevent the solutions from becoming saturated with or depleted of mineral ions (0.7 ml solution/mm2 enamel surface per cycle).
Twice daily, after the first and last demineralizing phase, the specimens in each group were brushed for 10 s (Oral-B Indicator; Proctor & Gamble, Schwalbach am Taunus, Germany) with either regular fluoride dentifrice (1,450 ppm F− [ppm] as NaF; Blend-a-med Frisch, Procter & Gamble, Schwalbach am Taunus, Germany) (group name, e.g., NaF1) or a high fluoride dentifrice (5000 ppm as NaF; Colgate Duraphat 5000 ppm Fluoride Toothpaste, Colgate-Palmolive Ltd, Guildford, UK) (e.g., NaF5) dentifrice slurry (Table 2).
To simulate the recommended brushing time of 2 min, specimens were perfused with distilled water to remove the slurry after another 110 s. The machine was adjusted to a constant brushing frequency of 60 strokes/min and a constant brushing load of 1.5 N. These settings ensured the prevention of brushing abrasion in the present study and simulated a relatively realistic brushing sequence at the lower end of the range [20]. Dentifrice slurries were prepared with deionized water in a ratio of 1:3 parts by weight (toothpaste:water) and refreshed every 2 days.
Determination of free fluoride and pH in the slurries
For the fluoride-containing dentifrices, total soluble [F−] in the slurries and their pH were determined as described previously [21, 22]. Two hundred milligrams of the toothpaste slurries (1 part toothpaste to 3 parts mineral solution, by weight) were diluted in 100 ml distilled water at room temperature. Four milliliters of the diluted slurries were centrifuged at 10,000 g for 10 min and 1 ml of the supernatant was added to 1 ml TISAB II (Thermo Fisher Scientific, Beverly, Mass., USA). To calibrate, the electrode four fluoride solutions (3.8 mg/l, 1.9 mg/l, 0.38 mg/l, and 0.19 mg/l) were prepared, since these concentrations are in the same range of the expected sample concentrations. After calibration, the fluoride concentration of the toothpastes were determined using a fluoride-sensitive electrode (type 96–09 BNC; Thermo Fisher Scientific). Analyses were made in triplicate.
Transversal microradiographic analysis
Slices of approximately 300 μm thickness from each specimen were obtained after initial demineralization (Baseline) and after the pH-cycling (pH-cycle) (Exakt GmbH, Norderstedt, Germany). Subsequently, the slices were ground and polished to a thickness of 100 μm (± 10 µm) using waterproof silicon carbide papers (FEPA grit sizes: 800, 1200, 2400, 4000; Struers). Microradiographical images of the slices were obtained as described previously (20 kV and 20 mA) [22].
Calculation of integrated mineral loss, lesion depth
Mineral loss (ΔZBaseline/ΔZpH-cycle) and the lesion depth (LDBaseline/LDpH-cycle) were calculated by using the transversal microradiography (TMR) software (Version 5.25 by Joop de Vries, Groningen, the Netherlands). The average mineral content of sound enamel was assumed to be 87 vol%, and the mineral density of sound enamel to be 2.88 g/cm3, as measured by previous studies [23, 24]. The lesion depth was calculated using a threshold of 95% of the mineral content of sound enamel (i.e., 82.7%). Thus, integrated mineral loss (ΔZ), lesion depth (LD), and “R”-value (being the ratio of mineral loss (∆Z) to lesion depth (LD)) could be calculated [25, 26].
Baseline mineral loss (ΔZBaseline) and lesion depth (LDBaseline) of initially demineralized surfaces were subtracted from the respective values after pH-cycling (ΔZpH-cycle, LDpH-cycle) [17, 27]. Changes in mineral loss (ΔΔZ = ΔZBaseline—ΔZpH-cycle) and lesion depth (ΔLD = LDBaseline—LDpH-cycle) were then calculated. For a more intuitive reading, ΔΔZST and ΔLDST were calculated as well, although values for sound surfaces were measured only after the pH-cycling [12]. For this, baseline values were assumed to be zero. Furthermore, graphics of mean mineral density profiles were prepared for all groups with the TMR/WIM Calculation Program.
Statistical analysis
Data were analyzed using SPSS statistical software (SPSS 25.0; SPSS, Munich, Germany). Variables were tested for normal distribution (Shapiro–Wilk test). Within one experimental group, changes in mineral loss and lesion depth after initial demineralization (ΔZBaseline/LDBaseline) and after pH-cycling (ΔZpH-cycle/LDpH-cycle) were analyzed using paired t-tests. Analysis of covariance (ANCOVA) for the regular and high fluoride dentifrices was used to detect differences in changes of mineral losses (∆∆ZDT, ∆∆ZST) and lesion depths (ΔLDDT, ΔLDST). For this, TMR and treatment were defined as fixed effects. Since for pre-demineralized lesions (DT), a correlation between ΔΔZ and [F−] was shown graphically in Fig. 2, evaluation was carried out using the Spearman rank correlation coefficient for pre-demineralized lesions. For this, only groups N [0 ppm], NaF [22600 ppm], SDF [35200 ppm], and CaF [45200 ppm] were used. All tests were performed at a 5% level of significance.
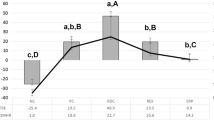
Means with confidence intervals (95%) of the changes in mineral loss (∆∆Z; A and B) and lesion depths (∆LD; C and D) of initially demineralized surfaces (ΔΔZDT/∆LDDT) and sound surfaces (ΔΔZST/∆LDST). Different letters indicate significant differences between treatments among specimens being brushed with 1450 ppm (e.g., SC1) (large caps) and with 5000 ppm (e.g., SC5) (small caps) (p < 0.05; ANCOVA). For initially demineralized specimens, a significantly lower change in mineral loss could only be observed for CaF1 compared with the high fluoride dentifrice (SC5), whereas for sound enamel surfaces the combined use of the regular fluoride dentifrice and all varnishes/solutions induced a significantly lower change in mineral loss compared with SC5. A single asterisk (*) indicates significant differences between regular and high fluoride dentifrices within one varnish treatment. Negative ΔΔZ values indicate demineralization, positive ΔΔZ values indicate remineralization. For a more intuitive reading, ΔΔZST/∆LDST values were calculated as well, although the values were measured only after pH cycling. For this, the baseline values were assumed to be zero
Results
After initial demineralization, treatment groups didh not differ significantly in mineral loss (p > 0.05; ANCOVA) and lesion depth (p > 0.05; ANCOVA). Mean (95% CI) ΔZbaseline,DT was 1879 (1734; 2024) vol% × µm and LDbaseline,DT was 97 (91; 102) µm. Due to losses during preparation, final TMR analysis was performed with 16–21 specimens per group (Table 3).
TMR—mineral loss
For mineral loss, significantly increased values between before and after pH-cycling were observed in SC1, NaF1, TCP1 and SC5, NaF5, SDF5 indicating further demineralization (p < 0.05; two-tailed paired t-test; Table 3), whereas only CaF1 and CaF5 showed (non-significantly) decreased ∆ZDT values (p > 0.05; two-tailed paired t-test; Table 3).
For comparisons between the groups, initially demineralized specimens of all interventions (except NaF5, CPP5, and SDF5) showed significantly lower further mineral loss (∆∆ZDT) than SC1 and SC5, respectively (p < 0.05; ANCOVA; Fig. 2). Furthermore, specimens of CaF1 and CaF5 showed significantly lower changes in mineral loss (∆∆ZDT) than NaF1 and NaF5, respectively (p < 0.05; ANCOVA; Fig. 2). For ∆∆ZST, all groups with additional interventions showed significantly lower further mineral loss (∆∆ZDT) than SC1 and SC5, respectively (p < 0.05; ANCOVA; Fig. 2). For comparison between regular and high fluoride dentifrices, a significantly lower change in mineral loss (∆∆ZDT and ∆∆ZST) for the high fluoride dentifrice could only be observed in the dentifrice only group (SC1 vs. SC5) (p < 0.05; ANCOVA; Fig. 2).
TMR—lesion depth
For lesion depth, significantly increased values between those after initial demineralization and after pH-cycling were observed in SC1, SDF1 and SC5, CPP5, SDF5 (p < 0.05; two-tailed paired t-test; Table 3). For comparisons between the groups, specimens of SC1 and SC5 showed a significantly higher changes in lesion depths (∆LDDT and ∆LDST) compared to all interventions (except for initially demineralized specimens of SDF5) (p < 0.05; ANCOVA; Fig. 2). For comparison between regular and high fluoride dentifrices, a significant lower increase in lesion depths (∆LDDT and ∆LDST) for the high fluoride dentifrice could only be observed in sound surfaces in the dentifrice only group (p < 0.05; ANCOVA; Fig. 2).
TMR—mineral density profiles.
All specimens revealed subsurface lesions without abrasive surface losses (Fig. 3, Supplementary Information Fig. 1, Supplementary Information Fig. 2). After pH-cycling, a second layer of demineralized tissue could be observed in initially demineralized specimens of SC1, SC5, NaF5, and CPP5. For sound surfaces, a second lesion body could only be observed in specimens of SC1.
Mean mineral density profiles of the initially demineralized enamel surfaces before (baseline, DT) and after pH cycling (pH-cycle, DT) as well as the profiles of sound surfaces (pH-cycle, ST). Left diagrams: combined use of regular fluoride dentifrices [1450 ppm as NaF] and fluoride varnishes. Right diagrams: combined use of regular fluoride dentifrices [5000 ppm as NaF] and fluoride varnishes. Lesions were assessed using the TMR/WIM calculation program. In initially demineralized specimens, a second layer of demineralized tissue could be observed in specimens of SC1, SC5, and NaF5. For sound surfaces, a second lesion body could only be observed in specimens of SC1
TMR—correlation analysis
A significant moderate correlation could be found between ΔΔZDT and F concentrations of the varnishes (0; 22,600; 35,400; 45,200) for both, the regular (rDT = 0.493) and the highly (rDT = 0.404) fluoridated dentifrices (p < 0.001).
Free fluoride content and pH of the dentifrice slurries
The mean free fluoride content (SD) [and the percentage of free fluoride in relation to given fluoride (SD)] were 1538 (35) ppm [116 (2) %] for the regular fluoride dentifrices and 5367 (96) ppm [107 (2)%] for the regular fluoride dentifrices (Table 2). The pH values were 6.9 and 8.1, respectively. As regards the fluoride content of the varnishes, values are given in the Table 1.
Discussion
The present study compared the effects of highly fluoride- and calcium-containing caries-preventive agents (> 22.000 ppm F, varnishes and solutions) being used in combination with either regular or high fluoride dentifrices on sound enamel and on artificial enamel lesions under net-demineralizing conditions. Compared with the standard control, the additional use of all fluoride varnishes and of a SDF solution significantly prevented further mineral loss in sound surfaces as well as in artificial caries lesions. However, for sound surface and for demineralized specimens, application of CaF (containing 45,200 ppm as NaF and CaF2) yielded to significantly less demineralization compared with the “conventional” NaF (containing 22,600 ppm as NaF) varnish partially rejecting our first hypothesis that no significant differences in mineral loss would be observed between the fluoride varnishes/solutions.
In the present study, the use of fluoride varnishes/solutions being used in combination with either regular or high fluoride dentifrices significantly hampered further mineral loss compared to the dentifrice only groups (SC1 and SC5). Although only a few (in vitro) studies compared different fluoride compounds for fluoride varnishes this is in line with a previous review on 13 clinical trials, showing a preventive fraction of 43% for fluoride varnishes when compared to placebos or no treatment [3]. Interestingly, until now, there is no data on the demineralization inhibiting effects of the combined use of high fluoride dentifrices (5000 ppm F−) and fluoride varnishes or SDF solution in vivo.
In recent in vivo [28], in situ [29], and in vitro [21] studies, a significantly higher caries-preventive effect could be observed for high fluoride dentifrices when compared to regular fluoride dentifrices. This is in line with the present results. Although several studies analyzed fluoride-free and regular fluoride dentifrices in combination with additional fluoride agents in different concentration [30, 31]; to our knowledge, regular and high fluoride dentifrices have not been compared on enamel before when additional fluoride agents (e.g., gel or varnish) have also been used. However, one in vitro study compared high fluoride dentifrice and regular fluoride dentifrices in combination with an additional application of fluoride gel on dentin [8] and two studies compared high fluoride dentifrices compared to regular fluoride dentifrices in combination with an additional application of fluoride gel [8, 32]. In both studies, no significant differences in the change of mineral losses between the high fluoride dentifrice (alone) as well as the regular fluoride dentifrice and the high fluoride dentifrice both in combination with fluoride gels could be observed. However, for all three groups, a significant difference could be observed when compared to regular fluoride dentifrices (alone). Thus, the results of the previous studies on fluoride gels/solutions [8, 32] and the present study on varnishes/solutions challenges the assumptions of an additional benefit when fluoride varnishes/solutions/gels are used in combination with high fluoride dentifrices instead of regular fluoride dentifrices. Furthermore, based on the results of the three studies, it seems plausible that patient preferences (i.e., additional efforts and costs to acquire the agents, taste of the available products) should be included when deciding for either high fluoride dentifrices (alone) or the combined use of regular fluoride dentifrices and further fluoride agents (e.g., gel or varnish).
In recent studies, the used pH-cycling brushing machine was capable to reveal a dose–response characteristic in bovine enamel [33] and dentin [21] similar to the anticipated clinical effects when dentifrices were investigated. Significant fluoride dose-responses between fluoride and change in mineral loss could be observed for highly as well as lowly demineralized enamel lesions in the range 0, 1100, and 5000 ppm [33] as well as for sound dentin surfaces and initially demineralized dentin lesions in the rage 0, 1450, 2800, 5000, and 12,500 ppm [21]. In the present study, a fluoride dose–response in the range 22,600, 35,400, and 45,200 ppm could also be observed under net-demineralizing condition after the single application of varnishes/solutions. However, this could only be observed for initially demineralized specimens and only in combination with the regular fluoride dentifrice, partially confirming the hypothesis that for initially demineralized lesions a significant correlation between mineral loss and fluoride concentration of the varnishes would be observed. Nonetheless, the results are in agreement with a previous pH-cycling study [34]. Although the present in vitro study (also) demonstrated a fluoride dose–response up to 45,200 ppm, the clinical implication of that finding remains unclear since until now a dose–response for fluoride varnishes (or gels) of different fluoride concentration have not been analyzed or observed in vivo [3]. This lack of knowledge might be explained by the unknown risks associated with a long-term use of such a regime (e.g., fluoride intoxication), especially considering the daily use of the high fluoride (5000 ppm F−) toothpastes. Even that accidental ingestion of fluoride is for sure more common by young children, the risks of such an intensive fluoride application approach has to be weighed against the additional anti-caries effect. Moreover, when using this approach, patients have to be clearly instructed to avoid frequently accidentally swallowing of the toothpaste.
Although the present model revealed a dose–response characteristic for varnishes, the dentifrices did not only differed in fluoride content but also in other inactive ingredients. It might, thus, be speculated that the remineralizing capacity of the varnishes was influenced by other ingredients (e.g., antimicrobial or abrasive ingredients) of the dentifrices. However, all specimens revealed subsurface lesions without abrasive surface losses and no antimicrobial interferences are expected in a chemical caries model. Nonetheless, it would be interesting to test dentifrices differing only in their fluoride content. In the present study, bovine enamel specimens were used to analyze anti-cariogenic effects and the initial caries lesions were created using a method described by Buskes et al. [1985]. Compared to natural initial caries lesion in human enamel, the lesion of the present study shows the same characteristics. However, they present normally more porous, though intact surface layer, covering a demineralized subsurface area, presenting 10–70 vol% of mineral content [35]; characteristics which were also observed here. On the one hand, they, thus, present higher rates of mineral changes, diffusion rates, and faster lesion formation [36, 37]. On the other hand, bovine teeth are easier to obtain, present the same mechanism of caries formation, and have a more homogeneous mineralization pattern, which results in a more consistent experimental response [38]. Therefore, they are considered as suitable substrate for anti-caries in vitro and in situ studies [22].
In previous anti-caries [5, 10] and anti-erosive [39] studies, varnishes were actively removed within 6–24 h after application. This is in contrast to the present study in which varnishes/solutions were not actively removed. However, currently a few pH-cycling studies can be found in the literature, in which varnishes were also not removed from the sample surfaces (e.g., [12, 13]) as it was done here. The present regime was used to simulate the clinical situation, in which varnishes are not actively removed by the dentist. They rather get removed by the natural action of occlusal contact to antagonists and the mechanical action of the brushing procedure. Furthermore, to simulate the real clinical situation as closely as possible controlled and constant linear strokes with brushing forces of 1.5 N were used twice daily. It seemed reasonable to assume that the varnishes would get removed from the specimen surfaces after some hours of simulated toothbrushing. Although linear strokes could be observed in vivo [40] and were used in vitro [41], the adjusted brushing force was at the lower range of previous in vitro (1.5–2.5 N [41]) and in vivo (1.5–3 N [40]) studies. Nonetheless, our (non-published) pre-studies indicated that in the used pH-cycling machine forces higher than 1.5 N would result in enamel surface abrasions. However, the brushing regime did not result in the same level of “cleanliness” in all groups. Due to differences in the “adhesive properties” of the varnishes in some groups (NaF, TCP, and CPP), small amounts of the varnishes could be observed even after the pH-cycling period. Thus, it might be speculated that in these groups slow-releasing fluoride depots—being physical barriers as well—were present the entire pH-cycling period. Although this has also been observed in a previous study [12], it has, consequently, to be highlighted that higher brushing forces or the removal of the varnishes/solutions prior pH-cycling would probably result in a lower enhancement of the demineralizing inhibitory effect as it has been observed here. Furthermore, it might be speculated that due to the observed differences in the “adhesive properties” of the varnishes/solutions the described effect would vary between the groups.
In conclusion, under the in vitro conditions chosen, all fluoride varnishes and the SDF solution significantly prevented further demineralization when compared with the standard control. Furthermore, a significant dose–response characteristic regards the fluoride content in fluoride varnishes could be revealed. A varnish with 45,200 ppm F as NaF plus CaF revealed the highest caries-preventive effect on enamel caries–like lesions under chosen net-demineralizing conditions. Furthermore, there was no difference in the demineralization-inhibitory capacity of fluoride varnish and the SDF solution when used in combination with either standard or high fluoride dentifrices.
Data availability
All data generated or analyzed during this study are included in this article [and/or] its supplementary material files. Further enquiries can be directed to the corresponding author.
References
Pessan JP, Toumba KJ, Buzalaf MAR (2011) Topical use of fluorides for caries control. Monogr Oral Sci 22:115–132. https://doi.org/10.1159/000325154
Ogard B, Seppa L, Rolla G (1994) Professional topical fluoride applications–clinical efficacy and mechanism of action. Adv Dent Res 8:190–201. https://doi.org/10.1177/08959374940080021001
Marinho VC, Worthington HV, Walsh T and Clarkson JE (2013) Fluoride varnishes for preventing dental caries in children and adolescents. Cochrane Database Syst Rev:CD002279. https://doi.org/10.1002/14651858.CD002279.pub2
Karlinsey RL, Mackey AC, Walker TJ, Frederick KE, Blanken DD, Flaig SM, Walker ER (2011) In vitro remineralization of human and bovine white-spot enamel lesions by NaF dentifrices: a pilot study. J Dent Oral Hyg 3:22–29
Mohd Said SN, Ekambaram M, Yiu CK (2017) Effect of different fluoride varnishes on remineralization of artificial enamel carious lesions. Int J Paediatr Dent 27:163–173. https://doi.org/10.1111/ipd.12243
Reynolds EC (1997) Remineralization of enamel subsurface lesions by casein phosphopeptide-stabilized calcium phosphate solutions. J Dent Res 76:1587–95. https://doi.org/10.1177/00220345970760091101
Attin T, Hartmann O, Hilgers RD, Hellwig E (1995) Fluoride retention of incipient enamel lesions after treatment with a calcium fluoride varnish in vivo. Arch Oral Biol 40:169–74. https://doi.org/10.1016/0003-9969(95)98804-8
Erdwey D, Meyer-Lueckel H, Esteves-Oliveira M, Apel C, Wierichs RJ (2021) Demineralization Inhibitory effects of highly concentrated fluoride dentifrice and fluoride gels/solutions on sound dentin and artificial dentin caries lesions in vitro. Caries Res 55:41–54. https://doi.org/10.1159/000509931
Rosenblatt A, Stamford TC, Niederman R (2009) Silver diamine fluoride: a caries “silver-fluoride bullet.” J Dent Res 88:116–25. https://doi.org/10.1177/0022034508329406
Delbem AC, Bergamaschi M, Sassaki KT, Cunha RF (2006) Effect of fluoridated varnish and silver diamine fluoride solution on enamel demineralization: pH-cycling study. J Appl Oral Sci 14:88–92. https://doi.org/10.1590/s1678-77572006000200005
Yu OY, Mei ML, Zhao IS, Li QL, Lo EC, Chu CH (2018) Remineralisation of enamel with silver diamine fluoride and sodium fluoride. Dent Mater 34:e344–e352. https://doi.org/10.1016/j.dental.2018.10.007
Wierichs RJ, Stausberg S, Lausch J, Meyer-Lueckel H, Esteves-Oliveira M (2018) Caries-Preventive Effect of NaF, NaF plus TCP, NaF plus CPP-ACP, and SDF varnishes on sound dentin and artificial dentin caries in vitro. Caries Res 52:199–211. https://doi.org/10.1159/000484483
Pithon MM, Dos Santos MJ, Andrade CS, Leao Filho JC, Braz AK, de Araujo RE, Tanaka OM, Fidalgo TK, Dos Santos AM, Maia LC (2015) Effectiveness of varnish with CPP-ACP in prevention of caries lesions around orthodontic brackets: an OCT evaluation. Eur J Orthod 37:177–82. https://doi.org/10.1093/ejo/cju031
Gluzman R, Katz RV, Frey BJ, McGowan R (2013) Prevention of root caries: a literature review of primary and secondary preventive agents. Spec Care Dentist 33:133–40. https://doi.org/10.1111/j.1754-4505.2012.00318.x
Twetman S, Keller MK (2016) Fluoride rinses, gels and foams: an update of controlled clinical trials. Caries Res 50(Suppl 1):38–44. https://doi.org/10.1159/000439180
Central German Ethics Committee (2003) The use of human body materials for the purpose of medical research (in German). https://www.zentrale-ethikkommission.de/stellungnahmen/koerpermaterialien/. Accessed 21 Nov 2021
Meyer-Lueckel H, Wierichs RJ, Gninka B, Heldmann P, Dorfer CE, Paris S (2015) The effect of various model parameters on enamel caries lesions in a dose-response model in situ. J Dent 43:1261–7. https://doi.org/10.1016/j.jdent.2015.08.003
Buskes JA, Christoffersen J, Arends J (1985) Lesion formation and lesion remineralization in enamel under constant composition conditions. A new technique with applications. Caries Res 19:490–6. https://doi.org/10.1159/000260887
Wierichs RJ, Kogel J, Lausch J, Esteves-Oliveira M, Meyer-Lueckel H (2017) Effects of self-assembling peptide P11–4, fluorides, and caries infiltration on artificial enamel caries lesions in vitro. Caries Res 51:451–459. https://doi.org/10.1159/000477215
Jansen EE, Meyer-Lueckel H, Esteves-Oliveira M, Wierichs RJ (2021) Do bleaching gels affect the stability of the masking and caries-arresting effects of caries infiltration-in vitro. Clin Oral Investig 25:4011–4021. https://doi.org/10.1007/s00784-020-03732-4
Wierichs RJ, Rupp K, Meyer-Lueckel H, Apel C, Esteves-Oliveira M (2020) Effects of dentifrices differing in fluoride content on remineralization characteristics of dentin in vitro. Caries Res 54:75–86. https://doi.org/10.1159/000504165
Esteves-Oliveira M, Santos NM, Meyer-Lueckel H, Wierichs RJ, Rodrigues JA (2017) Caries-preventive effect of anti-erosive and nano-hydroxyapatite-containing toothpastes in vitro. Clin Oral Investig 21:291–300. https://doi.org/10.1007/s00784-016-1789-0
Angmar B, Carlstrom D, Glas JE (1963) Studies on the ultrastructure of dental enamel. IV. The mineralization of normal human enamel. J Ultrastruct Res 8:12–23. https://doi.org/10.1016/s0022-5320(63)80017-9
de Josselin de Jong E, ten Bosch JJ and Noordmans J, (1987) Optimised microcomputer-guided quantitative microradiography on dental mineralised tissue slices. Phys Med Biol 32:887–99. https://doi.org/10.1088/0031-9155/32/7/008
Arends J, Dijkman T, Christoffersen J (1987) Average mineral loss in dental enamel during demineralization. Caries Res 21:249–54. https://doi.org/10.1159/000261028
Theuns HM, van Dijk JW, Driessens FC, Groeneveld A (1984) The surface layer during artificial carious lesion formation. Caries Res 18:97–102. https://doi.org/10.1159/000260756
Magalhaes AC, Moron BM, Comar LP, Wiegand A, Buchalla W, Buzalaf MA (2009) Comparison of cross-sectional hardness and transverse microradiography of artificial carious enamel lesions induced by different demineralising solutions and gels. Caries Res 43:474–83. https://doi.org/10.1159/000264685
Marinho VC, Higgins JP, Logan S and Sheiham A (2003) Topical fluoride (toothpastes, mouthrinses, gels or varnishes) for preventing dental caries in children and adolescents. Cochrane Database Syst Rev:CD002782. https://doi.org/10.1002/14651858.CD002782
Wierichs RJ, Musiol J, Erdwey D, Esteves-Oliveira M, Apel C, Meyer-Lueckel H (2020) Re- and demineralization characteristics of dentin depending on fluoride application and baseline characteristics in situ. J Dent 94:103305. https://doi.org/10.1016/j.jdent.2020.103305
Akabane S, Delbem AC, Pessan J, Garcia L, Emerenciano N, Goncalves DF, Danelon M (2018) In situ effect of the combination of fluoridated toothpaste and fluoridated gel containing sodium trimetaphosphate on enamel demineralization. J Dent 68:59–65. https://doi.org/10.1016/j.jdent.2017.10.013
PaesLeme AF, Dalcico R, Tabchoury CP, Del BelCury AA, Rosalen PL, Cury JA (2004) In situ effect of frequent sucrose exposure on enamel demineralization and on plaque composition after APF application and F dentifrice use. J Dent Res 83:71–5. https://doi.org/10.1177/154405910408300114
Fernandez CE, Tenuta LMA, Del BelCury AA, Nobrega DF, Cury JA (2017) Effect of 5,000 ppm fluoride dentifrice or 1,100 ppm fluoride dentifrice combined with acidulated phosphate fluoride on caries lesion inhibition and repair. Caries Res 51:179–187. https://doi.org/10.1159/000453624
Wierichs RJ, Westphal S, Lausch J, Meyer-Lueckel H, Esteves-Oliveira M (2018) Influence of highly concentrated fluoride dentifrices on remineralization characteristics of enamel in vitro. Clin Oral Investig 22:2325–2334. https://doi.org/10.1007/s00784-018-2333-1
Manarelli MM, Delbem AC, Lima TM, Castilho FC, Pessan JP (2014) In vitro remineralizing effect of fluoride varnishes containing sodium trimetaphosphate. Caries Res 48:299–305. https://doi.org/10.1159/000356308
Arends J, Christoffersen J (1986) The nature of early caries lesions in enamel. J Dent Res 65:2–11
Edmunds DH, Whittaker DK, Green RM (1988) Suitability of human, bovine, equine, and ovine tooth enamel for studies of artificial bacterial carious lesions. Caries research 22:327–36
Featherstone JD, Mellberg JR (1981) Relative rates of progress of artificial carious lesions in bovine, ovine and human enamel. Caries Res 15:109–14
Mellberg JR (1992) Hard-tissue substrates for evaluation of cariogenic and anti-cariogenic activity in situ. Journal of dental research 71(Spec):913–9
Magalhaes AC, Levy FM, Rios D, Buzalaf MA (2010) Effect of a single application of TiF(4) and NaF varnishes and solutions on dentin erosion in vitro. J Dent 38:153–7. https://doi.org/10.1016/j.jdent.2009.09.015
Ganss C, Schlueter N, Preiss S, Klimek J (2009) Tooth brushing habits in uninstructed adults–frequency, technique, duration and force. Clin Oral Investig 13:203–8. https://doi.org/10.1007/s00784-008-0230-8
Aykut-Yetkiner A, Attin T, Wiegand A (2014) Prevention of dentine erosion by brushing with anti-erosive toothpastes. J Dent 42:856–61. https://doi.org/10.1016/j.jdent.2014.03.011
Acknowledgements
This study was conducted as part of the doctoral thesis of J.M. We would like to thank the technical assistance of Mr. Michael Stiebritz for the preparation of the TMR-micrographs.
Funding
This study was funded by the authors and their institution.
Author information
Authors and Affiliations
Contributions
R.J.W., M.E.-O., and J.M. designed and planned the study; J.M. prepared the samples; R.J.W. and J.M. performed the measurements and statistical analysis; R.J.W. wrote the manuscript; T.G.W. and H.M.-L. commented on, and all authors revised the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
The study protocol conformed to the principles outlined in the German Ethics Committee’s statement for the use of human body material in medical research [16]. This study is reported according to the COPE guidelines.
Informed consent
For this type of study, formal consent is not required.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wierichs, R.J., Mester, J., Wolf, T.G. et al. Effects of the association of high fluoride- and calcium-containing caries-preventive agents with regular or high fluoride toothpaste on enamel: an in vitro study. Clin Oral Invest 26, 3167–3178 (2022). https://doi.org/10.1007/s00784-021-04299-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-021-04299-4