Abstract
The aim of this study was to compare the caries-preventive effect of a stabilized stannous fluoride/sodium fluoride dentifrice containing sodium hexametaphosphate with those of a regular, solely sodium fluoride-containing and amine fluoride-containing dentifrice on pre-demineralized bovine enamel specimens using a pH-cycling model. Bovine enamel specimens with two artificial lesions each were prepared. Baseline mineral loss of both lesions was analyzed using transversal microradiography (TMR). Eighty-five specimens with a mean (SD) baseline mineral loss of 3393 (683) vol% × µm were selected and randomly allocated to five groups (n = 13/15). Treatments during pH-cycling (28 days and 2 × 20 min demineralization/day) were: brushing twice daily with slurries of AmF (1400 ppm F−), NaF (1450 ppm F−), SnF2/NaF (1100 ppm F−/350 ppm F−), and fluoride-free (FF) dentifrices or they were immersed in distilled water and remained unbrushed (NB). Subsequently, from each specimen one lesion was covered with acid-resistant varnish, while the remaining lesion was demineralized for another 14 days. Differences in integrated mineral loss (∆∆Z) were calculated between values before and after pH-cycling (∆∆Z E1) as well as before pH-cycling and after second demineralization (∆∆Z E2) using TMR. Treatments AmF and NaF induced a significantly higher mineral gain (∆∆Z E1/∆∆Z E2) compared to treatments FF and NB (p < 0.05; ANOVA test). Except for treatments AmF and NaF no significant differences in mineral loss between before and after pH-cycling could be observed (p < 0.05; t test) [∆∆Z E1: AmF:1563 (767); NaF:1222 (1246); SnF2/NaF:258 (1259); FF:−52 (1223); NB:−151 (834)]. Both dentifrices with either AmF or NaF promoted remineralization, whereas SnF2/NaF dentifrice did not promote remineralization in a biofilm-free pH-cycling model.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A variety of clinical studies could demonstrate the benefits, safety and cost-effectiveness of various means of fluoride delivery [1]. Dentifrices, one of the most widely practiced and effective ways to deliver free or soluble fluoride, are probably the most common products in healthcare [2]. Several fluoride compounds can be utilized intending to provide numerous different benefits. Especially metal ions such as stannous combine different therapeutic effects to control caries [3] as well as oral malodor [4]. The first stannous fluoride (SnF2) dentifrices were combined with calcium pyrophosphate, sodium metaphosphate or other abrasive systems [5] in their formulations and provided up to 22–25 % reduction in dental caries compared to placebo dentifrices [3]. As the amounts of soluble/free fluoride in the first SnF2 dentifrices were limited further studies on alternative fluoride compounds were conducted and resulted in a shift to sodium fluoride based dentifrices. In 1987, data clearly demonstrated that there was a significant decline in caries, but still a high prevalence of gingivitis and gingival recession among adults [6]. Thus, the development of dentifrices provided not only a therapeutic effect against dental caries, but also against gingivitis came into focus. As SnF2 combines both effects as well as being effective against sensitivity [7] and dental erosion [8], the focus has again been placed on this fluoride compound. Several new formulas stabilizing SnF2 and increasing the amount of soluble/free fluoride have been investigated. The most frequently described compounds that have been included in the formulations are for example sodium gluconate [9], sodium fluoride [8], sodium hexametaphosphate [10] or amine fluoride [8]. Also the combination of sodium fluoride and stannous chloride has been used [11].
Although several studies analyzed the antierosive [11–13] and the antimicrobial [14] effects of the new SnF2 containing formulas only two in vitro study analyzed the anticaries effect of the new formulas [15, 16]. In the first study, no significant differences in the change of mineral loss between a stannous chloride containing dentifrice and the positive control (NaF), but for both compared with the fluoride-free (fluoride negative) control, were observed after 5 days pH-cycling [15]. Contrastingly, in the second pH-cycling study stabilized stannous fluoride/sodium fluoride dentifrice containing sodium hexametaphosphate revealed a significantly lower gain in surface microhardness compared to solely sodium fluoride-containing and amine fluoride-containing dentifrices after 20 days [16]. The outcome used in the second study is best suited as a complementary measure to direct techniques (e.g., transversal microradiography) [17, 18], but it cannot be used on lesions that have a well-mineralized surface layer [19]. Thus, the results on stabilized stannous fluoride/sodium fluoride dentifrice containing sodium hexametaphosphate need to be further investigated, since it is known that fluoride of amine and sodium fluoride-containing dentifrices protects further demineralization and enhances remineralization by forming a hypermineralized surface layer [20, 21].
Several pH-cycling models have widely been used to analyze caries-preventive agents. Artificial enamel and/or dentine lesions are cycled between a demineralizing and remineralizing solution, mimicking oral pH-fluctuation. Depending on the severity and duration of the demineralization challenge inhibition of mineral loss (net demineralization) or increased mineral uptake (net remineralization) can be studied [22]. In both ‘types’ of pH-cycling models no antimicrobial effects can be analyzed and only the soluble/free (bioavailable) fluorides reacts with the substrate and can be released to the de- and remineralizing solutions [23]. Insoluble and inactive fluorides do not affect mineral loss. Since the new formulas have been developed to stabilize SnF2 and to increase the amount of soluble/free fluoride, it is believed that these new formulations might show a similar remineralizing potential as sodium or amine fluoride-containing toothpastes. So far, this could only be shown for stannous chloride [15] but not for other SnF2 containing formulas.
Thus, the aim of this in vitro study was to compare the caries-preventive effect of a stabilized stannous fluoride/sodium fluoride dentifrice containing sodium hexametaphosphate with those of a regular, solely sodium fluoride-containing and amine fluoride-containing dentifrice on pre-demineralized bovine enamel specimens using a pH-cycling model. We hypothesized that no significant differences in mineral loss would be observed between all dentifrices containing similar fluoride concentrations, but for all compared to a fluoride-free control or non-brushing.
Materials and methods
Specimen preparation
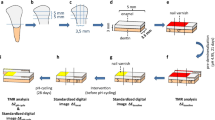
Bovine incisors were obtained from freshly slaughtered cattle (negative BSE test) and stored in 0.08 % thymol. Teeth were cleaned and 125 enamel blocks (5 × 3.5 × 3 mm) were prepared (Exakt 300; Exakt Apparatebau, Norderstedt, Germany). The enamel blocks were embedded in epoxy resin (Technovit 4071; Heraeus Kulzer, Hanau, Germany), ground flat and polished (4000 grit; silicon carbide, Phoenix Alpha, Wirtz-Buehler, Düsseldorf, Germany; Mikroschleifsystem Exakt, Exakt Apparatebau, Norderstedt, Germany) (Fig. 1).
Study design. Bovine enamel specimens with two artificial lesions each were prepared. Baseline mineral loss and lesion depth of both lesions were analyzed using transversal microradiography (TMR). After pH-cycling one lesion from each specimen was covered with acid-resistant varnish, while the remaining lesion was demineralized for another 14 days. Differences in integrated mineral loss (∆∆Z) and lesion depth (∆LD) were calculated between values before and after pH-cycling (∆∆Z E1 and ∆LDE1) as well as before pH-cycling and after second demineralization (∆∆Z E2 and ∆LDE2) using TMR
Lesion formation
Specimens were partially covered with acid-resistant varnish (sound control). In each of 125 specimens two artificial lesions were created [pH 4.95; 5 days] (Fig. 1) [24]. The samples were transversally sectioned to the orientation of the protective acid-resistant varnish (Trennschleifsystem Exakt 300; Exakt Apparatebau) and polished (4000 grit; Mikroschleifsystem Exakt) until thin (100 µm) plano-parallel sections were obtained. Transversal microradiography (TMR) was used to determine baseline mineral loss (ΔZ B1 and ΔZ B2) and lesion depth (LDB1 and LDB2). Microradiographic assessment was performed only in the central part of the lesion, at 100 µm distance from the lesion margin. Eighty-five specimens with a mean (SD) baseline mineral loss of 3393 (683) vol% × µm were selected and randomly allocated to five groups.
pH-cycling conditions
The pH-cycling lasted 28 days and conditions were chosen with a daily schedule of 2 cycles, where specimens were consecutively subjected to a demineralizing (0.3 h), a brushing and a remineralizing (11.6 h) phase. The remineralization solutions contained 1.5 mM CaCl2, 0.9 mM KH2PO4 and 20 mM Hepes, pH 7.0. The demineralization solution contained 0.6 µm methylhydroxydiphosphonate, 3 mM CaCl2, 3 mM KH2PO4 and 50 mM acetic acid adjusted to pH 4.95 [24]. The pH-cycling solutions were refreshed with each cycle. Each specimen was cycled in 10-ml aliquots of the solutions. Thus, the amounts of each solution were large enough to prevent the solutions from becoming saturated with or depleted of mineral ions.
Surface treatment
After the demineralization periods each specimen was manually brushed by one of the authors (HZ) for 10 s (Oral-B Indicator; Proctor and Gamble, Schwalbach am Taunus, Germany) with the dentifrice slurries described in Table 1 or not brushed and immersed in distilled water, as in group NB, the absolute negative control of the model. After brushing, the specimens remained immersed in the dentifrice slurries for further 10 s before the specimens were rinsed with distilled water for 30 s. Dentifrice slurries were prepared with deionized water in a ratio of 3:1 parts by weight and refreshed at the beginning of each experimental day. After pH-cycling one lesion from each specimen was covered with acid-resistant varnish, this area was denominated ‘effect 1’ (E1), while the remaining lesion was demineralized for another 14 days, representing the ‘effect 2’ (E2). The second demineralization was done with the same solution described previously [24]. The pH value was checked daily (pH-Meter GMH 3510; Greisinger, Regenstauf, Germany) and slight elevations were corrected with acetic acid or potassium hydroxide to maintain a constant pH between 4.98 and 5.02 during the demineralization period.
TMR
After the in vitro period thin plano-parallel sections were prepared again. Changes in mineral loss (ΔΔZ = ΔZ Baseline − ΔZ Effect) and lesion depth (ΔLD = LDBaseline − LDEffect) were calculated between values before and after pH-cycling for E1 (∆∆Z E1 and ΔLDE1) as well as before pH-cycling and after second demineralization for E2 (∆∆Z E2 and ΔLDE2) using transversal microradiographic images. Microradiographs of the enamel specimens were obtained and analyzed as described previously [25]. Furthermore, graphics of mean mineral density profiles were prepared for all groups with the TMR/WIM Calculation Program (v5.25; University of Groningen, the Netherlands).
Free fluoride analysis
Fluoride concentrations of all dentifrices were measured (Orion Autochemistry System 960; Fisher Scientific, Ulm, Germany) using a calibrated ion-specific electrode (type 96–09 BNC; Fisher Scientific) [26, 27]. For the measurements four fluoride solutions (3.8, 1.9, 0.38, and 0.19 mg/l) were prepared, since these concentrations are in the same range of the expected sample concentrations. The electrode potentials (mV) of the four standard solutions were measured and plotted against their concentrations (mg/l) in logarithmic scale. After calibration the fluoride concentration of the dentifrices were determined. For this, 200 mg of the dentifrices was diluted in 100 ml distilled water at room temperature. Four mL of each solution were centrifuged at 2500g and TISAB II (1:1; Fisher Scientific) was then added to control ionic strength. The percentage of free fluoride in relation to given total fluoride concentration (manufacture’s information) was determined. Two solutions for each dentifrice were prepared and analyzed in triplicates.
Electron microprobe analysis
The content of stannous (Sn) being incorporated in the enamel surface of specimens in group SnF2/NaF was measured using a JEOL Superprobe JXA 8900 electron microprobe at the Institute of Geosciences at Kiel University, equipped with five WD spectrometers. An accelerating voltage of 15 kV, a probe current of 15 nA and a probe diameter of about 5 µm were used. Characteristic X-rays were recorded using a TAP crystal for Na and Mg, a PETJ crystal for Cl and Ca and a PETH crystal for P and Sn. As standard materials natural tugtupite (Na, Cl), apatite (P), wollastonite (Ca), forsterite (Mg) and stannous oxide (Sn) were used. For both experimental setups (E1 and E2) three specimens of group SnF2/NaF were prepared and analyzed in triplicates. The percentage of SnF2 in relation to given total ions was determined. The calculated 1-sigma detection limit of Sn is about 200 ppm (0.02 wt%).
Statistical analysis
Data were analyzed using SPSS statistical software (SPSS 22.0; SPSS, Munich, Germany) and before analysis all variables were tested for normal distribution (Shapiro–Wilk test). Within one experimental group changes in mineral loss and lesion depth before and after pH-cycling (ΔZ B1 vs. ΔZ E1 and LDB1 vs. LDE1) and after pH-cycling and second demineralization (ΔZ B2 vs. ΔZ E2 and LDB2 vs. LDE2) were analyzed using t test. Analysis of variance (ANOVA) was used for pair-wise multiple-comparisons to detect differences in changes of mineral loss (∆∆Z E1, ∆∆ZE2 , ΔLDE1, ΔLDE2) between treatments. All tests were performed at a 5 % level of significance.
Results
After pre-demineralization specimens did not differ significantly in mineral loss between experimental groups (p > 0.05; Wilcoxon test). Mean (SD) baseline mineral loss was 3393 (683) vol% × µm. Due to preparation losses, final TMR analysis was performed with 13–15 specimens per group.
TMR—mineral density profiles
The mean mineral profiles of all groups for effects E1 and E2 can be seen in Fig. 2. After pH-cycling (E1) specimens of AmF and NaF revealed subsurface lesions without abrasive surface loss and specimens of SnF2/NaF, FF and NC showed less mineralized surface layers than specimens of AmF and NaF. After second demineralization (E2), a (slightly) further increase in mineral loss could be observed for specimens of all groups (Table 2). Furthermore, for specimens of group SnF2/NaF, FF and NB secondary lesion bodies beyond the original lesion fronts could be observed.
Mean mineral density profiles of the enamel specimens after pH-cycling (E1) and after pH-cycling plus 14 days demineralization (E2) were assessed. Specimens of AmF and NaF revealed subsurface lesions without abrasive loss after E1 and specimens of SnF2/NaF, FF and NC showed lower mineralized surface layers than specimens of AmF and NaF. After E2 a (slightly) further increase in mineral loss could be observed for specimens of all groups. Furthermore, for specimens of group SnF2/NaF, FF and NB secondary lesion bodies beyond the original lesion fronts could be observed
TMR—changes in mineral loss
After pH-cycling (E1) specimens of AmF, NaF and SnF2/NaF remineralized and specimens of FF and NB demineralized as compared to baseline. However, significant changes in lesion mineral content between baseline and E1 (i.e., remineralization) were only observed for AmF and NaF (p < 0.05, t test). After second demineralization (E2) although only AmF and NaF remineralized, significant changes in lesion mineral content were observed for all groups (p < 0.05, t test) (Table 2). For comparisons between the groups NaF and AmF showed significantly different ∆∆Z E1 and ∆∆Z E2 values compared to NB and FF (p < 0.05; ANOVA test) (Table 2).
TMR—changes in lesion depth
After pH-cycling (E1) specimens of AmF, NaF, SnF2/NaF and FF showed a decrease and specimens of NB showed an increase in lesion depth. However, significant changes in lesion depths between baseline and E1 measurements were only observed for NaF (p < 0.05, t test).
After second demineralization (E2) although only AmF and NaF showed a decrease in lesion depths, significant changes in lesion depths were observed for all groups except for AmF (p < 0.05, t test) (Table 2).
For comparisons between the groups no significant differences could be observed for ∆LDE1. After second demineralization NaF and AmF showed significantly different ∆LDE2 values compared to NB and FF (p < 0.05; ANOVA test), whereas no significant difference for ∆LDE2 could be observed between SnF2/NaF and NB or FF (Table 2).
Free fluoride and electron microprobe analysis
The percentages of free fluoride in relation to given total fluoride concentration (manufacture’s information) (SD) were 89 (2.5) % for AmF, 85 (0.8) % for NaF and 86 (0.8) % for SnF2/NaF. For the fluoride-free dentifrice no fluoride was measured. The maximum percentage of stannous in relation to total ions in the enamel surface layer was 0.27 wt% after pH-cycling and 0.25 wt% after second demineralization. No stannous could be detected at depths greater than 85 and 65 µm, respectively (Fig. 3).
The content of stannous (Sn) being incorporated in the enamel up to 85 µm depth of specimens in group SnF2/NaF was measured using a JEOL Superprobe JXA 8900 electron microprobe. For both experimental setups (E1 and E2) three specimens of group SnF2/NaF were prepared and analyzed in triplicates. The percentage (wt%) of stannous in relation to total ions present in enamel at each depth was determined
Discussion
The present in vitro study compared the effect of a stabilized stannous fluoride/sodium fluoride dentifrice containing sodium hexametaphosphate with those of a regular solely sodium fluoride-containing and amine fluoride-containing dentifrice on the prevention of enamel caries lesions progression in a pH-cycling model. Both dentifrices with either amine or sodium fluoride promoted remineralization significantly compared to fluoride-free dentifrice or non-brushing after pH-cycling as well as after a further demineralization. SnF2/NaF dentifrice could not promote considerable remineralization in this biofilm-free pH-cycling model partially rejecting our hypothesis that also SnF2/NaF containing dentifrice would significantly promote remineralization compared to fluoride-free dentifrice or non-brushing.
For specimens treated with AmF and NaF, a pronounced mineral gain was observed after pH-cycling and after second demineralization compared to SnF2/NaF, FF and NB treatments—showing significantly higher changes in mineral loss than FF and NB. In order to interfere in the dynamics of dental caries formation different “reservoirs” of fluoride have been described [28]. However, the adsorbed fluoride is presumed to exhibit the main effect for caries prevention [28]. In the presence of free or soluble fluoride, hydroxyapatite will behave as fluorhydroxyapatite during future dissolution episodes as it shifts the critical pH for demineralization approximately 0.5–1.0 units to a more acidic critical pH [29]. The more fluoride is incorporated in the structure of the crystals, the higher the decrease in the critical pH of tooth mineral. Fluoride of amine and sodium fluoride-containing dentifrices could probably easily be incorporated in the structure presumably shifting the critical pH below the pH of our demineralization solution, hampering further mineral loss and an increase in lesion depth. Contrastingly, fluoride of stannous fluoride/sodium fluoride-containing dentifrice might not have been incorporated in the structure so easily since SnF2 induces the formation of a relatively acid-resistant surface precipitate, limiting or delaying the direct contact of the acid with the mineral and presumably also the replacement of OH− by F− in hydroxyapatite [8, 13, 30]. The relatively acid-resistant surface precipitate seems to obstruct demineralization as well as remineralization. This is in agreement with our results showing almost no change in mineral loss and lesion depth in group SnF2/NaF. Nonetheless, when simulating the progression of enamel caries lesions in the worst-case scenario (second demineralization) this surface layer did not seem to protect the enamel for further mineral loss when compared to treatments AmF and NaF. Fluoride of amine or sodium fluoride forming ‘CaF2-like’ materials on the enamel surface protected the enamel surfaces at the first time and probably dissolved slowly over the time leading to a slight demineralization during 14 days of demineralization. Although the surface precipitate being formed by SnF2 probably dissolved slowly over the time as well, the surface precipitate inhibited the incorporation of F− during remineralization before resulting in a smaller pool of fluoride that effectively protects the crystal from dissolution during the demineralization period. Thus, significantly more mineral loss for treatment SnF2/NaF compared to AmF and NaF could be observed after second demineralization. Nonetheless, SnF2/NaF showed a trend to hamper further demineralization compared to FF and NB.
Several studies demonstrated that acidic dentifrices have a greater capacity to enhance the penetration of mineral ions during remineralization compared with neutral dentifrices [31]. Thus, the deposition of mineral ions into the body of the lesion increases, resulting in a (significantly) reduced lesion depth [32]. In the present study the fluoride-free dentifrice (FF) revealed an alkaline pH, whereas the three fluoride-containing dentifrices (AmF, NaF SnF2/NaF) revealed an acidic pH. The results of the AmF and NaF containing dentifrices were in agreement with previous studies [31, 32]; the more acidic dentifrice induced a higher remineralization than the less acidic one (while showing the same fluoride content). Interestingly, pH and fluoride (/free fluoride) content of the SnF2/NaF dentifrice were at the same level when compared to AmF and NaF; however, SnF2/NaF could not promote remineralization considerably. Therefore, it might be speculated that the effect of the acidic pH of the dentifrice (enhancing mineral diffusion) [31, 32] is overcome by the effect of the relative acid-resistant surface precipitate (hampering mineral diffusion) [8, 13, 30]. Furthermore, it might be speculated that the effect of increasing capacity to enhance remineralization with increasing acidity might be observed for certain fluoride compounds but not for all of them (e.g., SnF2). However, this hypothesis cannot be answered with the result of the present study.
In the mean mineral density profiles, lower mineralized surface layers were detected for non-brushed specimens as well as for specimens brushed with FF and SnF2/NaF. This might be interpreted as an indicator for abrasive losses during the pH-cycling period or the reduced presence of free fluoride at the sample–solution interface. In the present study, brushing was performed manually without an automatically controlled constant force and pace. Nonetheless, the author (HZ) was trained and calibrated prior to the pH-cycling period to brush all groups similarly. Therefore, abrasives losses would either be observed in all groups being brushed (which was not the case for AmF and NaF) or in none since abrasive ingredients of all dentifrices were similar. Furthermore, a lower mineralized surface layers could also be observed in specimens not being brushed at all. Thus, a complete abrasive surface loss in NB as well as in FF and SnF2/NaF can be excluded.
By forming a mineralized surface layer, fluoride of amine and sodium fluoride-containing dentifrices protected further demineralization and enhanced remineralization [20, 21]. Contrastingly, mean mineral density profiles showed that in the other groups a mineralized surface layer could only be observed in a very limited extent. A less mineralized surface layer was not only observed in the absence of fluoride (FF and NB), but also in presence of SnF2/NaF. Since fluoride incorporation in group SnF2/NaF was probably inhibited (as discussed above) less mineralization of the lesion was observed. Thus, not only FF and NB but also SnF2/NaF showed a less mineralized surface layer than specimens of AmF and NaF.
The model used in this study mimics the dynamics of enamel caries formation. Demineralizing challenges were followed by remineralizing challenges. The experiment was a basic research study using a relatively mild demineralizing model being intended to result in slight net demineralization when no treatment (non-brushing) was carried out. The number of cycles in the protocol was different from other pH-cycling studies using up to 6 cycles [33, 34], but still allowed fluoride to act on the demineralizing as well as on the remineralizing process of dental caries. In the second part of our study the specimens were demineralized for 14 days to mimic the (fast) progression of enamel caries lesions in the worst-case scenario and to investigate whether one of the fluoride compounds showed protective effect against further demineralization. This might be an unrealistic sequence, since in the oral environment of most of the individuals demineralization is interrupted several times a day [29]. However, we demineralized the specimens for further 14 days to compare fluoride compounds when remineralization cannot keep up with demineralization.
Former in vitro [34] and in situ studies [35–39] showed that ‘baseline substrate conditions’ directly affects lesion response. What all authors concluded is that treatment groups should therefore be well-balanced with respect to baseline mineral loss and lesion depth. In the present study, specimens with a mean (SD) baseline mineral loss of 3393 (683) vol% × µm were selected to compare three dentifrices differing in fluoride compounds on a wide range of pre-demineralized bovine enamel. At first sight this seems to be in contrast to the conclusion of the former studies, since ‘baseline substrate conditions’ certainly also influenced lesion response in the present study. Nonetheless, mean baseline mineral loss and lesion depth did not differ significantly between experimental groups. Thus, lesion response of the specimens within one experimental group probably differed, but between the experimental groups lesion responses were not affected differently.
Changes in mineral loss and lesion depth were calculated using TMR. With this technique mineral content of tooth material not being thicker than 100 µm can be quantified [40]. Using a polychromatic radiation as X-ray source an Al–Zn alloy step wedge can be used for mineral quantification since absorption spectra of both, tooth material and step wedge, are approximately similar [41]. Although the change in composition of the enamel and dentin does not affect the quantification, incorporation of polyvalent metal compounds may affect the quantification considerably [42]. Due to a higher absorption coefficient of stannous compared with hydroxyapatite [42], stannous incorporated in the enamel or deposited in the surface precipitate could result in a ‘falsely’ higher mineral content being interpreted as ‘enhanced’ remineralization. Previous studies demonstrated that a certain amount of stannous can be deposited as surface precipitates [30, 43] or incorporated in the enamel [43]. In the first study, investigating the effects of stannous (as SnCl2 solution) on etched enamel, the amount of stannous deposited in the surface precipitate was up to 8.9 wt% directly after application and up to 8.2 wt% after 6 days of water storage [30]. In the second study, using solutions with different concentration of SnCl2, 18.3 wt% stannous was detected as precipitate and 6 wt% incorporated into enamel [43]. The deposition as precipitates and the incorporation in the enamel structure were measured after cycling the specimens for 10 days. Contrastingly, in the present study the maximum percentage of stannous in relation to total ions present in enamel was much smaller, being of only 0.27 wt% after pH-cycling and 0.25 wt% after second demineralization. Even though, it is theoretically possible that even this very small amount of stannous could have influenced mineral quantification in the present investigation, it does not seem to have significantly changed our results. Actually if it would have influenced, this would be observable as enhanced mineralization for the SnF2/NaF group, resulting from the higher X-ray absorption. This was not the case. In fact, even with a supposedly higher absorption, the SnF2/NaF group showed less changes in mineral content (less remineralization in E1, or demineralization in E2) than both NaF and AmF groups and was not significantly different from FF. Thus indicating that the small amount of stannous incorporated to the enamel here did not notably influence the TMR results.
The anticariogenic effect of SnF2 is based on two mechanisms. Firstly, SnF2 delivers free or soluble fluoride to enhance remineralization as discussed above. Secondly, stannous ions, like other metals, exhibit an antibacterial and antiplaque effect. Stannous attaches to the bacterial surface, inhibits bacterial colonization, penetrates into the bacterial cytoplasm and interferes with the bacterial metabolism delaying bacterial growth [14, 44]. In this study a biofilm-free pH-cycling model was used. Thus, the antibacterial and antiplaque effect could not be analyzed. When analyzing both mechanisms simultaneously, it could previously be shown that stabilized stannous fluoride/sodium fluoride dentifrice containing sodium hexametaphosphate significantly reduces the 2-year caries increment relative to sodium fluoride [10]. It may, therefore, be assumed that in an in situ or in vivo study the tested SnF2/NaF dentifrice led to an enhanced remineralization as seen for the other fluoride compounds.
In conclusion, both dentifrices with either amine or sodium fluoride promoted remineralization, whereas a stabilized stannous fluoride/sodium fluoride dentifrice containing sodium hexametaphosphate dentifrice did not promote remineralization in a biofilm-free pH-cycling model.
References
Pessan JP, Toumba KJ, Buzalaf MA. Topical use of fluorides for caries control. Monogr Oral Sci. 2011;22:115–32. doi:10.1159/000325154.
Lippert F. An introduction to toothpaste—its purpose, history and ingredients. Monogr Oral Sci. 2013;23:1–14. doi:10.1159/000350456.
Mellberg JR. Fluoride dentifrices: current status and prospects. Int Dent J. 1991;41(1):9–16.
Dadamio J, Laleman I, Quirynen M. The role of toothpastes in oral malodor management. Monogr Oral Sci. 2013;23:45–60. doi:10.1159/000350472.
Muhler JC, Radike AW. Effect of a dentifrice containing stannous fluoride on dental caries in adults. II. Results at the end of 2 years of unsupervised use. J Am Dent Assoc. 1957;55(2):196–8.
Miller AJ. Oral health of United States adults: the national survey of oral health in D.S. employed adults and seniors: 1985–1986. National findings. National Institutes of Health. 1987;Publication no. NIH-87-2868.
Schiff T, He T, Sagel L, Baker R. Efficacy and safety of a novel stabilized stannous fluoride and sodium hexametaphosphate dentifrice for dentinal hypersensitivity. J Contemp Dent Pract. 2006;7(2):1–8.
Huysmans MC, Jager DH, Ruben JL, Unk DE, Klijn CP, Vieira AM. Reduction of erosive wear in situ by stannous fluoride-containing toothpaste. Caries Res. 2011;45(6):518–23. doi:10.1159/000331391.
Beiswanger BB, Doyle PM, Jackson RD, Mallatt ME, Mau M, Bollmer BW, et al. The clinical effect of dentifrices containing stabilized stannous fluoride on plaque formation and gingivitis—a six-month study with ad libitum brushing. J Clin Dent. 1995;6 Spec No:46–53.
Stookey GK, Mau MS, Isaacs RL, Gonzalez-Gierbolini C, Bartizek RD, Biesbrock AR. The relative anticaries effectiveness of three fluoride-containing dentifrices in Puerto Rico. Caries Res. 2004;38(6):542–50. doi:10.1159/000080584.
Faller RV, Eversole SL, Saunders-Burkhardt K. Protective benefits of a stabilised stannous-containing fluoride dentifrice against erosive acid damage. Int Dent J. 2014;64(Suppl 1):29–34. doi:10.1111/idj.12100.
Ganss C, Lussi A, Grunau O, Klimek J, Schlueter N. Conventional and anti-erosion fluoride toothpastes: effect on enamel erosion and erosion-abrasion. Caries Res. 2011;45(6):581–9. doi:10.1159/000334318.
Schlueter N, Klimek J, Ganss C. Effect of stannous and fluoride concentration in a mouth rinse on erosive tissue loss in enamel in vitro. Arch Oral Biol. 2009;54(5):432–6. doi:10.1016/j.archoralbio.2009.01.019.
Tinanoff N. Review of the antimicrobial action of stannous fluoride. J Clin Dent. 1990;2(1):22–7.
Faller RV, Eversole SL, Yan J. Anticaries potential of a stabilized stannous-containing sodium fluoride dentifrice. Am J Dent. 2010;23 Spec No B:32B–8B.
Lippert F, Newby EE, Lynch RJ, Chauhan VK, Schemehorn BR. Laboratory assessment of the anticaries potential of a new dentifrice. J Clin Dent. 2009;20(2):45–9.
White DJ, Faller RV, Bowman WD. Demineralization and remineralization evaluation techniques—added considerations. J Dent Res. 1992;71 Spec No:929–33.
Arends J, ten Bosch JJ. Demineralization and remineralization evaluation techniques. J Dent Res. 1992;71 Spec No:924–8.
Arends J, Schuthof J, Jongebloed WG. Microhardness indentations on artificial white spot lesions. Caries Res. 1979;13(5):290–7.
Ten Cate JM. In vitro studies on the effects of fluoride on de- and remineralization. J Dent Res. 1990;69: Spec No:614–9 (discussion 34-6).
Buchalla W. Histological and clinical Appearance of Caries. In: Meyer-Lueckel H, Paris S, Ekstrand K, editors. Caries management-science and clinical practice. Stuttgart: Thieme; 2013.
ten Cate JM, Featherstone JD. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit Rev Oral Biol Med. 1991;2(3):283–96.
Tenuta LM, Cury JA. Laboratory and human studies to estimate anticaries efficacy of fluoride toothpastes. Monogr Oral Sci. 2013;23:108–24. doi:10.1159/000350479.
Buskes JA, Christoffersen J, Arends J. Lesion formation and lesion remineralization in enamel under constant composition conditions. A new technique with applications. Caries Res. 1985;19(6):490–6.
Meyer-Lueckel H, Wierichs RJ, Schellwien T, Paris S. Remineralizing efficacy of a CPP-ACP cream on enamel caries lesions in situ. Caries Res. 2015;49(1):56–62. doi:10.1159/000363073.
Esteves-Oliveira M, Pasaporti C, Heussen N, Eduardo CP, Lampert F, Apel C. Rehardening of acid-softened enamel and prevention of enamel softening through CO2 laser irradiation. J Dent. 2011;39(6):414–21. doi:10.1016/j.jdent.2011.03.006.
Cury JA, Francisco SB, Simoes GS, Del Bel Cury AA, Tabchoury CP. Effect of a calcium carbonate-based dentifrice on enamel demineralization in situ. Caries Res. 2003;37(3):194–9.
Arends J, Christoffersen J. Nature and role of loosely bound fluoride in dental caries. J Dent Res. 1990;69 Spec No:601–5 (discussion 34-6).
Amaechi BT, van Loveren C. Fluorides and non-fluoride remineralization systems. Monogr Oral Sci. 2013;23:15–26. doi:10.1159/000350458.
Ellingsen JE. Scanning electron microscope and electron microprobe study of reactions of stannous fluoride and stannous chloride with dental enamel. Scand J Dent Res. 1986;94(4):299–305.
Kondo KY, Buzalaf MA, Manarelli MM, Delbem AC, Pessan JP. Effects of pH and fluoride concentration of dentifrices on fluoride levels in saliva, biofilm, and biofilm fluid in vivo. Clin Oral Investig. 2015;. doi:10.1007/s00784-015-1583-4.
Yamazaki H, Margolis HC. Enhanced enamel remineralization under acidic conditions in vitro. J Dent Res. 2008;87(6):569–74.
ten Cate JM, Buijs MJ, Miller CC, Exterkate RA. Elevated fluoride products enhance remineralization of advanced enamel lesions. J Dent Res. 2008;87(10):943–7.
ten Cate JM, Exterkate RA, Buijs MJ. The relative efficacy of fluoride toothpastes assessed with pH cycling. Caries Res. 2006;40(2):136–41. doi:10.1159/000091060.
ten Cate JM. In situ models, physico-chemical aspects. Adv Dent Res. 1994;8(2):125–33.
Lippert F, Lynch RJ, Eckert GJ, Kelly SA, Hara AT, Zero DT. In situ fluoride response of caries lesions with different mineral distributions at baseline. Caries Res. 2011;45(1):47–55. doi:10.1159/000323846.
Strang R, Damato FA, Creanor SL, Stephen KW. The effect of baseline lesion mineral loss on in situ remineralization. J Dent Res. 1987;66(11):1644–6.
Mellberg JR. Relationship of original mineral loss in caries-like lesions to mineral changes in situ. Short communication. Caries Res. 1991;25(6):459–61.
Schafer F, Raven SJ, Parr TA. The effect of lesion characteristic on remineralization and model sensitivity. J Dent Res. 1992;71 Spec No:811–3.
Thomas RZ, Ruben JL, de Vries J, ten Bosch JJ, Huysmans MC. Transversal wavelength-independent microradiography, a method for monitoring caries lesions over time, validated with transversal microradiography. Caries Res. 2006;40(4):281–91. doi:10.1159/000093186.
Herkstroter FM, Ten Bosch JJ. Wavelength-independent microradiography: a method for non-destructive quantification of enamel and dentin mineral concentrations using polychromatic X-rays. J Dent Res. 1990;69(8):1522–6.
Arndt UW, Creagh DC, Deslattes RD, Hubbell JH, Indelicato P, Kessler Jr. EG et al. Chapter 4.2. International Tables for Crystallography. 2006 p 191–258.
Schlueter N, Hardt M, Lussi A, Engelmann F, Klimek J, Ganss C. Tin-containing fluoride solutions as anti-erosive agents in enamel: an in vitro tin-uptake, tissue-loss, and scanning electron micrograph study. Eur J Oral Sci. 2009;117(4):427–34. doi:10.1111/j.1600-0722.2009.00647.x.
Rolla G, Ellingsen JE. Clinical effects and possible mechanisms of action of stannous fluoride. Int Dent J. 1994;44(1 Suppl 1):99–105.
Acknowledgments
This study was funded by the authors and their institution.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
R. J. Wierichs and H. Zelck contributed equally to the study.
Rights and permissions
About this article
Cite this article
Wierichs, R.J., Zelck, H., Doerfer, C.E. et al. Effects of dentifrices differing in fluoride compounds on artificial enamel caries lesions in vitro. Odontology 105, 36–45 (2017). https://doi.org/10.1007/s10266-016-0233-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10266-016-0233-x