Abstract
Recent reports of long-term bisphosphonate-treated patients developing cortical fractures have raised concerns that such fractures may relate to excessive suppression of bone turnover after prolonged use of these drugs. To evaluate the bone histology of patients presenting with cortical fractures after bisphosphonate therapy, we conducted a retrospective analysis of patients treated at Washington University Bone Health Program presenting with a history of low-energy cortical fractures (femoral shaft, pelvis, rib, metatarsal, and ankle), who had received bisphosphonates for at least two consecutive years and had undergone bone biopsy. Fifteen of 54 patients who underwent bone biopsy between November 2004 and March 2007 met the criteria. Of these, 10 patients had findings of suppressed trabecular bone remodeling, as demonstrated by lack of double tetracycline labels. There were no significant differences in bone density, clinical features, and biochemical features between those with suppressed turnover and the other five subjects with normal remodeling. However, the low-turnover group had received bisphosphonates (primarily alendronate) for a significantly longer duration (6.5 ± 0.6 vs. 3.9 ± 0.8 years, P = 0.02). Thus, about two-thirds of patients presenting with cortical fractures while on long-term treatment with bisphosphonates had suppressed turnover. Since the prevalence of such histological findings in nonfracture patients remains unknown, the impact of suppressed bone turnover on the development of cortical fractures cannot be determined. Considering the widespread use of bisphosphonates, it appears that the overall risk of cortical fractures is low. However, there may be a subset of as yet unidentified patients who could be predisposed to this complication.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The skeleton is a dynamic organ whose mechanical strength depends upon continued replacement of effete bone with new. This process, known as “remodeling” or “turnover,” involves the coupled activities of bone-resorbing osteoclasts and bone-forming osteoblasts. Thus, agents which inhibit osteoclast function also retard bone remodeling.
Bisphosphonates are the agents most prescribed for preventing and treating osteoporosis. They attenuate bone degradation, thereby increasing bone mineral density (BMD) and decreasing the incidence of fractures via inhibition of bone turnover [1–4]. While the protective effect of bisphosphonates against fragility fractures is established, safety concerns have recently emerged regarding long-term treatment with these drugs. Such concerns apply to alendronate in particular since this agent has been available for more than a decade throughout the world and it appears more potent than risedronate at inhibiting bone turnover at the doses used in clinical practice [5]. The prolonged suppression of resorption by continuous, long-term use of bisphosphonates also indirectly inhibits bone formation. A potential negative consequence of these effects is decreased ability to repair microdamage or rejuvenate old bone, thus predisposing to structural failure. Although this detrimental sequence of events remains to be established, some bisphosphonate-treated patients develop fractures at mainly cortical sites, such as the femoral shaft [6–11]. In many such individuals, bone turnover is severely suppressed [6, 7]. Typically, these individuals had received bisphosphonates for a long time, and some had comorbid conditions treated with other medications, such as glucocorticoids, which also posses bone-suppressive properties [6]. Hence, whether prolonged treatment with bisphosphonates excessively suppresses bone turnover, thereby compromising skeletal strength, remains to be determined.
To gain further insight on this issue, we retrospectively reviewed the clinical and pathological records of patients treated at the Bone Health Program of Washington University who underwent bone biopsy from November 2004 to March 2007 and who presented with low-energy cortical fractures while on bisphosphonate therapy. We found that two-thirds of patients who presented with this type of fracture during or after bisphosphonate treatment had histological evidence of suppressed bone turnover.
Subjects and Methods
Study Design
This is a retrospective review of patients in the Bone Health Program at Washington University School of Medicine.
Study Patients
Eligible patients were those who presented with low-energy fractures at mainly cortical sites, had received bisphosphonates for at least 2 years, and had a bone biopsy. Informed consent for data publication was obtained from all who could be contacted. In other circumstances, a waiver of consent was obtained from the Human Research Protection Office of Washington University in accordance with the guidelines for studies involving human subjects.
Clinical Data
Average daily calcium and vitamin D intake were estimated from the recorded consumption of supplements and foodstuffs. Smoking was expressed as the number of 20-cigarette packs smoked per day multiplied by the number of years of smoking (pack-years). Although there was no documentation of alcohol consumption, most patients were asked if they had a history of alcoholism. A family predisposition to osteoporosis was coded as positive if a primary blood relative had been diagnosed with osteoporosis, kyphosis, or fragility fractures in the absence of secondary causes. Body mass index (BMI) was calculated as the weight in kilograms divided by the square of the height in meters. The presence of a fracture was verified by review of radiological records by either one of two authors (R. A.-V., K. D.).
Bone Biopsy
All patients received 250 mg oral tetracycline four times daily on days 23–21 and 6–4 prior to biopsy, a standard double-labeling protocol for assessing dynamic histomorphometric indices of bone formation. All participants were specifically instructed to strictly comply with tetracycline administration. Transiliac bone biopsy was performed using a 5-mm internal diameter trephine (Bordier trephine; Lepine, Lyon, France). The nondecalcified specimen, fixed in 70% alcohol to preserve tetracycline fluorescence, was embedded in methylmethacrylate and sectioned using a rotary microtome equipped with a tungsten–carbide microtome blade. Sections were stained with Goldner trichrome to identify osteoid and osteoblasts, von Kossa to distinguish calcified from noncalcified bone (better assessment for osteoid), and tartrate-resistant acid phosphatase (TRAP) for evaluation of osteoclasts. The presence or absence of fluorescent tetracycline labels was determined by fluorescent microscopy. The nomenclature of the measured and calculated variables is in accordance with the standards established by the Committee on Bone Histomorphometry of the American Society for Bone and Mineral Research [12]. Biopsies of patients meeting inclusion criteria were independently examined by two investigators (D. N., S. T.). Based on the histological findings, patients were diagnosed as having suppressed bone turnover when fluorescent double tetracycline labels were absent in the trabecular bone, indicating lack of detectable newly formed mineralized bone (Fig. 1) [6].
Histological analysis of trabecular bone from representative normal turnover (A, C) and suppressed bone turnover (B, D) patients. A, B Gomori trichrome-stained sections. C, D Unstained sections viewed under fluorescence to identify tetracycline labels. The normal turnover patient has osteoid (red band around trabeculum) and a row of osteoblasts (between arrows) and both single and double tetracycline labels. The suppressed bone turnover patient has no osteoid lining trabeculae, no osteoblasts, and no single or double tetracycline labels
Biochemical Studies
Serum calcium and alkaline phosphatase were measured by an autoanalyzer; 25-hydroxyvitamin D (Bayer, Leverkusen, Germany), intact parathyroid hormone (iPTH, Bayer), and osteocalcin (CIS Bio International, Lyon, France) were assessed by radioimmunoassay; urinary N-telopeptide crosslinks (NTx) in a 24-h urine sample was assessed by ELISA (Vitros ECI, Bridgeport, CT) and normalized to creatinine. All biochemical studies were performed from samples obtained a few weeks prior to bone biopsy and at the time of first visit for a fracture evaluation. Except for two patients, all were still taking bisphosphonates at the time of sample collection. These tests were measured through the Clinical Laboratory of Barnes-Jewish Hospital in St. Louis.
BMD
BMD was assessed by dual-energy X-ray absorptiometry (Delphi; Hologic, Waltham, MA). The lumbar spine was examined in the anteroposterior projection, and its BMD was calculated as the average of L1–L4 vertebrae. The nondominant hip was used for proximal femur scans, and values were calculated on the total femur, femoral neck, trochanter, and intertrochanteric area. The coefficient of variability of this technique using the Hologic Delphi densitometer is 1.09% for the lumbar spine and 1.2% for the total femur in our center [13].
Statistical Analysis
Results were expressed as the mean ± SE. Group differences for continuous variables were compared using Student’s t-test for normally distributed variables and the Mann–Whitney test for those that are not normally distributed. Categorical variables (e.g., family history of osteoporosis) were compared using χ2. Data were managed using Excel 2000 (Microsoft, Redmond, WA) and analyzed using Statgraphic Plus 5.0 (Manugistic, Rockville, MD).
Results
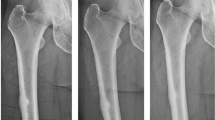
Among patients referred to the Bone Health Program of Washington University, 54 (44 women and 10 men) underwent bone biopsy from November 2004 to March 2007 for various indications. As detailed in Table 1, 15 of these patients (12 women and three men) presented with a history of fractures at mainly cortical sites, namely, femoral shaft (n = 6); femoral shaft, ribs, and metatarsals (n = 1); metatarsals (n = 2); ribs (n = 2); ribs and metatarsals (n = 1); pelvis (n = 1); fibula (n = 1); and ankle (n = 1). The radiographic image in Fig. 2 illustrates a transverse “chalk-stick” fracture of the femoral shaft (patient 2), which occurred in an otherwise normal-appearing cortical bone. Furthermore, patients 1, 2, 5, and 6 had a history of delayed fracture healing; and patient 5 had suffered a new non-union fracture of the femoral shaft 15 months following open reduction and internal fixation of the original fracture, while remaining on alendronate. Based on the absence of double tetracycline labels in the trabecular bone, 10 of the 15 patients were diagnosed as having suppressed remodeling (Table 1) in accordance with the definition suggested by Odvina et al. [6]. The majority of patients in the entire cohort had BMD that did not fulfill the World Health Organization’s criteria for osteoporosis [14–16], although most would have met the accepted guidelines for pharmacological treatment for osteoporosis at the time of diagnosis [16].
By design, all 15 patients had a history of bisphosphonate therapy. Eight of 10 in the suppressed turnover group were taking the drugs at the time of biopsy, while patients 1 and 4 had ceased treatment 1 and 2 years earlier, respectively. Patient 1 had been previously reported [7]. He underwent sequential biopsies which documented transition from high turnover to suppressed bone remodeling after 6 years of alendronate therapy. Fourteen patients received alendronate, and one (patient 14) had been treated with risedronate. Other than supplemental calcium and vitamin D, bisphosphonates were the only drugs with known skeletal effects administered to five of the 10 patients with suppressed bone turnover. The other five subjects in this group also received drugs with potential bone-suppressive effects including corticosteroid inhalers with (n = 1) and without (n = 2) a TNF-α inhibitor, oral corticosteroids with (n = 1) and without (n = 1) estrogen, and a selective estrogen receptor modulator (n = 1) [17–19]. However, four of five subjects with normal turnover also received additional drugs with bone remodeling–suppressive effects, including estrogen and oral corticosteroids (n = 1), a TNF-α inhibitor and testosterone (n = 1), and a steroid inhaler (n = 2).
The biopsies of 14 patients were sufficiently intact to perform detailed histomorphometric analysis (Table 2). Meaningful quantitative data could not be obtained from the trabecular compartment of the biopsy of patient 7. As expected, the number of osteoblasts and the extent of osteoid-covered surfaces were both significantly decreased in patients with suppressed bone turnover. While the number of trabecular osteoclasts was reduced in the biopsies of patients with suppressed remodeling, the difference was not statistically significant relative to the subjects with normal turnover. Despite the fact that some double tetracycline labels were present in the cortex of the majority of suppressed turnover biopsies, osteoblast number and osteoid surface mirrored those of the trabecular compartment, and in this case the number of osteoclasts was significantly reduced. On the other hand, bone volume/tissue volume, trabecular thickness, and cortical thickness did not differ among patients with different turnover states. Furthermore, there were no significant differences in histomorphometric findings between patients with suppressed bone turnover who were taking other medications that would potentially suppress remodeling and those who were only receiving bisphosphonates (not shown).
Table 3 shows the clinical features of the study population. There were no significant differences in age, BMI, BMD, calcium and vitamin D intake, or serum calcium, PTH, and 25-hydroxyvitamin D between patients with suppressed or normal turnover. Nonetheless, subjects with suppressed turnover had received bisphosphonates for a significantly longer period. Despite histomorphometric features of decreased bone formation, serum alkaline phosphatase was normal in patients with suppressed bone turnover and comparable to that of subjects with normal remodeling. Serum osteocalcin and urine NTx were determined in six and eight patients, respectively. Of the five suppressed turnover patients in whom serum osteocalcin was measured, two had levels beneath the laboratory’s reference range (7.2–27.9 ng/ml); and three of six patients in this group had urine NTx below the premenopausal reference range (19–63 nmol BCE/mmol creatinine) [20, 21]. Serum osteocalcin and urine NTx were measured in only one normal remodeling patient.
Discussion
Bone remodeling is an ever-occurring process characterized by the sequential appearance of osteoclasts and osteoblasts at a particular location in the mammalian skeleton. As such, there is a tethering of bone resorption and formation in which osteoblast activity requires that of preexisting osteoclasts. Thus, states of accelerated bone resorption, such as estrogen withdrawal or hyperparathyroidism, are also attended by enhanced formation, with bone mass reflecting the relative rates of each process. While inhibition of bone resorption in states of high remodeling increases bone mass, prolonged suppression of bone resorption eventuates in inhibited bone formation and, thus, suppressed skeletal turnover. Because the principal function of remodeling is probably to replace old bone with new, excessive and prolonged inhibition of this process may result in a skeleton with compromised mechanical strength relative to bone mass, as occurs with glucocorticoid therapy, which inhibits osteoblast function [21].
Bisphosphonates are stable pyrophosphate analogues which avidly bind bone mineral. These agents have revolutionized the treatment of osteoporosis, particularly in light of current evidence challenging the safety of hormone replacement therapy [22]. They reduce fracture incidence and are generally safe and effective for at least 10 years of continuous therapy [23, 24]. However, the long-term use of these drugs has been called into question after reports of suppressed bone turnover associated with low-energy cortical fractures [6, 7]. Administration of large doses of bisphosphonates to dogs increases “microcrack” density [25], and in humans, microdamage accumulation may increase fracture risk [26, 27]. Interestingly, alendronate appears to be more inductive of microdamage accumulation than risedronate, which may reflect their various binding affinities to hydroxyapatite and, thus, different uptake and persistence in bone [28].
Despite the development of biomarkers of bone turnover, the nondecalcified iliac crest biopsy is the most reliable indicator of the state of an individual patient’s bone remodeling status at the tissue and cell levels. This is particularly true when the patient is administered tetracycline before the procedure. These antibiotics avidly bind newly deposited bone mineral, and their presence is identified by florescent microscopy. Hence, administration of time-spaced courses of the antibiotic yields parallel fluorescent bands at sites of skeletal synthesis and, thus, enables quantification of the rate of bone formation. Bisphosphonates directly inhibit the activity of osteoclasts after these cells mobilize the drug from bone. This arrest of osteoclast function translates into blunted bone formation and general dampening of the remodeling process. Inhibitory effects on osteoblast activity and survival by bisphosphonates occur in vitro but at high concentrations relative to the therapeutic doses used in osteoporosis [29]. However, bisphosphonates bind to bone mineral and are released upon bone resorption, raising the possibility that local concentration of the active compound sufficient to inhibit osteoblastic cells may be reached after long-term exposure to bisphosphonates.
Two-thirds of our patients who developed cortical fractures had histological findings of suppressed bone turnover and had received bisphosphonate therapy for a significantly longer period of exposure than those with cortical fractures but with normal bone turnover. Our results are consistent with a previous report of nine alendronate-treated patients with attenuated remodeling who presented with fractures at mainly cortical sites [6]. Based on the magnitude of suppression of histological parameters of bone turnover observed in that series, the authors proposed a definition of severe suppression of bone turnover, to distinguish it from adynamic bone disease of renal failure. While these accumulated cases suggest that some patients who receive bisphosphonates (primarily alendronate) for several years may develop cortical fractures associated with diminished bone turnover, the retrospective nature of our analysis, as well as that of Odvina et al. [6], does not provide an unambiguous link between attenuated remodeling and increased risk of cortical fractures. In fact, one-third of our patients had normal bone formation, suggesting that factors other than suppressed bone turnover are involved in determining low-energy cortical fracture in bisphosphonate-treated patients. Furthermore, since sequential biopsies were obtained in only one patient, it is not possible to determine whether suppression of bone turnover is the consequence of prolonged bisphosphonate treatment or a preexisting condition. Moreover, half of our patients with suppressed bone turnover were receiving drugs, such as corticosteroids, estrogen, selective estrogen receptor modulators, or TNF-α inhibitors, which also inactivate remodeling. Notably, previous investigators reported that 4% of patients treated with alendronate for glucocorticoid-induced osteoporosis [30] and 3.3% of patients who received a combined therapy of alendronate and hormone replacement therapy [31] had no double tetracycline labels. However, such drug combinations do not always suppress bone turnover as four of five patients with detectable double tetracycline label were receiving the same additional medications. Nevertheless, it is possible that in some individuals the bone-suppressive effects of these drugs may be aggravated when administered with other medications, such as glucocorticoids, with bone turnover–suppressing activity [17, 18]. This posture also underscores the need for biochemical markers with the sensitivity and precision to identify individuals with preexisting low bone turnover as the presently available skeletal biomarkers are limited by analytical and biological variability [20].
Our study has limitations. Since this is a case series, prospective data on bone turnover markers before, during, and after therapy are not available. Likewise, bone biopsies were taken after several years of treatment, and only in one case was a pretreatment biopsy available. Finally, concern for extreme skeletal fragility in our patients limited the use of a larger needle to obtain bone biopsy samples. Although a larger bioptic would have been preferable for making an accurate determination of low turnover, considering the consistency of our findings using the different staining procedures (with two sections for each), we are confident that our assessment of bone turnover was accurate.
Considering the number of individuals administered bisphosphonates, the complications addressed in this series are uncommon. Most importantly, there is little question that the risk/benefit ratio in osteoporotic patients is in favor of bisphosphonate therapy. Our findings suggest that a subset of bisphosphonate-treated individuals may be predisposed to cortical fractures, which in most cases are associated with suppressed bone remodeling. The challenge is their identification before and during therapy.
References
Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, Bauer DC, Genant HK, Haskell WL, Marcus R, Ott SM, Torner JC, Quandt SA, Reiss TF, Ensrud KE (1996) Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 348:1535–1541
Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, Adami S, Weber K, Lorenc R, Pietschmann P, Vandormael K, Lombardi A (2000) Alendronate for the treatment of osteoporosis in men. N Engl J Med 343:604–610
Liberman UA, Weiss SR, Broll J, Minne HW, Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J, Favus M (1995) Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 333:1437–1443
Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, Palermo L, Prineas R, Rubin SM, Scott JC, Vogt T, Wallace R, Yates AJ, LaCroix AZ (1998) Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 280:2077–2082
Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, Kagan R, Chen E, Petruschke RA, Thompson DE, de Papp AE (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20:141–151
Odvina CV, Zerwekh JE, Rao DS, Maalouf N, Gottschalk FA, Pak CY (2005) Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90:1294–1301
Armamento-Villareal R, Napoli N, Panwar V, Novack D (2006) Suppressed bone turnover during alendronate therapy for high-turnover osteoporosis. N Engl J Med 355:2048–2050
Lenart BA, Lorich DG, Lane JM (2008) Atypical fractures of the femoral diaphysis in postmenopausal women taking alendronate. N Engl J Med 358:1304–1306
Schneider JP (2006) Should bisphosphonates be continued indefinitely? An unusual fracture in a healthy woman on long-term alendronate. Geriatrics 61:31–33
Goh SK, Yang KY, Koh JS, Wong MK, Chua SY, Chua DT, Howe TS (2007) Subtrochanteric insufficiency fractures in patients on alendronate therapy: a caution. J Bone Joint Surg Br 89:349–353
Kwek EB, Goh SK, Koh JS, Png MA, Howe TS (2008) An emerging pattern of subtrochanteric stress fractures: a long-term complication of alendronate therapy? Injury 39:224–231
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Armamento-Villareal RC, Napoli N, Klug T, Civitelli R (2004) The oxidative metabolism of estrogen modulates response to ERT/HRT in postmenopausal women. Bone 35:682–688
Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K, Genant HK, Palermo L, Scott J, Vogt TM (1993) Bone density at various sites for prediction of hip fractures. The Study of Osteoporotic Fractures Research Group. Lancet 341:72–75
Melton LJ III, Atkinson EJ, O’Fallon WM, Wahner HW, Riggs BL (1993) Long-term fracture prediction by bone mineral assessed at different skeletal sites. J Bone Miner Res 8:1227–1233
(1998) Osteoporosis: review of the evidence for prevention, diagnosis and treatment and cost-effectiveness analysis. Executive summary. Osteoporos Int 8(suppl 4):S3–S6
Mazziotti G, Angeli A, Bilezikian JP, Canalis E, Giustina A (2006) Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol Metab 17:144–149
Lange U, Teichmann J, Muller-Ladner U, Strunk J (2005) Increase in bone mineral density of patients with rheumatoid arthritis treated with anti-TNF-alpha antibody: a prospective open-label pilot study. Rheumatology (Oxford) 44:1546–1548
Allali F, Breban M, Porcher R, Maillefert JF, Dougados M, Roux C (2003) Increase in bone mineral density of patients with spondyloarthropathy treated with anti-tumour necrosis factor alpha. Ann Rheum Dis 62:347–349
Delmas PD (2000) Markers of bone turnover for monitoring treatment of osteoporosis with antiresorptive drugs. Osteoporos Int 11(suppl 6):S66–S76
Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C (2003) Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 48:3224–3229
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 288:321–333
Bone HG, Hosking D, Devogelaer JP, Tucci JR, Emkey RD, Tonino RP, Rodriguez-Portales JA, Downs RW, Gupta J, Santora AC, Liberman UA (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199
Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, Satterfield S, Wallace RB, Bauer DC, Palermo L, Wehren LE, Lombardi A, Santora AC, Cummings SR (2006) Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA 296:2927–2938
Mashiba T, Hirano T, Turner CH, Forwood MR, Johnston CC, Burr DB (2000) Suppressed bone turnover by bisphosphonates increases microdamage accumulation and reduces some biomechanical properties in dog rib. J Bone Miner Res 15:613–620
Sobelman OS, Gibeling JC, Stover SM, Hazelwood SJ, Yeh OC, Shelton DR, Martin RB (2004) Do microcracks decrease or increase fatigue resistance in cortical bone? J Biomech 37:1295–1303
Zioupos P (2001) Accumulation of in vivo fatigue microdamage and its relation to biomechanical properties in ageing human cortical bone. J Microsc 201:270–278
Nancollas GH, Tang R, Phipps RJ, Henneman Z, Gulde S, Wu W, Mangood A, Russell RG, Ebetino FH (2006) Novel insights into actions of bisphosphonates on bone: differences in interactions with hydroxyapatite. Bone 38:617–627
Orriss IR, Key ML, Colston KW, Arnett TR (2009) Inhibition of osteoblast function in vitro by aminobisphosphonates. J Cell Biochem 106:109–118
Chavassieux PM, Arlot ME, Roux JP, Portero N, Daifotis A, Yates AJ, Hamdy NA, Malice MP, Freedholm D, Meunier PJ (2000) Effects of alendronate on bone quality and remodeling in glucocorticoid-induced osteoporosis: a histomorphometric analysis of transiliac biopsies. J Bone Miner Res 15:754–762
Bone HG, Greenspan SL, McKeever C, Bell N, Davidson M, Downs RW, Emkey R, Meunier PJ, Miller SS, Mulloy AL, Recker RR, Weiss SR, Heyden N, Musliner T, Suryawanshi S, Yates AJ, Lombardi A (2000) Alendronate and estrogen effects in postmenopausal women with low bone mineral density. Alendronate/Estrogen Study Group. J Clin Endocrinol Metab 85:720–726
Acknowledgments
This work was supported in part by National Institutes of Health grant K12 HD01459 (Building Interdisciplinary Research Careers in Women’s Health, to R. A.-V.). These results were presented at the 29th annual meeting of the American Society for Bone and Mineral Research, Honolulu, HI, September 16–19, 2007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armamento-Villareal, R., Napoli, N., Diemer, K. et al. Bone Turnover in Bone Biopsies of Patients with Low-Energy Cortical Fractures Receiving Bisphosphonates: A Case Series. Calcif Tissue Int 85, 37–44 (2009). https://doi.org/10.1007/s00223-009-9263-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-009-9263-5