Abstract
Bone disease in long-term survivors after gastric cancer resection has received little research attention. This study aimed to investigate bone health after curative resection of gastric cancer and the consequences of high-dose vitamin D supplementation in patients with low levels of 25-(OH)-vitamin D. Disease-free patients at least 24 months after gastric cancer resection represented the study cohort. Serum markers of bone metabolism were assessed at baseline and at 3 and 12 months. Bone mineral density and presence of fractures were assessed by X-ray at baseline. Patients with 25-(OH)-vitamin D ≤30 ng/mL at baseline received 16,000 IU of vitamin D3 every 10 days during the 1-year follow-up. Forty patients were included in the study. Mean time from surgery was 48.9 (24–109) months. Vitamin D insufficiency and secondary hyperparathyroidism were observed in 38 and 20 patients, respectively. Densitometry showed osteoporosis in 14 women and seven men and prevalent fractures in 12 women and six men at baseline. After 3 months of vitamin D supplementation, 35 patients reached values of 25-(OH)-vitamin D over 30 ng/mL. After 12 months, 38 patients were in the normal range of 25-(OH)-vitamin D. At the same time, iPTH levels and markers of bone turnover (C-terminal cross-linked telopeptide of type-I collagen, serum concentrations of bone-specific alkaline phosphatase and osteocalcin) significantly decreased after vitamin D intervention. Oral administration of high doses of vitamin D is easily implemented and restored 25-(OH)-vitamin D and iPTH values, which are frequently disturbed after gastric cancer resection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A combination of radical surgery and adjuvant therapy has resulted in improved survivorship in patients with gastric cancer. Five-year survival rates in the range of 60–78% have been reported in recent trials [1]. Increasing attention is now being focused on the long-term sequelae of the therapy, particularly weight loss, nutritional deficiencies, and deterioration in quality of life. Patients with gastric cancer usually undergo more or less extensive gastric resections with different types of intestinal reconstruction that reduce absorption of minerals, proteins and vitamins. Adequate absorption of calcium and vitamin D are important for calcium–phosphorous homeostasis and vitamin D deficiency leads to hyperparathyroidism, bone loss, osteomalacia, osteoporosis and fractures [2]. Bone loss and the risk of osteoporosis and fractures can have a significant negative impact on the lives of long-term gastric cancer survivors [3].
The consequences of partial or total gastrectomy on bone metabolism have been studied for many years by several authors [4,5,6]. Some research has demonstrated that post-gastrectomy patients have bone metabolism alterations as a consequence of surgical intervention, with impaired absorption of calcium and rapid intestinal transit [7]. In iliac crest biopsies, gastrectomy has also been associated with severe osteomalacia, marrow fibrosis, and impaired calcium distribution, partially explained by secondary hyperparathyroidism and low calcium absorption [8].
However, only a few studies have evaluated the specific impact of surgery in long-term gastric cancer survivors [5, 9, 10]. Treatment and doses of vitamin D supplementation remain a contradictory topic that has to be resolved.
The present study investigated the prevalence of osteoporosis, fractures and bone metabolism changes in a cohort of long-term survivors after gastric cancer resection. The effects of an easily implemented oral administration of high doses of vitamin D in those patients with low levels of 25-(OH)-vitamin D were also assessed.
Materials and methods
Study design
A prospective, non-selected, observational, clinical cohort study was conducted at the Section of Gastrointestinal Surgery and the Musculoskeletal Unit in the Hospital del Mar, Barcelona, Spain. An intervention with oral supplements of vitamin D was performed in patients with low levels of 25-(OH)-vitamin D. The Ethics Committee of the institution approved the study and written informed consent was obtained from all participants.
Patients
We selected from our prospectively maintained database all patients with gastric cancer who underwent curative total or subtotal gastric resection between 2004 and 2013, and had survived at least 2 years without disease recurrence. A 70 cm Roux-en-Y loop reconstruction was performed in all cases. Patients with glomerular filtration rate (GFR) <45 mL/min/1.73 m2, chronic kidney disease (CKD) stage 3b, 4, and 5 according to KDOQI classification, chronic liver disease, rheumatoid arthritis, Paget bone disease, and concurrent or previous treatment with bisphosphonates, oral corticosteroids, or any other bone-active drugs were excluded. Patients eligible for the study were contacted by telephone and invited to participate.
Bone history
All patients were asked about osteoporosis risk factors, menopause status, and years since menopause. Previous non-fragility fractures, height decrease, falls and back pain were recorded. Dietary calcium intake was estimated using a weekly food-intake frequency questionnaire, validated for the Spanish population [11].
Measurements
Serum levels of 25-(OH)-vitamin D and iPTH
Plasma concentrations of 25-(OH)-vitamin D and iPTHwere measured at baseline and at 3 and 12 months after intervention with vitamin D supplements, when required. The 25-(OH)-vitamin D levels were analyzed using a competitive electrochemiluminescence protein-binding assay intended for the quantitative determination of total 25-(OH)-vitamin D in human serum and plasma. The assay employs a vitamin D binding protein as capture protein, which binds to both 25-OH VitD3 and 25-OH VitD2 (Cobas e602, Roche Diagnostics, Mannheim, Germany). Intra-assay coefficient of variation (CV) was 7.8 and 8.1% at mean concentrations of 15.7 and 26.2 ng/mL, respectively, using quality control material provided by Roche Diagnostics.
iPTH was analyzed with a solid-phase, two-site chemiluminescent enzyme-labeled immunometric assay (IMMULITE 2000 Siemens, Los Angeles, CA, USA). CV was 7.0% at mean concentrations of 47 pg/mL, using quality control material provided by Siemens.
Biochemical bone metabolism
Calcium (Ca) and phosphorus (P) and albumin levels were determined using a chemical autoanalyzer (MODULAR® ANALYTICS, Roche Diagnostics, Mannheim, Germany) with a CV value less than 5%.
Biochemical markers of bone turnover (BTM): serum concentrations of bone-specific alkaline phosphatase (BAP), osteocalcin (OC) and C-terminal cross-linked telopeptide of type-I collagen (CTX) were measured at baseline and at 3 and 12 months of follow-up. BAP was measured using the Access Ostase enzyme assay (Beckman Coulter, Fullerton, CA, USA), OC by a solid-phase two-site chemiluminescence assay (IMMULITE 2000 Siemens, Los Angeles, CA, USA), and CTX by chemiluminescence assay (ECLIA, MODULAR ANALYTICS E170, Roche Diagnostics, Mannheim, Germany).
Bone mineral density (BMD) analysis
At baseline, BMD at lumbar spine (L1–L4), femoral neck, and total hip was measured by dual-energy X-ray absorptiometry (DXA) using a Hologic QDR4500SL bone densitometer (Hologic, Waltham, MA, USA), and following the usual protocol provided by the manufacturer. The in vivo CV is 1.0% for the lumbar spine and 1.65% for femoral neck.
Assessment of vertebral fractures
Lateral X-ray films of the thoracic and lumbar spine were obtained at baseline to identify prevalent vertebral fractures (VF). Spine fractures were defined according to the semiquantitative method of Genant et al. [12, 13].
Intervention
According to the Endocrine Society Clinical Practice Guideline definition of 25-(OH)-vitamin D deficiency and insufficiency [14], patients with 25-(OH)-vitamin D concentration ≤30 ng/mL at the baseline visit were treated with oral vitamin D. An easy scheme with 0.266 mg (16,000 IU) of vitamin D3 (calcifediol, Hidroferol®, FAES FARMA) every 10 days during the 1-year follow-up was used. Moreover, patients with fragility fractures or diagnosis of osteoporosis by DXA were treated with intravenous bisphosphonate once a year.
Statistical analysis
Data are presented as mean (SD). One-tailed Student t test for paired measures was used to compare 25-(OH)-vitamin D levels at different time points. Correlation between pairs of quantitative variables was assessed by adjusting the distribution to a polynomial curve using the least square method. The p values <0.05 were considered statistically significant. All analysis was performed using IBM SPSS version 22 (SPSS Inc., Chicago, IL, USA).
Results
Forty patients (20 men and 20 women) out of 123 who had undergone gastric cancer resection were included in the study after signing the informed consent document. Eighty-three patients were excluded for reasons depicted in Fig. 1. Baseline and bone-related characteristics of study participants are shown in Tables 1 and 2.
Vitamin D concentrations at baseline
In the study population, baseline levels of 25-(OH)-vitamin D were 13.4 ± 9.1 ng/mL; 38 patients had concentrations <30 ng/mL (vitamin D insufficiency), 29 < 20 ng/mL (vitamin D deficiency), and 21 < 10 ng/mL (severe vitamin D deficiency). All patients had serum calcium and serum phosphorus levels within the normal range, despite low levels of 25-(OH)-vitamin D.
A weak but significant correlation was observed between 25-(OH)-vitamin D concentrations at baseline and iPTH levels (R 2 = 0.190; p = 0.005) (Fig. 2). No significant correlations were observed between 25-(OH)-vitamin D concentrations at baseline and other variables such as age, body mass index, and time since surgery.
iPTH levels at baseline
In the study population, levels of iPTH were 90.2 ± 68.4 pg/mL (range 23–377); 21 patients had concentrations >70 pg/mL in the range of hyperparathyroidism, all with normal serum levels of corrected total calcium. Overall, 13 patients with severe vitamin D deficiency had levels of iPTH >70 pg/mL. Three patients had very high values of iPTH and were evaluated to rule out primary hyperparathyroidism.
Restoration of 25-(OH)-vitamin D levels and iPTH levels after 3 and 12 months of intervention
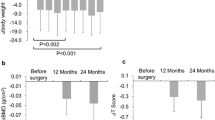
Significant increases in 25-(OH)-vitamin D levels were achieved after 3 and 12 months of vitamin D supplementation (Fig. 3). At 3 months, 35 patients reached values over 30 ng/mL, five had values within the range of insufficiency and no one remained in the range of vitamin D deficiency or severe deficiency. After 12 months, all patients were in the normal range of 25-(OH)-vitamin D except two who reported non-adherence to treatment. Moreover, five patients reached high 25-(OH)-vitamin D values (>100 ng/mL) but maintaining serum calcium in the normal range.
The iPTH levels decreased after vitamin D intervention. Mean levels were 58.6 ± 33.0, and only 11 patients maintained concentrations >70 pg/mL at 3 months. A fall in iPTH levels was observed in all patients. The response of vitamin D and iPTH levels after 3 and 12 months of supplementation did not show any correlation with BMI and vitamin D at baseline.
Calcium intake, BMD, and fractures at baseline
The mean calcium intake was 720 mg per day, range (121–1454). Only six patients reached the 1000–1200 mg of recommended calcium intake [15, 16]. Mean DXA values at baseline are shown in Table 2. In men, only one patient had normal BMD values at all sites measured, 12 patients had osteopenic values, and seven had osteoporosis, according to WHO criteria [17]. In women, only one had normal BMD values, five had osteopenia, and 14 had osteoporosis. The majority of fragility fractures were observed in women (12 vs. six in men), as shown in Table 3. Only two men had non-vertebral fractures and four had vertebral fractures, one grade 1 and three grade 3 according to Genant’s criteria in the spine X-ray. Moreover, an analysis of bone-related characteristics at baseline according to the presence or the absence of bone fracture found significant differences only for iPTH and BMD values, and not for age, BMI, calcium intake, or 25(OD)-Vit D (Table 4).
Biochemical markers of bone turnover at baseline and after 3 and 12 months of intervention and BMD after 12 months of intervention
As defined in the protocol, all patients with osteoporosis received treatment with intravenous bisphosphonate and, as expected, the BTM values decreased after 3 and 12 months (Table 5). Six patients with levels of BAP >30 had levels of 25-(OH)-vitamin D in the range of severe deficiency.
BMD increased significantly after 12 months with intravenous bisphosphonate, but there was no difference in the group without osteoporosis that received only vitamin D supplementation (Table 5). No new fractures occurred during the follow-up.
Discussion
Our study found a high prevalence of vitamin D deficiency with secondary hyperparathyroidism and osteopenia/osteoporosis in almost all long-term survivors after gastric cancer resection. The main consequence of these bone metabolism disorders is the high prevalence of fractures. Even severe deficiency after surgery was reversed with high doses of oral vitamin D supplements.
Long-term survivors after gastric cancer resections are under systematic controls by oncologists and surgeons to rule out recurrence, and they often receive treatment with vitamin B12 to prevent anemia. However, they are not usually evaluated for other chronic disease such as osteoporosis. Bone loss is one of the adverse health effects in cancer survivors [18, 19]. Bone metabolism disorders in patients undergoing other types of surgical procedures, such as bariatric surgery, have been known for many years [20]. Although the majority of guidelines have recommended calcium and vitamin D supplementation after gastrointestinal surgery [21], there are no recommendations about doses and timing. In our study, we supplemented every 10 days with high doses of calcifediol, and we achieved levels of 25-(OH)-vitamin D in a normal range without any adverse effects and with normalization of iPTH levels. The dose of vitamin D supplementation selected was based on previous studies from our group in patients with bariatric surgery [22] and breast cancer [23].
Lim and colleagues published a study with 133 patients and found a prevalence of osteoporosis and reported fractures of 39.6 and 18%, respectively [10]. In our study, the prevalence was higher, especially among women, because age and time since gastrectomy were also higher. Therefore, time since menopause was added as an important risk factor for bone loss in women. Although Lim et al. [10] reported 13.5% of patients with high serum alkaline phosphatase (AP) as suspected osteomalacia, they did not analyze 25-(OH)-vitamin D to confirm the diagnosis [10]. Our study found a high prevalence of secondary hyperparathyroidism, with six patients having high levels of BAP. No bone biopsy was performed to establish the diagnosis of osteomalacia. In a prospective study, Baek et al. [24] did not find statistical differences in vitamin D levels before and 12 months after gastrectomy; they explained that their patients were instructed to increase calcium and vitamin D intake. Based on their results, Baek et al. [24] recommended supplementation with vitamin D to avoid secondary hyperparathyroidism. In a similar study, Heiskanen et al. [5] showed significantly lower BMD values in 18 patients after gastrectomy than that of the control group and also found low levels of 25 (OH)-vitamin D. However, they did not analyze iPTH levels to find secondary hyperparathyroidism.
The current study provides additional data to support the use of supplementation with a very easy and safe scheme of 16,000 IU of oral vitamin D in order to avoid or reverse hyperparathyroidism and secondary bone loss. Therefore, the DXA values obtained after treatment with vitamin D supplementation or intravenous bisphosphonates were as expected. Treatment for osteoporosis relies on antiresorptive or anabolic drugs (e.g., bisphosphonates or denosumab and teriparatide, respectively). Vitamin D is used to avoid secondary hyperparathyroidism and to achieve 25(OH)-vitamin D >30 ng/mL, the threshold at which PTH levels began to increase [14].
Glatzle et al. [7] obtained good results using a complicated scheme combining extremely high intramuscular and oral doses of vitamin D. However, a recent randomized clinical trial in community-dwelling men and women [25] showed an increased incidence of falls and no benefit in lower extremity function, dissuading clinicians from using the extremely high doses of vitamin D used by Glatzle et al. [7].
Safety of vitamin D supplementation is an important issue. The use of calcitriol is not usually recommended, because it could increase hypercalcemia. Cholecalciferol and calcifediol are quite safe; hypercalcemia is very unusual at regular and even high oral doses, and happens only as a prescription or medication error [26]. In our study, five patients had 25-(OH)-vitamin D values over 100 ng/mL but no participant had hypercalcemia. In all five cases, calcium values were between 9.5 and 9.8 mg/dL and phosphorus between 3.8 and 3.9 mg/dL. When 25(OH) vitamin D values after supplementation exceed 100 ng/mL, action would be required in the presence of hypercalcemia. If not present or asymptomatic, only reduction of vitamin D dose is required; but if symptomatic hypercalcemia is present, treatment with zoledronic acid or denosumab, intravenous saline serum and furosemide must also be prescribed. Another safety aspect is the risk of falls when high doses of vitamin D are used. Although the occurrence of falls was not a specific objective of our study, the investigators asked about falls at each follow-up visit (3 and 12 months after supplementation) and none of the patients reported falling.
The resorption marker CTX showed a moderate decrease in patients with vitamin D supplementation alone and very high reduction in patients with osteoporosis and intravenous bisphosphonate treatment, as expected [27] On the other hand, bone formation markers showed a clear and significant decrease in patients with intravenous bisphosphonates. Our results are consistent with a previous study where authors looked at the short-term changes in bone metabolism after one year of gastrectomy [24] and confirmed the effect of long-term vitamin D deficiency and secondary hyperparathyroidism on BTM, and also showed the reversibility of this situation with vitamin D supplementation.
One important aspect of our study is the high prevalence of fragility fractures, especially in women. Glatzle et al. [7] also showed a high prevalence of vertebral deformity in women as more time elapsed since gastrectomy. Based on these results, an important recommendation would be to use thoracic and lumbar lateral X-rays to perform an active search for vertebral fragility fractures in all patients before and after gastrectomy during their annual gastric cancer check-up.
Our main study limitation was the small sample size, which did not allow for a broad analysis of all bone metabolism factors. However, the selection of a homogenous sample of patients with long-term survival without any treatment or other disease that could affect bone metabolism was an important strength of the study. We ruled out many factors, including alcoholism, use of corticosteroids, or many other drugs that have some influence in bone metabolism, in order to assess how the gastrectomy itself affects skeletal bone. Another limitation was the lack of specific data on microalbuminuria in order to rule out early kidney damage that it might influence the 25-(OH)-Vit D levels.
In summary, the current study highlights the importance of analyzing bone health and diagnosing vitamin D deficiency or insufficiency and the secondary hyperparathyroidism that may occur several years after gastrectomy. The study showed that a simple scheme of high doses of oral vitamin can restore normal 25-(OH)-vitamin D and iPTH values with no adverse effects.
References
Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ, CLASSIC trial investigators (2014) Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 15:1389–1396
Heaney RP (2013) Health is better at serum 25(OH)D above 30 ng/mL. J Steroid Biochem Mol Biol 136:224–228
Coleman R, Body JJ, Aapro M, Hadji P, Herrstedt J, Group EGW (2014) Bone health in cancer patients: ESMO clinical practice guidelines. Ann Oncol 25:iii124–iii137
Nicolaysen R, Ragard R (1955) The calcium and phosphorus metabolism in gastrectomized patients. Scand J Clin Lab Invest 7:298–299
Heiskanen JT, Kroger H, Paakkonen M, Parviainen MT, Lamberg-Allardt C, Alhava E (2001) Bone mineral metabolism after total gastrectomy. Bone 28:123–127
Bisballe S, Eriksen EF, Melsen F, Mosekilde L, Sorensen OH, Hessov I (1991) Osteopenia and osteomalacia after gastrectomy: interrelations between biochemical markers of bone remodelling, vitamin D metabolites, and bone histomorphometry. Gut 32:1303–1307
Glatzle J, Piert M, Meile T, Besenthal I, Schafer JF, Konigsrainer A, Zittel TT (2005) Prevalence of vertebral alterations and the effects of calcium and vitamin D supplementation on calcium metabolism and bone mineral density after gastrectomy. Br J Surg 92:579–585
Krause M, Keller J, Beil B, van Driel I, Zustin J, Barvencik F, Schinke T, Amling M (2015) Calcium gluconate supplementation is effective to balance calcium homeostasis in patients with gastrectomy. Osteoporosis Int 26:987–995
Lim JS, Lee JI (2011) Prevalence, pathophysiology, screening and management of osteoporosis in gastric cancer patients. J Gastric Cancer 11:7–15
Lim JS, Kim SB, Bang HY, Cheon GJ, Lee JI (2007) High prevalence of osteoporosis in patients with gastric adenocarcinoma following gastrectomy. World J Gastroenterol 13:6492–6497
Gonzalez-Macias J, Del Pino-Montes J, Olmos JM, Nogues X, en nombre de la Comision de Redaccion de las Guias de Osteoporosis de la SEIOMM (2015) Clinical practice guidelines for posmenopausal, glucocorticoid-induced and male osteoporosis. Spanish Society for Research on Bone and Mineral Metabolism (3rd updated version 2014). Rev Clin Esp 215:515–526
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. In. Geneva: World Health Organization. Osteoporos Int 4:368–381
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine Society (2011) Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930
Moyer VA, LeFevre ML, Siu AL (2013) Vitamin D and calcium supplementation to prevent fractures in adults. Ann Intern Med 159:856–857
Ross AC (2011) The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr 14:938–939
NIH Consensus Development Panel on Osteoporosis Prevention D, Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Lustberg MB, Reinbolt RE, Shapiro CL (2012) Bone health in adult cancer survivorship. J Clin Oncol 30:3665–3674
Stava CJ, Jimenez C, Hu MI, Vassilopoulou-Sellin R (2009) Skeletal sequelae of cancer and cancer treatment. J Cancer Surviv 3:75–88
Coates PS, Fernstrom JD, Fernstrom MH, Schauer PR, Greenspan SL (2004) Gastric bypass surgery for morbid obesity leads to an increase in bone turnover and a decrease in bone mass. J Clin Endocrinol Metab 89:1061–1065
American Gastroenterological Association (2003) American Gastroenterological Association medical position statement: guidelines on osteoporosis in gastrointestinal diseases. Gastroenterology 124:791–794
Prieto-Alhambra D, Servitja S, Javaid MK, Garrigos L, Arden NK, Cooper C, Albanell J, Tusquets I, Diez-Perez A, Nogues X (2012) Vitamin D threshold to prevent aromatase inhibitor-related bone loss: the B-ABLE prospective cohort study. Breast Cancer Res Treat 133:1159–1167
Lanzarini E, Nogues X, Goday A, Benaiges D, de Ramon M, Villatoro M, Pera M, Grande L, Ramon JM (2015) High-dose Vitamin D supplementation is necessary after bariatric surgery: a prospective 2-year follow-up study. Obes Surg 25:1633–1638
Baek KH, Jeon HM, Lee SS, Lim DJ, Oh KW, Lee WY, Rhee EJ, Han JH, Cha BY, Lee KW, Son HY, Kang SK, Kang MI (2008) Short-term changes in bone and mineral metabolism following gastrectomy in gastric cancer patients. Bone 42:61–67
Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, Staehelin HB, Meyer OW, Theiler R, Dick W, Willett WC, Egli A (2016) Monthly high-dose vitamin d treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med 176:175–183
Heaney RP, Davies KM, Chen TC, Holick MF, Barger-Lux MJ (2003) Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am J Clin Nutr 77:204–210
Iwamoto J, Uzawa M, Sato Y, Takeda T, Matsumoto H (2010) Effect of alendronate on bone mineral density and bone turnover markers in post-gastrectomy osteoporotic patients. J Bone Miner Metab 28:202–208
Acknowledgements
The authors wish to acknowledge Joan Sancho, MD, for expert help in the statistical analysis, and Elaine Lilly, PhD, for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None to be disclosed by any of the authors.
About this article
Cite this article
Climent, M., Pera, M., Aymar, I. et al. Bone health in long-term gastric cancer survivors: A prospective study of high-dose vitamin D supplementation using an easy administration scheme. J Bone Miner Metab 36, 462–469 (2018). https://doi.org/10.1007/s00774-017-0856-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00774-017-0856-1