Abstract
This study was conducted to evaluate the anti-obesity effects of long-term taurine supplementation in a mild obese ICR mouse model and to study the mechanism by which taurine induces weight loss. Three groups of male ICR mice were fed a normal chow diet, a high-fat diet (HFD), or an HFD supplemented with 2% taurine in drinking water for 28 weeks. Body weight was measured every week. Metabolic, behavioral, and physiological monitoring were carried out using PhenoMaster at 28 weeks. Interscapular brown fat (BAT), inguinal white fat tissue (WAT), and quadriceps muscle were analyzed and compared to assess the change of gene expression related to adipogenesis. Taurine supplementation showed the trend of anti-obesity effect in ICR mice fed an HFD for 28 weeks. HFD-fed mice did not show significant difference of oxygen consumption (VO2), energy expenditure (EE), respiratory exchange rate (RER), and locomotive activity compared with those of normal chow diet fed mice. The expression of adipogenesis-related genes such as PPAR-α, PPAR-γ, C/EBP-α, C/EBP-β, and AP2 increased in BAT and WAT, but not in muscle tissue. Taurine supplementation showed the downregulation of these genes in WAT but not in BAT or muscle. Consistently, the expression of taurine transporter (TauT) and adipocyte-specific genes such as adiponectin, leptin, and IL-6 was regulated in a similar pattern by taurine supplementation. Long-term taurine supplementation causes weight loss, most likely by inhibiting adipogenesis in WAT. TauT expression may be involved in the expression of various genes regulated by taurine supplementation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Approximately 0.1% of the human body weight consists of taurine, making it one of the most abundant amino acids. Taurine plays various physiological roles in body (Lambert et al. 2015) and has been investigated as a beneficial molecule to reduce metabolic dysfunctions such as dyslipidemia, insulin resistance, and hyperglycemia, which are mainly associated with obesity (Kim et al. 2012). Thus, the anti-obesity effect of taurine has attracted much interest from many researchers as a potentially safe agent to reduce weight in the era of global obesity (Lifshitz and Lifshitz 2014; Murakami 2017). Taurine is believed to have an anti-obesity effect. White adipose tissue actively synthesizes taurine (Ide et al. 2002), and its synthesis activity changes in the process of differentiation and hypertrophy of adipocytes (Tsuboyama-Kasaoka et al. 2006; Ueki and Stipanuk 2009). In particular, obese people have a lower content of taurine in the body (Rosa et al. 2014). People who consume a lot of seafood containing high levels of taurine are much less likely to have metabolic diseases such as obesity, diabetes, dyslipidemia, and hypertension than people who do not (Yamori et al. 2001, 2010; Sagara et al. 2015). In particular, animal studies have showed that taurine effectively reduces or delays obesity in mice fed a high-fat diet (Lin et al. 2013; Batista et al. 2013). These observations indirectly suggest that taurine scarcity in the body could induce metabolic dysfunctions such as obesity and dyslipidemia, and that taurine is a very important and beneficial substance. Thus, it is important to further elucidate the molecular mechanism by which taurine inhibits metabolic dysfunction.
Long-term treatment with taurine have exerted its beneficial effects such as anti-obesity, anti-hyperglycemia, and anti-atherosclerosis in leptin-deficient obese (ob/ob) animal model or in high-fat diet (HFD)-induced obese mouse models using C57BL/6 mice (Murakami et al. 2000; Borck et al. 2018). In most studies, obese animals were treated for short term with diet or drinking water containing 5% or 3% taurine (Murakami et al. 2016; Du et al. 2010; Ribeiro et al. 2012). The amount of taurine used to treat obese animals in these studies is not practical as a dietary regimen in normal human life. The treatment amount of taurine must be lowered and the anti-obesity effects reevaluated before taurine can be used to treat obese humans. Furthermore, the molecular mechanisms by which taurine ameliorates obesity have been studied by many researchers. Taurine seems to be involved in stimulation of lipid metabolism (Kim et al. 2012) and energy expenditure (Batista et al. 2013) or in inhibition of oxidative stress and inflammation (Rosa et al. 2014). In addition, the degree of obesity in most obese model mice of C57BL/6 is so severe that they develop metabolic diseases such as diabetes and dyslipidemia. Thus, these mouse models may not be suitable to mimic mild obesity in humans.
In this study, we investigated anti-obesity effects of chronic treatment using 2% taurine drinking water on HFD-fed ICR mice and the molecular mechanism by which taurine ameliorates mild obesity.
Materials and methods
Animals and diets
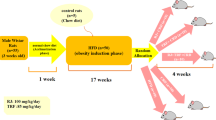
Thirty, male, 4-week old ICR mice were randomly subdivided into three groups, housed in a specific pathogen-free (SPF) facility with a 12 h light/dark cycle, and given ad libitum access to food and water. The first group was fed a normal chew diet (Normal, n = 10); the second group was fed a high-fat diet (HFD) (HFD, n = 10); the third group was fed an HFD supplemented with 2% taurine in the drinking water (HFD + TAU, n = 10). All animal protocols were approved by the Committee on Animals of Kyung Hee University Hospital at GANGDONG (KHNMC AP 2016-009). The normal diet was purchased from Orient Bio (Korea). The HFD, Research Diets D12451 diet (45 kcal % fat), was purchased from Nara Biotech (Seoul, Korea). Taurine was obtained from the Institute of Dong-A Pharmaceuticals (YongIn, Korea). Purified water containing 2% taurine was provided as drinking water for taurine supplementation to the HFD + TAU group, while the Normal and HFD groups received purified water.
Food uptake, activity, metabolic parameters, and body composition
Mouse body weight was monitored weekly for 28 weeks. Metabolic monitoring was assessed in a resting state using the PhenoMaster System (TSE systems GmbH, Bad Homburg, Germany). Energy expenditures including CO2 production (VCO2) and O2 consumption (VO2) were monitored for 48 h. The mice were free to consume food and water. The respiratory exchange ratio (RER) was defined as the ratio of carbon dioxide volume versus oxygen volume (VCO2/VO2). Food uptake and locomotor activity were also measured. An LF50 body composition analyzer (Bruker, Germany) was used to determine body composition (lean body mass, total body fat, and fluid) in mice. Animals were given 4–6 h to acclimate to the metabolic caging prior to beginning data collection, which took place over a 24 h period. Data collected (respiratory exchange ratio (RER), VO2, VCO2, energy expenditure (EE), food uptake, drinking, and activity) were separately averaged over the light and dark periods. Animals were maintained on a 12 h light–dark cycle, continued to consume a standard rodent chow diet, and were provided with water ad libitum. All procedures were approved and ethical consent was provided by the Animal Care Committee at Seoul University College of Veterinary Medicine, Korea Mouse Phenotyping Center (KMPC).
Tissue and blood of mice
Inguinal white fat tissue, interscapular brown fat tissue, and quadriceps muscle were harvested from euthanized mice by cervical dislocation, instantly frozen in liquid nitrogen, and kept at − 80 °C until analysis. Total RNA was extracted from the inguinal white fat tissue, interscapular brown fat tissue, and quadriceps muscle using Trizol (Thermo Fisher Scientific Korea, Seoul). Blood was obtained by heart puncture prior to sacrifice. The blood levels of glucose, total triglyceride, total cholesterol, and high-density lipoproteins cholesterol (HDL-C) were measured by automated clinical chemistry analyzer, FUJI DRI-CHEM NX500 (FUJIFILM, Japan).
Quantitative real-time RT-PCR
cDNA was synthesized from RNA using a commercial cDNA synthesis kit (Thermo Fisher Scientific Korea, Seoul) according to the manufacturer’s instructions. Quantitative real-time RT-PCR was performed using an Applied Biosystem™ Real-Time PCR system (Applied Biosystems, Carlsbad, CA, USA) with the primer sequences shown in Table 1. The relative mRNA expression of the target gene was calculated using the ΔΔCt method and was normalized to 18S rRNA as an internal control.
Statistical analysis
Experimental data are expressed as mean ± standard error of the mean (SEM). Differences between three groups were analyzed using the nonparametric Kruskal–Wallis test. If a statistical difference was detected (P < 0.05), post hoc pairwise group comparisons were performed using Dunn’s test with Bonferroni multiple-testing correction (Dunn 1964). Differences between two groups of day and night was compared by the Mann–Whitney test. Prism software v.5 (Graphpad Software, San Diego, CA) was used for statistical analysis and graphing. Differences were considered statistically significant at P<0.05.
Results
Anti-obesity effect of taurine in high fat diet-induced mildly obese ICR mice
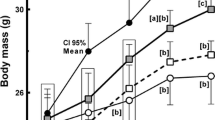
ICR mice are not generally regarded as diet-induced obese (DIO) rodents but, rather, dietary-resistant (DR) strains (Speakman et al. 2007; Zhuhua et al. 2015). To induce mild obesity in these mice, ICR mice were fed a high fat diet (HFD) for 28 weeks to mimic mild human obesity without severe metabolic diseases such as diabetes and hyperlipidemia. HFD-fed mice did not show any metabolic diseases from blood analyses of blood glucose, total cholesterol, or triglycerides (Supplementary data). However, HFD significantly increased animal body weight over 28 weeks of feeding compared to mice receiving a normal diet, which did not induce severe obesity (Fig. 1a). Taurine supplementation (2% in drinking water) showed the trend of anti-obesity in HFD-fed ICR mice after 24 weeks of taurine feeding. At 28 weeks of feeding, the mean body weights (mean ± SEM) of HFD-fed mice and normal mice were 55.90 ± 2.705 g and 45 ± 1.208 g, respectively. Long-term taurine supplementation (2% in drinking water) in HFD-fed mice showed the trend of weight loss in HFD-induced mildly obese ICR mice compared with that of HFD-fed mice (55.9 ± 2.705 g vs. 49.33 ± 1.131 g). In accordance with the body weight change, body composition analysis showed that fat mass significantly increased and lean mass showed the decreasing trend in HFD mice, and the change of body composition was reversed by taurine supplementation with no statistical significance. Taken together, these findings suggest that long-term taurine supplementation (2% in drinking water) may lead to loss of increased fat mass in HFD-fed mice (Fig. 1b).
Effect of taurine on body weight loss in high-fat diet (HFD)-fed ICRice. a ICR mice were fed a normal chew diet, HFD, or HFD + taurine (2% in drinking water) for 28 weeks (n = 10/group). b Body composition of the three groups (n = 4) was analyzed with an LF50 body composition analyzer after each meal for 28 weeks. Differences between three groups were analyzed using the nonparametric Kruskal–Wallis test. If a statistical difference was detected (P < 0.05), post hoc pairwise group comparisons were performed using Dunn’s test with Bonferroni multiple-testing correction. Differences were considered statistically significantly at P < 0.05. ***P < 0.001; **P < 0.01; *P < 0.05; #P < 0.05; ns not significant. To show the trend of weight loss, P value of body weight and body composition was indicated at 28 weeks. *High-fat diet (HFD)-fed group versus normal diet-fed group, #HFD + TAU group versus HFD group
Effects of taurine on the metabolic, behavioral, and physiological activity of HFD-fed mice
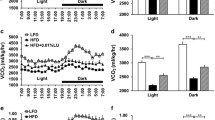
To understand the mechanism by which taurine induced weight loss in the mildly obese mouse model, the mice were first analyzed for metabolic, behavioral, and physiological activity using a metabolic cage. The respiratory exchange rate (RER) is calculated as the ratio of the amounts of carbon dioxide (CO2) produced during metabolism and oxygen (O2) used. It is an indicator of which foods, carbohydrates, or fats are being metabolized to provide the body with energy. Oxidation of fatty acids requires more O2 and produces less CO2 than oxidation of carbohydrates, leading to a lower RER. Thus, as shown in Fig. 2, VCO2 in HFD group was significantly decreased compared to that of normal group. Taurine supplementation could not recover the slightly decreased level of VCO2. The level of RER, VO2 and energy expenditure (EE) also showed the similar pattern as VCO2 pattern without statistical significance. Behavioral activity such as activity, drinking and food uptake also did not show a significant difference between three groups. All parameters measured during the day were lower than night, suggesting that the mice were more active at night than during the day.
Effect of taurine on the metabolic, behavioral, and physiological activities of HFD-fed mice. Metabolic monitoring of mice in each group (n = 4) was performed in a metabolic cage. Energy expenditure and respiratory exchange ratio (RER) were monitored for 48 h. Food uptake, drinking and locomotor activity were also measured. Differences between three groups were analyzed using the nonparametric Kruskal–Wallis test. If a statistical difference was detected (P < 0.05), post hoc pairwise group comparisons were performed using Dunn’s test with Bonferroni multiple-testing correction. Between-group differences of day and night were compared using the Mann–Whitney U test Differences were considered statistically significantly at P < 0.05. *P < 0.05, #P < 0.05; ns not significant, *High-fat diet (HFD)-fed group versus normal diet-fed group, #day group versus night group
Effects of taurine on expression of adipogenesis-related transcription factors and adipocyte-specific genes
First, to determine whether HFD induces adipogenesis in different tissues, the transcriptional expression levels of adipogenesis-related transcription factors of PPAR-α, PPAR-γ, C/EBP-α, C/EBP-β, and AP2 were measured and compared in inguinal white fat tissue, interscapular brown fat tissue, and quadriceps muscle tissues (Fig. 3). The expression of transcription factors was significantly increased by HFD in the two fat tissues but was not increased in muscle. Taurine supplementation induced the downregulation of the increased mRNA expression levels of transcription factors PPAR-γ, C/EBP-α, C/EBP-β, and AP2 in WAT, but it did not downregulate the mRNA expression level of transcriptional factors in BAT and quadriceps muscle. To verify the inhibitory effect of taurine on adipogenesis, the mRNA levels of adipocytes-specific genes were investigated (Fig. 4). First, the mRNA expression levels of leptin, adiponectin, and IL-6 were compared in the three tissues. The expression of leptin and adiponectin in WAT and BAT was significantly higher than that in muscle, while the IL-6 mRNA level in BAT was significantly lower than that of WAT and muscle (Fig. 4a). The mRNA expression levels of their genes were significantly increased by HFD in both WAT and BAT. Inconsistent with the HFD-regulated expression levels of adipogenesis-related transcription factors, HFD increased the expression level of adipocyte-specific genes leptin, adiponectin, and IL-6 in both WAT and BAT. Taurine supplementation decreased the mRNA expression levels of these genes in WAT but not in BAT. All of these results indirectly suggest that taurine supplementation inhibits WAT adipogenesis more specifically than BAT and muscle adipogenesis through the regulation of transcription factor expression.
Effect of taurine on transcriptional expression of adipogenesis-related transcription factors in tissues. The mRNA expression levels of PPAR-α, PPAR-γ, C/EBP-α, C/EBP-β, and AP2 were evaluated from inguinal white fat tissue, interscapular brown fat tissue, and quadriceps muscle tissues of the three groups (n = 5). N normal diet group, H high-fat diet (HFD), H + T HFD + taurine (2% in drinking water). Differences between three groups were analyzed using the nonparametric Kruskal–Wallis test. If a statistical difference was detected (P < 0.05), post hoc pairwise group comparisons were performed using Dunn’s test with Bonferroni multiple-testing correction. The P value of two groups was indicated to show the trend of anti-adipogenesis in WAT. Differences were considered statistically significantly at P < 0.05. **P < 0.01; *P < 0.05; ns not significant
Effects of taurine on transcriptional expression of adipocyte-specific genes in tissues. a The mRNA expression levels of leptin, adiponectin, and IL-6 were evaluated and compared from inguinal white fat tissue, interscapular brown fat tissue, and quadriceps muscle tissues of the normal diet group (n = 5). b Differential regulation of adipocyte-specific genes in WAT and BAT by taurine. Taurine supplementation suppresses HFD-induced expression of adiponectin, leptin, and IL-6 in WAT but not in BAT. Differences between three groups were analyzed using the nonparametric Kruskal–Wallis test. If a statistical difference was detected (P < 0.05), post hoc pairwise group comparisons were performed using Dunn’s test with Bonferroni multiple-testing correction. Differences were considered statistically significantly at P < 0.05. *P < 0.05; ns not significant
Effect of taurine on expression of taurine transporter (TauT)
To explain one of the molecular mechanisms by which taurine differentially inhibits adipogenesis, the expression level of the taurine transporter gene was investigated in the three tissues (Fig. 5). The TauT mRNA expression level was not different in the three tissues. The transcriptional expression level of TauT was increased in HFD mice in the three tissues, and the level of TauT was decreased by taurine supplementation in WAT but not in BAT or muscle. This result suggests that taurine may be required by tissues in response to HFD, and that the transcriptional expression level is more effectively regulated by taurine in WAT than in BAT or muscle.
Effect of taurine on the transcriptional expression of taurine transporter (TauT) in tissues. a TauT mRNA level of the normal diet group was compared in WAT, BAT, and muscle. The mRNA expression level of TauT in the three tissues was not significantly different. b BAT, c WAT, d muscle. The modulation of TauT mRNA level by taurine supplementation was compared in the three tissues of the three groups. HFD increased the mRNA expression of TauT in BAT, WAT, and muscle, but taurine supplementation decreased the TauT mRNA expression in WAT but not in BAT and muscle. Differences between three groups were analyzed using the nonparametric Kruskal–Wallis test. If a statistical difference was detected (P < 0.05), post hoc pairwise group comparisons were performed using Dunn’s test with Bonferroni multiple-testing correction. Differences were considered statistically significantly at P < 0.05. **P < 0.01; *P < 0.05; ns not significant
Discussion
In this study, we investigated whether taurine supplementation induces weight loss in a high-fat diet (HFD)-fed ICR mouse model mimicking human mild obesity. ICR mice were fed a normal chow diet, HFD alone, or HFD supplemented with 2% taurine drinking water for 28 weeks. Taurine significantly reduced weight in HFD-fed ICR obese model mice. To understand the molecular mechanisms by which taurine induces weight loss in ICR mice, we investigated whether taurine increases energy expenditure. Thus, using a TSE PhenoMaster system, we monitored the basal behaviors and physiological parameters of activity, food intake, respiratory exchange rate (PER), energy expenditure (EE), VO2 (oxygen consumption rate), and VCO2. We then further examined whether taurine inhibited adipogenesis in muscle and white and brown fat tissues by assessing the expression levels of transcription factors related to adipogenesis and adipocyte-specific genes.
Although taurine supplementation did not significantly change energy expenditure compared to that of control, HFD group tended to reduce energy expenditure (EE), and taurine supplementation partially restored EE decreased by HFD. Our results contrast with a previous study reporting that taurine supplementation increased resting energy expenditure and prevented HFD-induced obesity in C57BL/6 (Tsuboyama-Kasaoka et al. 2006). Taurine treatment also increased body temperature compared with that of the control obese group in monosodium glutamate (MSG)-induced obesity in rats (Cao et al. 2016). The increase of body temperature in the taurine-treated group can be partly explained by the increased brown fat weight and decreased white fat weight of mice in the taurine group, even though brown fat weight does not necessarily correlate with body temperature. However, in this study, four mice in each group were measured for energy expenditure because of the limited facility of the TSE PhenoMaster system. If more mice were tested, significant differences may be revealed.
When we assessed the inhibitory effect of taurine on adipogenesis in the three tissues, WAT, BAT, and muscle, taurine significantly inhibited the adipogenesis in WAT but not in BAT or muscle. This result is consistent with a previous report (Lin et al. 2013), who showed that taurine supplementation (5%) inhibited weight gain of subcutaneous, epididymal, mesenteric, and retroperitoneal WAT but did not inhibit weight gain of BAT or the gastrocnemius muscle. Furthermore, WAT of taurine-treated mice had a higher concentration of taurine, whereas the taurine content of plasma and liver was not significantly different between groups (Lin et al. 2013). This indirectly means that the taurine transporter (TauT) of WAT has an expression control system that is different from that of the liver. Consistent with this result, in our study (shown in Fig. 4), TauT in WAT was regulated differently from that of BAT and muscle by taurine supplementation. In addition, taurine is converted to taurine chloramine (Tau-Cl) by immune cells at the site of inflammation. We suggested in a previous report that Tau-Cl inhibited differentiation of preadipocytes into adipocytes in a dose-dependent manner (Kim et al. 2013). It suppresses the production of reactive oxygen species (ROS) and proinflammatory cytokines (Schuller-Levis and Park 2004). Taurine supplementation is known to affect food intake and locomotor activity in rats by acting as an agonist of receptors of inhibitory gamma-aminobutyric acid and glycinergic neurotransmitter systems (Albrecht and Schousboe 2005). It can partly explain the mechanism for the anti-obesity effect of taurine. However, in this study, taurine supplementation (2% in drinking water) did not reduce food intake or increase locomotor activity. Thus, we would like to exclude the possibility that the anti-obesity effect of taurine in this study may be due to anorexigenic effects in the central nervous system.
In this study, we used HFD-fed ICR mice mimicking mild obesity in humans. HFD-induced obesity of mice varies depending on the genetic background. For instance, the C57BL/6 mouse is a representative diet-induced obese (DIO) rodent because it greatly increases body weight in response to HFD. On the other hand, many rodents are categorized as dietary-resistant (DR) strains because HFD does not induce obesity (Speakman et al. 2007). Therefore, many researchers prefer C57BL/6 as an animal model of human obesity to test anti-obesity effects of their potential agents. Although ICR mice are not preferred as an obesity model, they have been used as diabetes or metabolic disease models fed a combined diet of high fat and other molecules such as fructose and have the potential to be used as models for metabolic disease studies (Zhuhua et al. 2015). In our preliminary study, ICR mice fed an HFD increased in weight to the level of mild obesity, representing a model that mimics moderate obesity of humans without severe metabolic disease. Regardless of mild obesity, the blood glucose, total cholesterol, high-density lipoprotein-cholesterol (HDLC), and triglyceride levels from the sera of HFD-fed ICR mice was not significantly different from that of normal diet-fed mice. Thus, HFD-fed ICR mice in this study were regarded as a model that mimics moderate obesity of humans without severe metabolic disease.
In conclusion, all the data indirectly suggests that long-term taurine supplementation in a mildly obese ICR mouse model may contribute to weight loss by specifically inhibiting adipogenesis in white fat tissue but not in BAT and muscle and not by increasing energy expenditure. Our results suggest that long-term taurine uptake in normal life may help reduce body weight in mildly obese people. However, clinical trials are needed to determine if the anti-obesity effect of taurine is also present in humans. TauT expression may be more specifically regulated by taurine supplementation in WAT than in BAT and muscle and may be involved in the expression of various genes regulated by taurine supplementation.
References
Albrecht J, Schousboe A (2005) Taurine interaction with neurotransmitter receptors in the CNS: an update. Neurochem Res 30(12):1615–1621. https://doi.org/10.1007/s11064-005-8986-6
Batista TM, Ribeiro RA, da Silva PM, Camargo RL, Lollo PC, Boschero AC, Carneiro EM (2013) Taurine supplementation improves liver glucose control in normal protein and malnourished mice fed a high-fat diet. Mol Nutr Food Res 57(3):423–434. https://doi.org/10.1002/mnfr.201200345
Borck PC, Vettorazzi JF, Branco RCS, Batista TM, Santos-Silva JC, Nakanishi VY, Boschero AC, Ribeiro RA, Carneiro EM (2018) Taurine supplementation induces long-term beneficial effects on glucose homeostasis in ob/ob mice. Amino Acids 50(6):765–774. https://doi.org/10.1007/s00726-018-2553-3
Cao PJ, Jin YJ, Li ME, Zhou R, Yang MZ (2016) PGC-1alpha may associated with the anti-obesity effect of taurine on rats induced by arcuate nucleus lesion. Nutr Neurosci 19(2):86–93. https://doi.org/10.1179/1476830514Y.0000000153
Du H, You JS, Zhao X, Park JY, Kim SH, Chang KJ (2010) Antiobesity and hypolipidemic effects of lotus leaf hot water extract with taurine supplementation in rats fed a high fat diet. J Biomed Sci 17(Suppl 1):S42. https://doi.org/10.1186/1423-0127-17-S1-S42
Dunn OJ (1964) Multiple comparisons using rank sums. Technometrics 6:11
Ide T, Kushiro M, Takahashi Y, Shinohara K, Cha S (2002) mRNA expression of enzymes involved in taurine biosynthesis in rat adipose tissues. Metabolism 51(9):1191–1197
Kim KS, Oh DH, Kim JY, Lee BG, You JS, Chang KJ, Chung HJ, Yoo MC, Yang HI, Kang JH, Hwang YC, Ahn KJ, Chung HY, Jeong IK (2012) Taurine ameliorates hyperglycemia and dyslipidemia by reducing insulin resistance and leptin level in Otsuka Long-Evans Tokushima fatty (OLETF) rats with long-term diabetes. Exp Mol Med 44(11):665–673. https://doi.org/10.3858/emm.2012.44.11.075
Kim KS, Ji HI, Chung H, Kim C, Lee SH, Lee YA, Yang HI, Yoo MC, Hong SJ (2013) Taurine chloramine modulates the expression of adipokines through inhibition of the STAT-3 signaling pathway in differentiated human adipocytes. Amino Acids 45(6):1415–1422. https://doi.org/10.1007/s00726-013-1612-z
Lambert IH, Kristensen DM, Holm JB, Mortensen OH (2015) Physiological role of taurine–from organism to organelle. Acta Physiol (Oxf) 213(1):191–212. https://doi.org/10.1111/apha.12365
Lifshitz F, Lifshitz JZ (2014) Globesity: the root causes of the obesity epidemic in the USA and now worldwide. Pediatr Endocrinol Rev 12(1):17–34
Lin S, Hirai S, Yamaguchi Y, Goto T, Takahashi N, Tani F, Mutoh C, Sakurai T, Murakami S, Yu R, Kawada T (2013) Taurine improves obesity-induced inflammatory responses and modulates the unbalanced phenotype of adipose tissue macrophages. Mol Nutr Food Res 57(12):2155–2165. https://doi.org/10.1002/mnfr.201300150
Murakami S (2017) The physiological and pathophysiological roles of taurine in adipose tissue in relation to obesity. Life Sci 186:80–86. https://doi.org/10.1016/j.lfs.2017.08.008
Murakami S, Kondo Y, Nagate T (2000) Effects of long-term treatment with taurine in mice fed a high-fat diet: improvement in cholesterol metabolism and vascular lipid accumulation by taurine. Adv Exp Med Biol 483:177–186. https://doi.org/10.1007/0-306-46838-7_19
Murakami S, Fujita M, Nakamura M, Sakono M, Nishizono S, Sato M, Imaizumi K, Mori M, Fukuda N (2016) Taurine ameliorates cholesterol metabolism by stimulating bile acid production in high-cholesterol-fed rats. Clin Exp Pharmacol Physiol 43(3):372–378. https://doi.org/10.1111/1440-1681.12534
Ribeiro RA, Santos-Silva JC, Vettorazzi JF, Cotrim BB, Mobiolli DD, Boschero AC, Carneiro EM (2012) Taurine supplementation prevents morpho-physiological alterations in high-fat diet mice pancreatic beta-cells. Amino Acids 43(4):1791–1801. https://doi.org/10.1007/s00726-012-1263-5
Rosa FT, Freitas EC, Deminice R, Jordao AA, Marchini JS (2014) Oxidative stress and inflammation in obesity after taurine supplementation: a double-blind, placebo-controlled study. Eur J Nutr 53(3):823–830. https://doi.org/10.1007/s00394-013-0586-7
Sagara M, Murakami S, Mizushima S, Liu L, Mori M, Ikeda K, Nara Y, Yamori Y (2015) Taurine in 24-h urine samples is inversely related to cardiovascular risks of middle aged subjects in 50 populations of the world. Adv Exp Med Biol 803:623–636. https://doi.org/10.1007/978-3-319-15126-7_50
Schuller-Levis GB, Park E (2004) Taurine and its chloramine: modulators of immunity. Neurochem Res 29(1):117–126
Speakman J, Hambly C, Mitchell S, Krol E (2007) Animal models of obesity. Obes Rev 8(Suppl 1):55–61. https://doi.org/10.1111/j.1467-789X.2007.00319.x
Tsuboyama-Kasaoka N, Shozawa C, Sano K, Kamei Y, Kasaoka S, Hosokawa Y, Ezaki O (2006) Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology 147(7):3276–3284. https://doi.org/10.1210/en.2005-1007
Ueki I, Stipanuk MH (2009) 3T3-L1 adipocytes and rat adipose tissue have a high capacity for taurine synthesis by the cysteine dioxygenase/cysteinesulfinate decarboxylase and cysteamine dioxygenase pathways. J Nutr 139(2):207–214. https://doi.org/10.3945/jn.108.099085
Yamori Y, Liu L, Ikeda K, Miura A, Mizushima S, Miki T, Nara Y, Disease WH-C, Alimentary Comprarison Study G (2001) Distribution of twenty-four hour urinary taurine excretion and association with ischemic heart disease mortality in 24 populations of 16 countries: results from the WHO-CARDIAC study. Hypertens Res 24(4):453–457
Yamori Y, Taguchi T, Mori H, Mori M (2010) Low cardiovascular risks in the middle aged males and females excreting greater 24-hour urinary taurine and magnesium in 41 WHO-CARDIAC study populations in the world. J Biomed Sci 17(Suppl 1):S21. https://doi.org/10.1186/1423-0127-17-S1-S21
Zhuhua Z, Zhiquan W, Zhen Y, Yixin N, Weiwei Z, Xiaoyong L, Yueming L, Hongmei Z, Li Q, Qing S (2015) A novel mice model of metabolic syndrome: the high-fat-high-fructose diet-fed ICR mice. Exp Anim 64(4):435–442. https://doi.org/10.1538/expanim.14-0086
Acknowledgements
The authors thank Dr. Kang Jong-Sun at Sungkyunkwan University School of Medicine for critical reading and comments.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education, Science, and Technology (Grant number 2017R1D1AB03031409). This research was partly supported by the Korea Mouse Phenotyping Project (2013M3A9D5072550) of the Ministry of Science, ICT, and Future Planning through the National Research Foundation.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts.
Research involving human participants and/or animals
All animal protocols were approved by the Committee on Animals of Kyung Hee University Hospital at GANGDONG (KHNMC AP 2016-009). There are no human participants.
Informed consent
Human tissues and sera were never used in this study. No informed consent is required.
Additional information
Handling Editor: S. W. Schaffer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
726_2018_2659_MOESM1_ESM.pptx
Supplementary material 1 Comparison of levels of glucose and lipid of Normal, HFD and HFD (2% taurine drinking water)-fed mice (n = 10). As described in Methods, after sacrifice at 28 weeks, the blood levels of glucose, total triglyceride, total cholesterol, and high-density lipoproteins cholesterol (HDL-C) were measured by automated clinical chemistry analyzer, FUJI DRI-CHEM NX500 (FUJIFILM, Japan). Differences between three groups were analyzed using the nonparametric Kruskal–Wallis test (PPTX 60 kb)
Rights and permissions
About this article
Cite this article
Kim, K.S., Jang, M.J., Fang, S. et al. Anti-obesity effect of taurine through inhibition of adipogenesis in white fat tissue but not in brown fat tissue in a high-fat diet-induced obese mouse model. Amino Acids 51, 245–254 (2019). https://doi.org/10.1007/s00726-018-2659-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-018-2659-7