Abstract
Aromatase inhibitor (AI)-related bone loss is associated with increased fracture rates. Vitamin D might play a role in minimising this effect. We hypothesised that 25-hydroxy-vitamin D concentrations [25(OH)D] after 3 months supplementation might relate to bone loss after 1 year on AI therapy. We conducted a prospective cohort study from January 2006 to December 2011 of a consecutive sample of women initiating AI for early breast cancer who were ineligible for bisphosphonate therapy and stayed on treatment for 1 year (N = 232). Serum 25(OH)D was measured at baseline and 3 months, and lumbar spine (LS) bone mineral density at baseline and 1 year. Subjects were supplemented with daily calcium (1 g) and vitamin D3 (800 IU) and additional oral 16,000 IU every 2 weeks if baseline 25(OH)D was <30 ng/ml. Linear regression models were fitted to adjust for potential confounders. After 1 year on AI therapy, 232 participants experienced a significant 1.68 % [95 % CI 1.15–2.20 %] bone loss at LS (0.017 g/cm2 [0.012–0.024], P < 0.0001). Higher 25(OH)D at 3 months protected against LS bone loss (−0.5 % per 10 ng/ml [95 % CI −0.7 to −0.3 %], adjusted P = 0.0001), and those who reached levels ≥40 ng/ml had reduced bone loss by 1.70 % [95 % CI 0.4–3.0 %; adjusted P = 0.005] compared to those with low 25(OH)D levels (<30 ng/ml). We conclude that improved vitamin D status using supplementation is associated with attenuation of AI-associated bone loss. For this population, the current Institute of Medicine target recommendation of 20 ng/ml might be too low to ensure good bone health.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aromatase inhibitors (AI) are routinely used in the adjuvant treatment of women with hormone receptor-positive early breast cancer [1, 2], and will become more prevalent with exemestane showing a 65 % reduction in primary prevention of breast cancer [3]. Third generation AI (anastrozole, exemestane and letrozole) have superior efficacy and better safety than tamoxifen [4, 5], both as first-line choice and when switching therapy [6, 7]. However, AI therapy has significant unwanted effects and anastrozole, exemestane and letrozole led to an increased risk of developing osteoporosis and fragility fractures in the registration trials [5, 8–10]. The mechanism for the accelerated bone loss is thought to be, at least in part, profound suppression of oestrogen synthesis; the end result is increased osteoclast activation and net bone resorption.
In addition to the effects of AI, the population of women with early breast cancer has a high prevalence of vitamin D insufficiency (as defined by serum concentration of 25-hydroxy-vitamin D [25(OH)D] <30 ng/ml): we have shown that in our community (Barcelona, Spain) the prevalence of vitamin D insufficiency among patients treated for early breast cancer is 88.1 %, with 21.2 % having severe vitamin D deficiency, defined by serum concentrations of 25(OH)D <10 ng/ml [11]. In a number of observational studies, low levels of serum [25(OH)D] are associated with an increased risk of hip fracture [12, 13]. Furthermore, in some [14] but not all [15, 16] studies, vitamin D supplementation reduces risk of future fracture and has non-skeletal effects on a number of tissues [17]. We recently published an observational study showing that a target concentration of 40 ng/ml 25(OH)D may prevent development of AI-induced arthralgia [18], a syndrome highly associated with therapy discontinuation in clinical settings. These findings conflict with the latest recommendations by the Institute of Medicine (IOM) [19], which proposed 20 ng/ml as a target threshold for bone health.
However, it is not known if vitamin D status affects the rate of bone loss in patients commencing AI therapy. Therefore, we aimed to test the hypothesis that vitamin D concentrations after 3 months of oral supplementation would be inversely related to bone loss as measured by DXA after 1 year of AI therapy in normal or osteopenic women not treated with bisphosphonates. Secondly, we studied the association between improvement in [25(OH)D] concentration at 3 months from baseline and bone loss.
Methods
Details on study design, recruitment methods, and study population have been fully explained elsewhere [11, 20] and are briefly summarised below.
Study design and participants
We conducted a prospective cohort study from January 2006 to December 2011. All postmenopausal women diagnosed with early breast cancer and candidates for AI treatment attending our outpatient Breast Cancer Unit (Barcelona, Spain) from 2006 to the end of 2010 were consecutively invited to participate in the B-ABLE cohort study and recruited after informed consent. Patients were selected for treatment with AI according to the current American Society of Clinical Oncology (ASCO) recommendations [21]. Patients with history of any bone disease, rheumatoid arthritis, metabolic or endocrine diseases, prior diagnosis of Paget’s bone disease or osteomalacia, concurrent or prior treatment with bisphosphonates, oral corticosteroids, or any other bone-active drug except tamoxifen were excluded.
Patients with 25(OH)D concentration <30 ng/ml at the recruitment visit, were treated with oral calcium (1 g) and vitamin D (800 IU) supplements daily and additional oral 16,000 IU or 0.266 mg of vitamin D3 (cholecalciferol, Hidroferol®, FAES FARMA) every 2 weeks throughout the year of study. Those with baseline vitamin D ≥30 ng/ml received only the oral calcium and vitamin D daily supplements of 800 IU/day.
Patients were then stratified by bone mineral density (BMD) at the lumbar spine (LS), femoral neck (FN), and total hip (TH), and assigned to the corresponding therapeutic regimen. Weekly bisphosphonate therapy (either risedronate or alendronate, randomly assigned) was provided to patients with osteoporosis [T score < −2.5] or with a T score ≤ −2.0 at any site plus 1 major risk factor or prevalent fragility fractures.
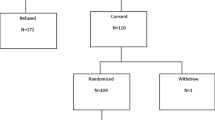
For the current analyses, we studied the population of women who, according to this therapeutic regimen, were not treated with bisphosphonates [see CONSORT diagram, Fig. 1].
Power estimation
The available sample of 232 women completing the study follow-up, ensures >85 % power to estimate a difference of 0.5 standard deviations in bone loss between the group of women achieving levels ≥40 mg/dl (about 20 % of participants according to our previous experience [18]) and those with serum levels <40 mg/dl after 3 months of supplementation.
Measurements
Serum concentrations of 25(OH)D
At baseline and at 3 months follow-up, plasma concentrations of 25(OH)D were determined using competitive immunoluminometric direct assay with direct-coated magnetic microparticles (DiaSorin Iberia SA, Madrid, Spain). The detection threshold of the tool is 4.0 ng/ml, intra-assay coefficient of variation (CV) is 3.4 %, and inter-assay CV is 7.6 %. Our laboratory is part of the vitamin D external quality assessment programme of the College of American Pathologists.
Bone mineral density
At baseline and at 1 year, BMD was measured at the LS (L1–L4), FN and TH using a dual-energy X-ray (DXA) densitometer QDR 4500 SL® (Hologic, Waltham, MA, USA), following the usual protocol in our unit. In our department, the in vivo CV of this technique ranges from 1.0 % at LS to 1.65 % at FN.
Percentage BMD loss was estimated according to the following formula: (baseline BMD − BMD at 1 year follow-up)/baseline BMD.
Calcium daily intake
Dietary calcium intake was estimated using a validated weekly food-intake frequency questionnaire [22]. Calcium supplements use was also recorded.
Covariates
For potential further adjustments in multivariate models, we collected information at baseline on the following: age, years since menopause, body mass index (BMI), smoking (current/ex/never), prior tamoxifen therapy, AI used (exemestane/letrozole), chemotherapy and radiotherapy. Season when blood samples were drawn was also registered.
Ethics approval
The study protocol was approved by the corresponding ethics committee (Hospital del Mar‘s Human Research Ethics Committee) and written informed consent was obtained from all participants.
Statistical analysis
We used paired T tests to assess changes in BMD at the three sites measured. The association between BMD loss at LS and vitamin D concentrations at 3 months was assessed using linear regression. Multivariate models were fitted to adjust for the season when serum samples were drawn, age, years since menopause, prior tamoxifen use, BMI, dietary calcium intake, and type of AI used (exemestane vs letrozole). Models for absolute bone loss were further adjusted for baseline BMD. As baseline vitamin D had been used to determine whether the participants should be supplemented with 800 IU daily or an additional 16,000 IU every other week, we did not adjust the whole cohort analyses for baseline 25(OH)D. However, we did so in the multivariate models for the population receiving high-dose vitamin D supplementation. Secondly, similar linear regression models were used to assess the existing relationship between vitamin D increment after 3 months of supplementation and BMD loss at 1 year (both absolute and bone loss rates).
All analyses were two-tailed, and p values were considered significant when <0.05. Statistical analyses were performed using Stata for Mac version 10 and R for Mac version 2.9.1, using the foreign, car, Hmisc, sciplot, Design and mass packages.
Results
Of the 326 women recruited between January 2006 and December 2010, 324 (99.4 %) have completed a year of follow-up. After risk fracture assessment, 94 (28.8 %) were initiated on bisphosphonates per protocol (see “Study design and participants”). The remaining 232 (71.2 %) were only given calcium and vitamin D supplements, constituting the population of this study (see Flowchart in Fig. 1). Baseline characteristics of this population are presented in Table 1.
Only 21 (9.0 %) participants had baseline 25(OH)D ≥30 ng/ml, and so were treated with calcium 1,000 mg and 800 IU of vitamin D3 per day; the remaining 211 (90.9 %) had different degrees of vitamin D insufficiency, and were additionally prescribed 16,000 IU of vitamin D3 orally every 2 weeks. After 3 months of supplementation, mean (standard deviation, SD) 25(OH)D concentrations were 42.0 (22.4) ng/ml; 67 (28.9 %) women remained at <30 ng/ml, 60 (25.9 %) had levels between 30 and <40 ng/ml, and 105 (45.2 %) achieved 25(OH)D ≥40 ng/ml.
After 1 year on AI therapy, participants had a significant bone loss at the 3 sites measured (see Table 2).
Among women with baseline vitamin D deficiency, there was no significant association between baseline vitamin D concentrations and BMD loss (adjusted P = 0.16). However, vitamin D concentrations after 3 months of supplementation were inversely associated with bone loss at LS: for each 10 ng/ml increase in serum [25(OH)D] there was a 0.5 % [95 % CI 0.26–0.75; P < 0.001] reduction in bone loss at LS, equivalent to 0.005 g/cm2 [95 % CI 0.002–0.007], or 0.13 SD. This remained significant after adjustment for season, BMI, calcium intake, AI used (exemestane vs letrozole), age, years since menopause and baseline BMD (P < 0.001) (see Table 3). Among those with baseline vitamin D insufficiency, BMD loss was also significantly reduced, by 0.5 % over the year per each 10 ng/ml increase in 25(OH)D concentrations at 3 months [95 % CI 0.2–0.7; P < 0.001], and still significant after multivariate adjustment for the same covariates plus baseline vitamin D (P < 0.001).
In addition, the 105 patients (45.3 % of the total study population) who achieved a vitamin D ≥40 ng/ml threshold at 3 months had less BMD loss at LS than those who reached lower concentrations (<30 ng/ml): 1.7 % [95 % CI 0.4–3.0; P = 0.010], equivalent to 0.017 g/cm2 (0.44 SD) [see Fig. 2]. This remained significant after multivariate adjustment (P = 0.007) [Table 3]. This association was also seen in those with baseline 25(OH)D < 30 ng/ml, after adjustment for baseline concentrations (P = 0.03) [Table 3].
Vitamin D increments after 3 months of supplements (defined as 25(OH)D at 3 months − 25(OH)D at baseline) in the whole population were also protective for LS bone loss: for each 10 ng/ml increase in serum 25(OH)D concentrations, bone loss was significantly reduced, by 0.6 % [95 % CI 0.4–0.8 %; P < 0.001] (equivalent to 0.005 g/cm2 [95 % CI 0.003–0.007], 0.13 SD) [Fig. 3]. This remained significant in multivariate adjusted models (P < 0.001) and when we repeated the analyses only for those with baseline vitamin D insufficiency at baseline [Table 3].
Discussion
As expected, in patients on AI therapy for a year, we found significant bone loss at the three sites measured: TH, FN and LS.
Baseline vitamin D concentrations were not significantly related to bone loss. By contrast, vitamin D after 3 months of supplementation was inversely correlated to LS bone loss at 1 year follow-up, independently of baseline 25(OH)D concentrations and of initial BMD. In addition, patients who reached a threshold of 25(OH)D ≥40 ng/ml at 3 months had a significant reduction of 1.7 % (almost half standard deviation according to our data) in bone loss rates compared to those who stayed at vitamin D serum levels <30 ng/ml.
Vitamin D increments at 3 months were also inversely correlated to bone loss rates at LS: each 10 ng/ml increase in vitamin D translated into a 0.6 % bone loss reduction.
Most of the big clinical trials have evaluated bone loss rates as a main side effect of AI therapy, and almost all of them reported significant bone loss at LS and hip. Rates of bone density change after 1 year of AI treatment ranged from −1.66 % [23] to −7.40 % [24], with wide variation in between depending on baseline characteristics of the patients studied. At least two studies have reported significant bone loss among patients switching from tamoxifen to AI therapy: Hines et al. [23] reported a 1.66 % bone loss rate at LS in patients after 1 year on Letrozole, and Coleman et al. [25] reported a slightly higher bone loss rate in patients who switched to exemestane and were on it for a year: −2.70 %BMD reduction at LS. The average bone loss rate observed in our population was in the lower range (−1.68 % at LS), which could be due to several reasons, such as a longer time since menopause at baseline or previous tamoxifen use.
Extensive data is available on the efficacy of bisphosphonates [26–28] and denosumab [29] to prevent bone loss and fractures in patients with low bone mass or with clinical risk factors for fracture, and clinical guidelines have been published on whom to treat with anti-resorptive agents [30]. These reports recommend that patients at low risk for fractures should be supplemented with calcium and vitamin D, although the dosage recommended (calcium 1 g/day and vitamin D 400 to 800 IU daily) is probably too low to attain adequate levels in those with vitamin D deficiency at baseline: almost 30 % of participants in this study did not reach a concentration of 30 ng/ml at 3 months of much higher dose supplementation (16,000 IU every 2 weeks and 800 IU daily). In addition, the possibility that calcium supplements might be related to an increase in cardiovascular events has raised safety concerns about their use [31]. Hence, high-dose vitamin D supplements, not accompanied by calcium, might be more useful in these patients to achieve the target levels of 40 ng/ml.
Consistent with our data, one recent small pilot trial including 60 participants has shown a borderline-significant protective effect (P = 0.06) of high-dose vitamin D supplementation on AI-induced bone loss [32], but these results require confirmation in bigger studies. We report here that vitamin D repletion can have a protective effect on bone loss among low-risk patients who did not require bisphosphonate therapy. In addition, we show that a threshold of ≥40 ng/ml after 3 months of supplementation can be a reasonable target, as our data appear to show a relationship to a significant decrease in bone loss rate, compared to those who remained at insufficient (below 30 ng/ml) levels, and almost 50 % of patients receiving our supplementation protocol achieved that threshold. Both the supplement dosage and the threshold suggested here are clearly higher than those proposed by the last IOM report, which advised a recommended dietary allowance (RDA) of 600 to 800 IU of vitamin D, and a 20 ng/ml target 25(OH)D concentration. These conflicting results provide a rationale for an individualised vitamin D supplementation regimen depending on patient characteristics and antecedents. Therefore, at least for this population of women on AI treatment, our data suggest that 25(OH)D levels of 40 ng/ml might be a more reasonable therapeutic target. Interestingly enough, 40 ng/ml is the same threshold found to prevent AI-induced arthralgia in our previous work [18]. The combined benefit of bone loss attenuation and decreased AI-associated arthralgia strengthens the case for this higher level as the optimal threshold.
Furthermore, we also found in our analyses that vitamin D increments (defined as the difference in vitamin D concentrations between 3 months and baseline) were inversely related to bone loss. This supports the hypothesis that Vitamin D repletion can play a protective role against AI-induced bone loss. The fact that baseline vitamin D concentrations did not predict bone loss, and that vitamin D increments predicted it independently of baseline levels, suggests that the achieved levels at 3 months are a clinically important measurement in deciding whether a higher dose supplementation should be prescribed or not. However, these are novel findings, which need replication in further studies.
Congruent with our results, vitamin D status has been related to BMD [33], and most of the trials and available meta-analyses have shown that vitamin D supplementation is protective for fractures [34, 35]. Besides, vitamin D can have other beneficial effects on bone health, as some trials have reported that it can protect from falls [17]. Nevertheless, some concerns have been recently raised in a clinical trial, where elderly patients given 500,000 IU vitamin D3 once yearly were at higher falls risk than those treated with placebo [36]; however, almost half of patients were probably vitamin D replete with a minority (<5 %) deficient and so while of concern these findings can not be generalised to patients with vitamin D deficiency or insufficiency. Moreover, the administration regime with very high peak levels after each dose, might also contribute to this paradoxical effect.
Strengths and limitations
Our study has several limitations. As this is an observational study, causality for the described association between vitamin D concentrations and bone loss cannot be ensured. Thus, we cannot exclude confounding such as higher vitamin D being a surrogate of higher outdoors activity, which could lead to a reduced bone loss. However, the biological plausibility and the strength of the association observed support our results. A randomised clinical trial is, however, required to confirm them.
After this first year of follow-up, we have not enough statistical power to address the most important outcome in this context: the potential preventive effect of vitamin D on the occurrence of incident fractures. In our data, only five new fractures have been observed so far.
Our data were collected in a clinical setting, not in a randomised clinical trial, and patients were recruited consecutively, which make them more likely to be representative of the population treated with AI in actual practise. Thus, one can assume that the external validity of our results is high.
Conclusions
Our results suggest that Vitamin D higher concentrations after 3 months of supplementation are protective for AI-induced bone loss. A target threshold of ≥40 ng/ml, far above the 20 ng/ml target suggested by the last IOM report, could be recommended for these patients in order to protect them from bone loss. However, a randomised clinical trial is warranted to confirm these results.
References
Coombes RC, Kilburn LS, Snowdon CF, Paridaens R, Coleman RE, Jones SE, Jassem J, Van de Velde CJ, Delozier T, Alvarez I, Del Mastro L, Ortmann O, Diedrich K, Coates AS, Bajetta E, Holmberg SB, Dodwell D, Mickiewicz E, Andersen J, Lonning PE, Cocconi G, Forbes J, Castiglione M, Stuart N, Stewart A, Fallowfield LJ, Bertelli G, Hall E, Bogle RG, Carpentieri M, Colajori E, Subar M, Ireland E, Bliss JM (2007) Survival and safety of exemestane versus tamoxifen after 2–3 years’ tamoxifen treatment (intergroup exemestane study): a randomised controlled trial. Lancet 369(9561):559–570
Burstein HJ, Prestrud AA, Seidenfeld J, Anderson H, Buchholz TA, Davidson NE, Gelmon KE, Giordano SH, Hudis CA, Malin J, Mamounas EP, Rowden D, Solky AJ, Sowers MR, Stearns V, Winer EP, Somerfield MR, Griggs JJ (2010) American society of clinical oncology clinical practice guideline: update on adjuvant endocrine therapy for women with hormone receptor-positive breast cancer. J Clin Oncol 28(23):3784–3796
Goss PE, Ingle JN, Ales-Martinez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, McTiernan A, Robbins J, Johnson KC, Martin LW, Winquist E, Sarto GE, Garber JE, Fabian CJ, Pujol P, Maunsell E, Farmer P, Gelmon KA, Tu D, Richardson H (2011) Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. doi:10.1056/NEJMoa1103507
Coates AS, Keshaviah A, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens R, Castiglione-Gertsch M, Gelber RD, Colleoni M, Lang I, Del Mastro L, Smith I, Chirgwin J, Nogaret JM, Pienkowski T, Wardley A, Jakobsen EH, Price KN, Goldhirsch A (2007) Five years of letrozole compared with tamoxifen as initial adjuvant therapy for postmenopausal women with endocrine-responsive early breast cancer: update of study BIG 1–98. J Clin Oncol 25(5):486–492
Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS (2005) Results of the ATAC (arimidex, tamoxifen, alone or in combination) trial after completion of 5 years’ adjuvant treatment for breast cancer. Lancet 365(9453):60–62
Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, Hilfrich J, Kwasny W, Menzel C, Samonigg H, Seifert M, Gademann G, Kaufmann M, Wolfgang J (2005) Switching of postmenopausal women with endocrine-responsive early breast cancer to anastrozole after 2 years’ adjuvant tamoxifen: combined results of ABCSG trial 8 and ARNO 95 trial. Lancet 366(9484):455–462
Forbes JF, Cuzick J, Buzdar A, Howell A, Tobias JS, Baum M (2008) Effect of anastrozole and tamoxifen as adjuvant treatment for early-stage breast cancer: 100-month analysis of the ATAC trial. Lancet Oncol 9(1):45–53
Crivellari D, Sun Z, Coates AS, Price KN, Thurlimann B, Mouridsen H, Mauriac L, Forbes JF, Paridaens RJ, Castiglione-Gertsch M, Gelber RD, Colleoni M, Lang I, Del Mastro L, Gladieff L, Rabaglio M, Smith IE, Chirgwin JH, Goldhirsch A (2008) Letrozole compared with tamoxifen for elderly patients with endocrine-responsive early breast cancer: the BIG 1–98 trial. J Clin Oncol 26(12):1972–1979
Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, Mackey JR, Beckmann MW, Clack G (2008) Effect of anastrozole on bone mineral density: 5-year results from the anastrozole, tamoxifen, alone or in combination trial 18233230. J Clin Oncol 26(7):1051–1057
Doughty JC (2008) A review of the big results: the breast international group 1–98 trial analyses. Breast 17(Suppl 1):S9–S14
Nogues X, Servitja S, Pena MJ, Prieto-Alhambra D, Nadal R, Mellibovsky L, Albanell J, Diez-Perez A, Tusquets I (2010) Vitamin D deficiency and bone mineral density in postmenopausal women receiving aromatase inhibitors for early breast cancer. Maturitas 66:291–297
Cauley JA, Parimi N, Ensrud KE, Bauer DC, Cawthon PM, Cummings SR, Hoffman AR, Shikany JM, Barrett-Connor E, Orwoll E (2010) Serum 25-hydroxyvitamin D and the risk of hip and nonspine fractures in older men. J Bone Miner Res 25(3):545–553. doi:10.1359/jbmr.090826
Cauley JA, Lacroix AZ, Wu L, Horwitz M, Danielson ME, Bauer DC, Lee JS, Jackson RD, Robbins JA, Wu C, Stanczyk FZ, LeBoff MS, Wactawski-Wende J, Sarto G, Ockene J, Cummings SR (2008) Serum 25-hydroxyvitamin D concentrations and risk for hip fractures. Ann Intern Med 149(4):242–250
Trivedi DP, Doll R, Khaw KT (2003) Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ 326(7387):469. doi:10.1136/bmj.326.7387.469
Smith H, Anderson F, Raphael H, Maslin P, Crozier S, Cooper C (2007) Effect of annual intramuscular vitamin D on fracture risk in elderly men and women—a population-based, randomized, double-blind, placebo-controlled trial. Rheumatology (Oxford) 46(12):1852–1857
Grant AM, Avenell A, Campbell MK, McDonald AM, MacLennan GS, McPherson GC, Anderson FH, Cooper C, Francis RM, Donaldson C, Gillespie WJ, Robinson CM, Torgerson DJ, Wallace WA (2005) Oral vitamin D3 and calcium for secondary prevention of low-trauma fractures in elderly people (randomised evaluation of calcium or vitamin D, record): a randomised placebo-controlled trial. Lancet 365(9471):1621–1628
Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, Orav JE, Stuck AE, Theiler R, Wong JB, Egli A, Kiel DP, Henschkowski J (2009) Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 339:b3692
Prieto-Alhambra D, Javaid MK, Servitja S, Arden NK, Martinez-Garcia M, Diez-Perez A, Albanell J, Tusquets I, Nogues X (2011) Vitamin D threshold to prevent aromatase inhibitor-induced arthralgia: a prospective cohort study. Breast Cancer Res Treat. doi:10.1007/s10549-010-1075-9
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA (2011) The 2011 report on dietary reference intakes for calcium and vitamin d from the institute of medicine: what clinicians need to know. J Clin Endocrinol Metab 96(1):53–58
Servitja S, Nogues X, Prieto-Alhambra D, Martinez-Garcia M, Garrigós L, Pena MJ, de Ramon M, Diez-Perez A, Albanell J, Tusquets I (2012) Bone health in a prospective cohort of postmenopausal women receiving aromatase inhibitors for early breast cancer. Breast 21(1):95–101
Winer EP, Hudis C, Burstein HJ, Wolff AC, Pritchard KI, Ingle JN, Chlebowski RT, Gelber R, Edge SB, Gralow J, Cobleigh MA, Mamounas EP, Goldstein LJ, Whelan TJ, Powles TJ, Bryant J, Perkins C, Perotti J, Braun S, Langer AS, Browman GP, Somerfield MR (2004) American Society of Clinical Oncology technology assessment on the use of aromatase inhibitors as adjuvant therapy for postmenopausal women with hormone receptor-positive breast cancer: status report. J Clin Oncol 23(3):619–629
Orozco Lopez P, Zwart Salmeron M, Vilert Garrofa E, Olmos Dominguez C (2004) Prediction of the total calcium intake from consumption of milk products in Spain adult population. INDICAD study 2001. Aten Primaria 33(5):237–243.
Hines SL, Mincey B, Dentchev T, Sloan JA, Perez EA, Johnson DB, Schaefer PL, Alberts S, Liu H, Kahanic S, Mazurczak MA, Nikcevich DA, Loprinzi CL (2009) Immediate versus delayed zoledronic acid for prevention of bone loss in postmenopausal women with breast cancer starting letrozole after tamoxifen-N03CC. Breast Cancer Res Treat 117(3):603–609. doi:10.1007/s10549-009-0332-2
Gnant M, Mlineritsch B, Luschin-Ebengreuth G, Kainberger F, Kassmann H, Piswanger-Solkner JC, Seifert M, Ploner F, Menzel C, Dubsky P, Fitzal F, Bjelic-Radisic V, Steger G, Greil R, Marth C, Kubista E, Samonigg H, Wohlmuth P, Mittlbock M, Jakesz R (2008) Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol 9(9):840–849
Coleman RE, Banks LM, Girgis SI, Kilburn LS, Vrdoljak E, Fox J, Cawthorn SJ, Patel A, Snowdon CF, Hall E, Bliss JM, Coombes RC (2007) Skeletal effects of exemestane on bone-mineral density, bone biomarkers, and fracture incidence in postmenopausal women with early breast cancer participating in the intergroup exemestane study (IES): a randomised controlled study. Lancet Oncol 8(2):119–127
Markopoulos C, Tzoracoleftherakis E, Polychronis A, Venizelos B, Dafni U, Xepapadakis G, Papadiamantis J, Zobolas V, Misitzis J, Kalogerakos K, Sarantopoulou A, Siasos N, Koukouras D, Antonopoulou Z, Lazarou S, Gogas H Management of anastrozole-induced bone loss in breast cancer patients with oral risedronate: results from the arbi prospective clinical trial. Breast Cancer Res 12(2):R24.
Bundred NJ, Campbell ID, Davidson N, DeBoer RH, Eidtmann H, Monnier A, Neven P, von Minckwitz G, Miller JC, Schenk NL, Coleman RE (2008) Effective inhibition of aromatase inhibitor-associated bone loss by zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: ZO-FAST study results. Cancer 112(5):1001–1010. doi:10.1002/cncr.23259
Van Poznak C, Hannon RA, Mackey JR, Campone M, Apffelstaedt JP, Clack G, Barlow D, Makris A, Eastell R (2010) Prevention of aromatase inhibitor-induced bone loss using risedronate: the sabre trial. J Clin Oncol 28(6):967–975
Ellis GK, Bone HG, Chlebowski R, Paul D, Spadafora S, Fan M, Kim D (2009) Effect of denosumab on bone mineral density in women receiving adjuvant aromatase inhibitors for non-metastatic breast cancer: subgroup analyses of a phase 3 study. Breast Cancer Res Treat 118(1):81–87. doi:10.1007/s10549-009-0352-y
Reid DM, Doughty J, Eastell R, Heys SD, Howell A, McCloskey EV, Powles T, Selby P, Coleman RE (2008) Guidance for the management of breast cancer treatment-induced bone loss: a consensus position statement from a UK expert group. Cancer Treat Rev 34(Suppl 1):S3–S18
Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR (2011) Calcium supplements with or without vitamin d and risk of cardiovascular events: reanalysis of the women’s health initiative limited access dataset and meta-analysis. BMJ 342:d2040
Rastelli AL, Taylor ME, Gao F, Armamento-Villarreal R, Jamalabadi-Majidi S, Napoli N, Ellis MJ (2009) Vitamin D and aromatase inhibitor-induced musculoskeletal symptoms (AIMSS): a phase II, double-blind, placebo-controlled randomized trial. Breast Cancer Res Treat 129:107–116. doi:10.1007/s10549-011-1644-6
Bischoff-Ferrari HA, Kiel DP, Dawson-Hughes B, Orav JE, Li R, Spiegelman D, Dietrich T, Willett WC (2009) Dietary calcium and serum 25-hydroxyvitamin D status in relation to BMD among U.S. adults. J Bone Miner Res 24(5):935–942. doi:10.1359/jbmr.081242
Bischoff-Ferrari HA, Willett WC, Wong JB, Stuck AE, Staehelin HB, Orav EJ, Thoma A, Kiel DP, Henschkowski J (2009) Prevention of nonvertebral fractures with oral vitamin d and dose dependency: a meta-analysis of randomized controlled trials. Arch Intern Med 169(6):551–561
Nieves JW, Barrett-Connor E, Siris ES, Zion M, Barlas S, Chen YT (2008) Calcium and vitamin d intake influence bone mass, but not short-term fracture risk, in Caucasian postmenopausal women from the national osteoporosis risk assessment (NORA) study. Osteoporos Int 19(5):673–679. doi:10.1007/s00198-007-0501-2
Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC (2010) Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 303(18):1815–1822
Acknowledgments
This project has been partially funded by the Instituto Carlos III (FIS Grants 2010, Expedient number PI10/01464). Daniel Prieto-Alhambra receives support from the IDIAP Jordi Gol and Institut Catala de la Salut (“4a Convocatòria d’una estada a una Unitat de Recerca de l’IMIM o de l’ASPB”). The Department of Medical Oncology was supported by “ISCIII/FEDER-Subdirección General de Evaluación y Fomento de la Investigación (PI06 PI10/01464)”, which is part of the “Plan Nacional de Investigación Científica, Desarrollo e Innovación Tecnológica (I+D+I), iniciativa Ingenio 2010, Programa Consolider”, Instituto de Salud Carlos III/FEDER, Spain (RD06/0020/0109; RD06/0020/0019). The Internal Medicine Department and the URFOA IMIM receive support from the RETICEF (Red Temática de Investigación Cooperativa en Envejecimiento y Fragilidad, Instituto Carlos III, Government of Spain). Dr MK Javaid and Professor NK Arden receive support from the NIHR (National Institute of Health Research), Musculoskeletal BRU, Oxford. The authors thank Isabel Aymar for her technical assistance in the DXA measurements.
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prieto-Alhambra, D., Servitja, S., Javaid, M.K. et al. Vitamin D threshold to prevent aromatase inhibitor-related bone loss: the B-ABLE prospective cohort study. Breast Cancer Res Treat 133, 1159–1167 (2012). https://doi.org/10.1007/s10549-012-2013-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-012-2013-9