Abstract
Our aim is to investigate the potential therapeutic value of morin against osteoporosis and elucidate the mechanism of action. Osteoporosis was induced in rats by a subcutaneous injection of dexamethasone (DEX) for 5 weeks. Body weight was regularly monitored. Body mineral density (BMD) was determined at proximal femurs using dual energy X-ray absorptiometry. Pathological examination was performed by hematoxylin and eosin staining. The relative expression of osteogenic and bone resorption markers was determined by real-time polymerase chain reaction and Western blotting, respectively. Activation of the MAPK signaling pathway was analyzed by Western blotting. Body weight and BMD were both significantly decreased in osteoporotic rats, although BMD was partially restored by intraperitoneal morin administration. Morin treatment also increased the number of trabecular bones in DEX-induced rats. Mechanistically, morin reversed the decrease of osteogenic markers and increase of bone resorption markers, which might eventually be mediated by modulation of MAPK signaling cascades. Here, we uncovered the therapeutic effect of morin against osteoporosis and demonstrated its suppressive action on the MAPK pathway in this disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is a disease with the risk of fracture associated with accumulative bone weakness, and the most common reason for a broken bone in the elderly population [1]. According to the World Health Organization, bone density at the hip measured by dual-energy X-ray absorptiometry of 2.5 standard deviations below that of a young adult is defined as osteoporosis. Osteoporosis is relatively asymptomatic until the occurrence of fracture with minor or no stress [2]. The etiology of osteoporosis may be attributed to decreased bone mass and increased bone loss. In women, bone loss is frequently exacerbated due to lower levels of estrogen after the menopause [3]. Osteoporosis also occurs in the context of other diseases and corresponding treatments such as alcoholism, anorexia, hyperthyroidism, kidney disease and oophorectomy [4]. Epidemiologic investigations have accumulatively identified a number of medicines including some antiseizure medications, chemotherapy, proton pump inhibitors, selective serotonin reuptake inhibitors and glucocorticosteroids that contribute to the incidence of osteoporosis [5]. Notably, glucocorticoids are widely used for anti-inflammatory therapeutics in a variety of immune-mediated diseases, such as rheumatic disease and organ transplantation [6]. According to the Global Longitudinal Study of Osteoporosis in Women (GLOW), approximately 2.7–4.6% women aged ≥55 years receive glucocorticoid therapy, which is associated with a variety of catastrophic side-effects including glucocorticoid-induced osteoporosis (GIOP), a common cause of secondary osteoporosis. It is recommended that all patients receiving glucocorticoids undergo general evaluations and protective measures for preventive purpose. The clinical management of osteoporosis relies on calcium/vitamin D supplementation and anti-osteoporotic drugs such as bisphosphonates (alendronate, etidronate, risedronate, zoledronic acid) and teriparatide, an anabolic agent [7].
The MAPK pathway has been identified as the most critical signaling in bone biology and mediates the majority of extracellular signaling cues [8]. Mechanistic analysis demonstrates that the TAK1-MKK3/6-p38 MAPK axis phosphorylates Runx2 and promotes its association with the coactivator CREB-binding protein to assemble the master transcription factors in the osteoblast genetic program [9]. Therefore, intensive investigations have focused on specific inhibition of the MAPK signaling cascade in this disease for therapeutic purposes.
Morin is a natural compound isolated from Maclura pomifera, Maclura tinctoria and the leaves of Psidium guajava and possesses broad-spectrum therapeutic potential against a variety of human diseases [10]. Cumulative evidence suggests this compound has antioxidant, antidiabetic, anti-inflammatory, antitumoral, antihypertensive, antibacterial, hypouricemic and neuroprotective properties. For instance, Sithara et al. demonstrated that morin inhibited proliferation of SW480 colorectal cancer cells via apoptosis induced by reactive oxygen species formation and Warburg effect uncoupling [11]. Similar anti-tumor activity was also observed in lung cancer cells, where morin treatment inhibited cell viability, growth and migration by suppression of miR-135b and inducing its target CCNG2 [12]. Tian et al. revealed the protective effects of morin on lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting TLR4/NF-κB and activating the Nrf2/HO-1 signaling pathways [13]. In addition, He et al. demonstrated that morin hydrate promoted inner ear neural stem cell survival and differentiation and protected the cochlea against neuronal hearing loss [14]. In a preclinical in vitro study, morin was identified as a weak inhibitor of fatty acid synthase [15]. Morin was also found to inhibit amyloid formation by islet amyloid polypeptide and disaggregate amyloid fibers, which might hold clinical promise for neurodegenerative diseases such as Alzheimer’s and Parkinson’s [16]. Notably, Ma et al. demonstrated that morin attenuated ovalbumin-induced airway inflammation by modulating oxidative stress-responsive MAPK signaling [17]. This immediately prompted us to investigate the potential therapeutic value of this compound in a GIOP animal model. Our study provided evidence for the first time in support of the clinical exploitation of morin against glucocorticoid-induced osteoporosis.
Methods
Animals
Sprague Dawley (SD) rats (5-month-old, female) were housed in a standard specific pathogen-free environment with consistent temperature and humidity. The animals had free access to drinking water and standard laboratory rodent diet ad libitum. The animal study was performed in strict accordance with the protocol approved by the Ethics Committee of Tianjin Medical University General Hospital. The animals were randomly divided into 4 groups—control group; daily subcutaneous injection of dexamethasone (0.1 mg/kg, Tianjin Xinzheng, Tianjin, China) for 5 weeks [DEX group]; daily subcutaneous injection of dexamethasone plus an intraperitoneal injection of morin (5 mg/kg, Sigma, MO, USA) [DEX + ML group]; and daily subcutaneous injection of dexamethasone plus an intraperitoneal injection of morin (10 mg/kg) [DEX + MH group]. The body weight and bone mineral density (BMD) of all the experimental subjects were measured before and after treatment. The right femurs were collected from the sacrificed rats at the endpoint for further analysis.
BMD measurement
BMD was determined at proximal femurs in vivo using Hologic QDR 4500 dual energy X-ray absorptiometry (Hologic, Bedford, MA, USA) immediately before and after treatments. The measurements were performed at least three times and the average values were calculated.
Hematoxylin and eosin stain (H&E)
All rats were killed by cervical dislocation with 30 mg/kg pentobarbital and the right proximal tibias were resected. Bone samples were subsequently subjected to fixation with 4% formaldehyde for 24 h and dehydrated in a serial ethanol solution, hyalinized in xylene and embedded in molten paraffin at 62 °C overnight. Blocks were cut into 4-μm sections, which were then stained with H&E and analyzed with Image-Pro Plus software (version 6.0; Media Cybernetics, Inc., Rockville, MD, USA).
Real-time PCR
Total RNA from the proximal femoral tissues was extracted using TriZol reagent (Invitrogen, CA, USA). The integrity was quality-checked using a BioAnalyzer 2100 (Agilent, CA, USA). RNA was reversely transcribed into cDNA with a High-Capacity DNA Reverse Transcription Kit (ThermoFisher, MA, USA). Real-time PCR was performed using the SYBR Green Real-time PCR Master Mix (Tiangen, Beijing, China) on a HT7900 Real-time PCR System (ABI, CA, USA). Relative expression of target genes was normalized to endogenous GAPDH and calculated using the 2−∆∆Ct method. All primers used in this study are listed as follows:
ALP-Forward: 5′-AGCCTTCGTTGCTGTGGAGA-3′.
ALP-Reverse: 5′-TGGTGTCATAAGGATGGTGG-3′.
RANKL-Forward: 5′-ACGCCAACATTTGCTTCAGG-3′.
RANKL-Reverse: 5′ GTTGGACACCTGGACGCTAA-3′.
Ost-Forward: 5′-ATGAGGACCCTCTCTCTG-3′.
Ost-Reverse: 5′-CTGCCAGGTCAGAGAGGCACA-3′.
Runx2-Forward: 5′-TACTCTGCCGAGCTACGAAAT-3′.
Runx2-Reverse: 5′- GAGGATTTGTGAAGACCGTTAT-3′.
TRACP-Forward: 5′-CTCTCCTGGCTCACTGTACAGC-3′.
TRACP-Reverse: 5′-CCATAATCTGCACGGTTCTG-3′.
GAPDH-Forward: 5′-GGTGGACCTCATGGCCTACA-3′.
GAPDH-Reverse: 5′-CTCTCTTGCTCTCAGTATCCT-3′.
Elisa
Serum carboxyl-terminal telopeptide of type I collagen (CTX) was measured using an ELISA assay kit (Blue Gene, China) to evaluate the levels of osteoclastic markers.
Western blotting
Total protein from the proximal femoral tissues was prepared in ice-cold RIPA lysis buffer supplemented with cOmplete™ Protease Inhibitor Cocktail (Roche, Basel, Swiss). The protein concentration was determined using a BCA Protein Assay Kit (Pierce, MA, USA). The samples were resolved by 10% SDS-PAGE gel and transferred onto PVDF membrane (Roche). After brief blocking with 5% skim milk at room temperature for 1 h, the membrane was hybridized with indicated primary antibodies (anti-ALP, 1:1,000, ab354; anti-Ost, 1:1,000, ab121285; anti-RUNX2, 1:1,000, ab23981; anti-RANKL, 1:1,000, ab 9957; anti-CTX, 1:1,000, ab126785; anti-TRACP, 1:1,000, ab126785; anti-p-JNK, 1:1,000, ab124956; anti-JNK, 1:1,000, ab179461; anti-p-ERK, 1:1,000, ab50011; anti-ERK, 1:1,000, ab54230; anti-p-P38, 1:1,000, ab4822; anti-P38, 1:1,000, ab31828; anti-GAPDH, 1:2,000, ab9485; Abcam, Cambridge, UK) at 4 °C overnight. Membranes were then incubated with horseradish peroxidase-conjugated secondary antibody (anti-mouse, ab6728, 1:5,000; anti-rabbit, ab6721, 1:5,000; Abcam) at room temperature for 1 h. Protein blots were visualized with an enhanced chemiluminescence system (ECL, Millipore, MO, USA). The relative protein blot intensity was determined by the densitometry scan. Loading was controlled by endogenous GAPDH.
Statistical analysis
Data are presented as the mean ± SD. Statistical analyses were performed with Student’s t test and one-way analysis of variance with pairwise comparison by Fisher’s least significant difference test using SPSS19 software (IBM, New York, NY, USA). Values of P < 0.05 were considered statistical different.
Results
Morin alleviated glucocorticoid-induced osteoporosis
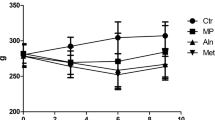
We first established the glucocorticoid-induced osteoporosis rat model via a subcutaneous injection of DEX following the previously described protocol [18]. The success in establishing the animal disease model was validated by measuring both body weight and BMD. The molecular structure is illustrated in Fig. 1a. Consistent with previous reports, body weight was significantly decreased upon DEX exposure compared to the control group (Fig. 1b); however, treatment with morin (both low and high dose) showed no remarkable influence on rat body weight. In line with the loss of body weight, the BMD index was significantly decreased in the DEX rats, which was partially reversed by administration of a high dose of morin (Fig. 1c). Therefore, our preliminary results suggested that a high concentration of morin demonstrated a significant beneficial effect on glucocorticoid-induced osteoporosis in a rat model.
Effects of morin on the changes in bone mineral density and body weight in glucocorticoid-induced osteoporosis (GIOP) rats. The molecular structure of morin (a). The initial and final body weights of the four groups (b); the initial and final BMD of the four groups (c). Data are shown as mean ± SD. **P < 0.01 versus the control group; ##P < 0.01 versus the DEX group. BW body weight, BMD bone mineral density, DEX dexamethasone, ML morin low, MH morin high
Effects of morin on histological changes in GIOP rats
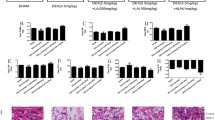
Our previous data clearly showed morin improved BMD in osteoporotic rats. Next, we sought to characterize the histological changes in the GIOP rats in response to morin administration. The femur tissue sections were obtained from the sacrificed experimental animals and subjected to H&E staining. As shown in Fig. 2, there was a significant reduction of trabecular bones in DEX-induced osteoporotic rats compared to the control group (Fig. 2a, b), and co-administration with a low concentration of morin led to negligible changes compared to the DEX group (Fig. 2c). Notably, a high dose of morin significantly increased the amount of trabecular bones to a comparable level with normal rats (Fig. 2d). Quantitative analysis was performed and is shown in Fig. 2e. Consistent with its beneficial effects on restoration of the BMD index, our histological examination clearly demonstrated that morin treatment also increased trabecular bones in osteoporosis.
Effects of morin on histological changes in GIOP rats. H&E staining of femur tissue sections from rats in the control group (a), DEX group (b), DEX + ML 5 mg/kg group (c), and DEX + MH 10 mg/kg group (d). Quantitative analysis was performed (d). Morphologic changes of the femoral metaphysis stained with H&E method were observed under light microscopy. Compared with control rats, there was a reduction of trabecular bones in the DEX group and DEX + ML group; however, in the DEX + MH group rats, a decrease of trabecular bones in the DEX group and DEX + ML group was confirmed. Magnification 200 ×, scale bar 200 μm. DEX dexamethasone, ML morin low, MH morin high
Effects of morin on molecular markers of osteoporosis
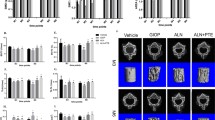
Our previous data demonstrated that morin improved osteoporosis in respect to both BMD and trabecular bones. Next, we sought to further characterize the detailed changes in crucial bone metabolism at the molecular level. Total RNA was extracted from the proximal femoral tissues, and the osteogenic markers including bone-specific alkaline phosphatase (ALP), osteocalcin (Ost), Runt-related transcription factor 2 (RUNX2), and bone resorption markers including Receptor activator for nuclear factor-κ B ligand (RANKL) and tartrate-resistant acid phosphatase-5b (TRACP) were determined by real-time PCR. The CTX content was measured by ELISA. As shown in Fig. 3a–c, all osteogenic markers were dramatically decreased in DEX rats, which were slightly restored by a low concentration of morin and significantly induced by a high dose of morin. In sharp contrast, all bone resorption markers were markedly up-regulated in the DEX group, but were moderately suppressed by low morin administration and significantly reduced by a high dose of morin (Fig. 3d–f). Our quantitative results suggest that the improvement in osteoporosis by morin might be attributed to its induction of osteogenic factors and inhibition of bone resorption factors. We further consolidated the corresponding alterations at protein level as shown in Fig. 4a, b.
Effects of morin on the expression of ALP (a), Ost (b), Runx2 (c), RANKL (d), CTX (e) and TRACP (f). mRNA expression levels were significantly decreased in rats exposed to DEX; however, morin dose-dependently restored these changes. The rats exposed to DEX showed significantly increased CTX content in the serum. Data are represented as mean ± SD (n = 8). **P < 0.01 compared with the control group; ##P < 0.01 compared with the DEX group. ALP bone-specific alkaline phosphatase, Ost osteocalcin, Runx2 Runt-related transcription factor 2, RANKL receptor activator for nuclear factor-κB ligand, CTX carboxy-terminal telopeptide of type I collagens, TRACP tartrate-resistant acid phosphatase-5b, DEX dexamethasone, ML morin light, MH morin high
Effects of morin on protein expression of ALP, Osx, Runx2 (a) and RANKL, CTX and TRACP (b). Protein expression level was significantly decreased in rats exposed to DEX; however, morin dose-dependently restored these changes. Data are represented as mean ± SD (n = 8). **P < 0.01 compared with the control group; ##P < 0.01 compared with the DEX group. ALP bone-specific alkaline phosphatase, Ost osteocalcin, Runx2 Runt-related transcription factor 2, RANKL receptor activator for nuclear factor-κB ligand, CTX carboxy-terminal telopeptide of type I collagens, TRACP tartrate-resistant acid phosphatase-5b, DEX dexamethasone, ML morin light, MH morin high
Morin treatment inhibited MAPK signaling in osteoporosis
In view of the critical role of activated MAPK signaling in osteoporosis, we further evaluated the potential impact of morin treatment on the MAPK signaling axis. Consistent with previous reports, we demonstrated significant activation of the MAPK pathway as indicated by the phosphorylation of JNK, ERK and P38 but not the total protein of these factors in our GIOP rats compared to the control group (Fig. 5a, b). The active signaling cascade was slightly inhibited by a low concentration of morin, but significantly suppressed by a high dose of morin. Our results suggest that morin improved osteoporosis, which might be mediated by modulation of the MAPK pathway.
Relative expression of p-JNK, JNK, p-ERK, ERK, p-P38, p38 to GAPDH using Western blotting. Morin restored the increase of these three proteins caused by DEX. Data are represented as mean ± SD (n = 8). **P < 0.01 compared with the control group; ##P < 0.01 compared with the DEX group. p-JNK phosphorylated Jun N-terminal kinase, JNL c-Jun N-terminal kinase, p-ERK phosphorylated extracellular regulated protein kinases, ERK extracellular regulated protein kinases, p-P38 phosphorylated P38, DEX dexamethasone, ML morin low, MH morin high
Discussion
The canonical MAPK signaling pathway has been increasingly recognized as being involving in the pathogenesis of osteoporosis [8]. In view of a previous report that morin might attenuate airway inflammation by modulating oxidative stress-responsive MAPK signaling [17], we sought to investigate the potential therapeutic benefit of morin in osteoporosis and attempted to elucidate the underlying molecular mechanism. Osteoporosis was successfully modeled in glucocorticoid-induced rats, which evidently manifested in both loss of body weight and reduction of BMD. An intraperitoneal injection of a high dose of morin significantly improved the osteoporotic symptoms. Pathologic analysis further demonstrated that morin treatment increased the number of trabecular bones in osteoporotic rats. Mechanistically, the osteogenic molecules were dramatically deceased and bone resorption molecules were markedly increased at both transcript and protein level in osteoporosis animals, and were significantly restored by a high dose of morin. In support of the critical role of the activated MAPK signaling pathway in osteoporosis, we demonstrated remarkable phosphorylation cascades in DEX rats, which were readily suppressed by administration with morin. Therefore, we unambiguously demonstrated the therapeutic benefits of morin against osteoporosis in a disease animal model and further elucidated that morin inhibited over-activation of the MAPK pathway in this disease. Our report highlighted the potential clinical application of morin in the treatment of osteoporosis and MAPK signaling as target candidates for further therapeutic exploitation for this disease.
A previous study by Ma et al. showed that morin attenuated ovalbumin-induced airway inflammation by modulating oxidative stress-responsive MAPK signaling [17]. This immediately prompted us to investigate the potential therapeutic effect of morin against osteoporosis, wherein overactivated MAPK signaling played a critical role. A study by Greenblatt et al. suggested that the p38 MAPK pathway was essential for both skeletogenesis and bone homeostasis in mice [8]. Gambogic acid has also been shown to have the potential to inhibit osteoclast formation and ovariectomy-induced osteoporosis by suppressing the JNK, p38 and Akt signaling pathways [19]. In support of this notion, our results preliminarily provided evidence that a high dose of morin significantly inhibited MAPK signaling and consequently ameliorated osteoporosis in the animal model. Further mechanistic and pre-clinical investigations are urgently warranted.
For the purpose of therapeutic exploitation for osteoporosis, the MAPK signaling cascades served as perfect target candidates. A number of compounds have been shown to possess potential suppressive effects on this pathway. For instance, Lee et al. reported that phloretin promoted osteoclast apoptosis in murine macrophages and inhibited estrogen deficiency-induced osteoporosis in rats [20]. Xu et al. suggested that hydrogen sulfide protected MC3T3-E1 osteoblastic cells against H2O2-induced oxidative damage and highlighted its implication for the treatment of osteoporosis [21]. The active component from traditional Chinese medicine, ginsenoside Rb1, has also been shown to inhibit osteoclastogenesis by modulating the NF-κB and MAPK pathways [18]. Thummuri et al. demonstrated that thymoquinone prevented RANKL-induced osteoclastogenesis activation and osteolysis in an in vivo model of inflammation by suppressing NF-κB and MAPK signaling [22]. Li et al. provided evidence that bergapten exerted inhibitory effects on diabetes-related osteoporosis via regulation of the PI3 K/AKT, JNK/MAPK and NF-κB signaling pathways in osteoprotegerin knockout rats [23]. Ablation of p38α MAPK signaling in osteoblast lineage cells via specific inhibitors significantly protected rats from bone loss induced by estrogen deficiency [24]. In line with all the above-mentioned results, we presented evidence that morin specifically inhibited MAPK signaling cascades, which mechanistically contributed to its therapeutic benefits against osteoporosis.
Conclusions
In summary, we exploited the potential pre-clinical application of morin in an osteoporotic rat model. Our study highlighted the therapeutic value of this compound to improve both BMD and trabecular bone amount, which might be mechanistically mediated by inhibition of the MAPK pathway.
References
den Uyl D, Bultink IE, Lems WF (2011) Advances in glucocorticoid-induced osteoporosis. Curr Rheumatol Rep 13:233–240. https://doi.org/10.1007/s11926-011-0173-y
Rachner TD, Khosla S, Hofbauer LC (2011) Osteoporosis: now and the future. Lancet 377:1276–1287. https://doi.org/10.1016/S0140-6736(10)62349-5
Martinez Perez JA, Palacios S, Garcia FC, Perez M (2011) Assessing osteoporosis risk factors in Spanish menopausal women. Gynecol Endocrinol 27:807–813. https://doi.org/10.3109/09513590.2010.540599
Schurer C, Wallaschofski H, Nauck M, Volzke H, Schober HC, Hannemann A (2015) Fracture risk and risk factors for osteoporosis. Dtsch Arztebl Int 112:365–371. https://doi.org/10.3238/arztebl.2015.0365
Sambrook P, Cooper C (2006) Osteoporosis. Lancet 367:2010–2018. https://doi.org/10.1016/S0140-6736(06)68891-0
Seibel MJ, Cooper MS, Zhou H (2013) Glucocorticoid-induced osteoporosis: mechanisms, management, and future perspectives. Lancet Diabetes Endocrinol 1:59–70. https://doi.org/10.1016/S2213-8587(13)70045-7
Kenanidis E, Potoupnis ME, Kakoulidis P, Leonidou A, Sakellariou GT, Sayegh FE, Tsiridis E (2015) Management of glucocorticoid-induced osteoporosis: clinical data in relation to disease demographics, bone mineral density and fracture risk. Expert Opin Drug Saf 14:1035–1053. https://doi.org/10.1517/14740338.2015.1040387
Greenblatt MB, Shim JH, Zou W, Sitara D, Schweitzer M, Hu D, Lotinun S, Sano Y, Baron R, Park JM, Arthur S, Xie M, Schneider MD, Zhai B, Gygi S, Davis R, Glimcher LH (2010) The p38 MAPK pathway is essential for skeletogenesis and bone homeostasis in mice. J Clin Invest 120:2457–2473. https://doi.org/10.1172/JCI42285
Kwon HS, Johnson TV, Tomarev SI (2013) Myocilin stimulates osteogenic differentiation of mesenchymal stem cells through mitogen-activated protein kinase signaling. J Biol Chem 288:16882–16894. https://doi.org/10.1074/jbc.M112.422972
Park C, Lee WS, Go SI, Nagappan A, Han MH, Hong SH, Kim GS, Kim GY, Kwon TK, Ryu CH, Shin SC, Choi YH (2014) Morin, a flavonoid from moraceae, induces apoptosis by induction of BAD protein in human leukemic cells. Int J Mol Sci 16:645–659. https://doi.org/10.3390/ijms16010645
Sithara T, Arun KB, Syama HP, Reshmitha TR, Nisha P (2017) Morin inhibits proliferation of SW480 colorectal cancer cells by inducing apoptosis mediated by reactive oxygen species formation and uncoupling of warburg effect. Front Pharmacol 8:640. https://doi.org/10.3389/fphar.2017.00640
Yao D, Cui H, Zhou S, Guo L (2017) Morin inhibited lung cancer cells viability, growth, and migration by suppressing miR-135b and inducing its target CCNG2. Tumour Biol 39:1010428317712443. https://doi.org/10.1177/1010428317712443
Tian Y, Li Z, Shen B, Zhang Q, Feng H (2017) Protective effects of morin on lipopolysaccharide/d-galactosamine-induced acute liver injury by inhibiting TLR4/NF-kappaB and activating Nrf2/HO-1 signaling pathways. Int Immunopharmacol 45:148–155. https://doi.org/10.1016/j.intimp.2017.02.010
He Q, Jia Z, Zhang Y, Ren X (2017) Morin hydrate promotes inner ear neural stem cell survival and differentiation and protects cochlea against neuronal hearing loss. J Cell Mol Med 21:600–608. https://doi.org/10.1111/jcmm.13005
Tian WX (2006) Inhibition of fatty acid synthase by polyphenols. Curr Med Chem 13:967–977
Noor H, Cao P, Raleigh DP (2012) Morin hydrate inhibits amyloid formation by islet amyloid polypeptide and disaggregates amyloid fibers. Protein Sci 21:373–382. https://doi.org/10.1002/pro.2023
Ma Y, Ge A, Zhu W, Liu YN, Ji NF, Zha WJ, Zhang JX, Zeng XN, Huang M (2016) Morin attenuates ovalbumin-induced airway inflammation by modulating oxidative stress-responsive MAPK signaling. Oxid Med Cell Longev 2016:5843672. https://doi.org/10.1155/2016/5843672
Zhang X, Chen K, Wei B, Liu X, Lei Z, Bai X (2016) Ginsenosides Rg3 attenuates glucocorticoid-induced osteoporosis through regulating BMP-2/BMPR1A/Runx2 signaling pathway. Chem Biol Interact 256:188–197. https://doi.org/10.1016/j.cbi.2016.07.003
Ma J, Ma Y, Liu X, Chen S, Liu C, Qin A, Fan S (2015) Gambogic acid inhibits osteoclast formation and ovariectomy-induced osteoporosis by suppressing the JNK, p38 and Akt signalling pathways. Biochem J 469:399–408. https://doi.org/10.1042/BJ20150151
Lee EJ, Kim JL, Kim YH, Kang MK, Gong JH, Kang YH (2014) Phloretin promotes osteoclast apoptosis in murine macrophages and inhibits estrogen deficiency-induced osteoporosis in mice. Phytomedicine 21:1208–1215. https://doi.org/10.1016/j.phymed.2014.04.002
Xu ZS, Wang XY, Xiao DM, Hu LF, Lu M, Wu ZY, Bian JS (2011) Hydrogen sulfide protects MC3T3-E1 osteoblastic cells against H2O2-induced oxidative damage-implications for the treatment of osteoporosis. Free Radic Biol Med 50:1314–1323. https://doi.org/10.1016/j.freeradbiomed.2011.02.016
Thummuri D, Jeengar MK, Shrivastava S, Nemani H, Ramavat RN, Chaudhari P, Naidu VG (2015) Thymoquinone prevents RANKL-induced osteoclastogenesis activation and osteolysis in an in vivo model of inflammation by suppressing NF-KB and MAPK signalling. Pharmacol Res 99:63–73. https://doi.org/10.1016/j.phrs.2015.05.006
Li XJ, Zhu Z, Han SL, Zhang ZL (2016) Bergapten exerts inhibitory effects on diabetes-related osteoporosis via the regulation of the PI3 K/AKT, JNK/MAPK and NF-kappaB signaling pathways in osteoprotegerin knockout mice. Int J Mol Med 38:1661–1672. https://doi.org/10.3892/ijmm.2016.2794
Thouverey C, Caverzasio J (2015) Ablation of p38alpha MAPK signaling in osteoblast lineage cells protects mice from bone loss induced by estrogen deficiency. Endocrinology 156:4377–4387. https://doi.org/10.1210/en.2015-1669
Funding
This work was supported by grants from the National Natural Science Foundation of China (No. 81501915).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Research involving human participants
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
Not applicable.
Rights and permissions
About this article
Cite this article
Wang, C., Wan, X., Li, Y. et al. Morin protects glucocorticoid-induced osteoporosis through regulating the mitogen-activated protein kinase signaling pathway . J Nat Med 72, 929–936 (2018). https://doi.org/10.1007/s11418-018-1228-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11418-018-1228-4