Abstract
Taurine (Tau) is involved in beta (β)-cell function and insulin action regulation. Here, we verified the possible preventive effect of Tau in high-fat diet (HFD)-induced obesity and glucose intolerance and in the disruption of pancreatic β-cell morpho-physiology. Weaning Swiss mice were distributed into four groups: mice fed on HFD diet (36 % of saturated fat, HFD group); HTAU, mice fed on HFD diet and supplemented with 5 % Tau; control (CTL); and CTAU. After 19 weeks of diet and Tau treatments, glucose tolerance, insulin sensitivity and islet morpho-physiology were evaluated. HFD mice presented higher body weight and fat depots, and were hyperglycemic, hyperinsulinemic, glucose intolerant and insulin resistant. Their pancreatic islets secreted high levels of insulin in the presence of increasing glucose concentrations and 30 mM K+. Tau supplementation improved glucose tolerance and insulin sensitivity with a higher ratio of Akt phosphorylated (pAkt) related to Akt total protein content (pAkt/Akt) following insulin administration in the liver without altering body weight and fat deposition in HTAU mice. Isolated islets from HTAU mice released insulin similarly to CTL islets. HFD intake induced islet hypertrophy, increased β-cell/islet area and islet and β-cell mass content in the pancreas. Tau prevented islet and β-cell/islet area, and islet and β-cell mass alterations induced by HFD. The total insulin content in HFD islets was higher than that of CTL islets, and was not altered in HTAU islets. In conclusion, for the first time, we showed that Tau enhances liver Akt activation and prevents β-cell compensatory morpho-functional adaptations induced by HFD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a complex disease characterized by reduced insulin secretion and increased insulin resistance (Cnop et al. 2005). The interaction between environmental and genetic factors contributes to the onset of obesity and this syndrome is one of the most important contributors to T2DM development (Kahn et al. 2001).
In plasma from prediabetic and diabetic patients, as well as in experimental models of diabetes mellitus (DM), reduced levels of Tau have been observed (Franconi et al. 1995; Anuradha and Balakrishnan 1999; Colivicchi et al. 2004; Tsuboyama-Kasaoka et al. 2006). Decreased plasma Tau levels in DM were also associated with hyperglycemia and disruption of osmoregulation (Hansen 2001). Tau supplementation improved insulin sensitivity and normalized glycemia, insulinemia, hypertension and dyslipidemia in T2DM experimental models (Anuradha and Balakrishnan 1999; Nakaya et al. 2000; Nandhini and Anuradha 2002; Tsuboyama-Kasaoka et al. 2006). Tau also exerted a protective effect upon β-cell apoptosis in streptozotocin-diabetic rats and non-obese diabetic mice, increasing the life span in these rodents (Chang 2000; Di Leo et al. 2004; Arany et al. 2004). Thus, Tau treatment has a potential therapeutic action in DM (Anuradha and Balakrishnan 1999; Nakaya et al. 2000; Nandhini and Anuradha 2002; Tsuboyama-Kasaoka et al. 2006). Here, using the HFD mice model of obesity, which causes increased glycemia after 1 week of diet treatment, and insulin resistance and glucose intolerance at around 8 weeks (Winzell et al. 2007; Ahren and Pacini 2002; Araujo et al. 2007; De Souza et al. 2007), we evaluated the potential preventive effect of Tau upon HFD-induced alterations in body weight and fat accumulation, glucose tolerance and insulin sensitiveness, as well as in pancreatic islet morpho-physiology. For the first time, now we demonstrate that Tau supplementation enhances liver insulin sensitivity and prevents β-cell morpho-functional alterations induced by HFD without interfering with body weight gain and increased fat deposition.
Materials and methods
Materials
125I human insulin was purchased from Genesis (São Paulo, SP, Brazil) and routine reagents were from Sigma-Aldrich Chemicals (St Louis, MO, USA).
Animals
All experiments were approved by the Ethics Committee at UNICAMP. Three-week-old female and male Swiss mice were obtained from the colony at UNICAMP. The mice were maintained on a 12-h light/dark cycle (lights on 0600–1800 hours), controlled temperature (22 ± 1 °C) and allowed free access to food and water after weaning until 6 months of age. The mice were distributed into four groups: mice that received a diet containing 17 % of protein without (CTL) or with 5 % Tau in their drinking water (CTAU); or mice submitted to a high-fat diet containing 36 % of saturated fat without (HFD) or with Tau supplementation (HTAU). Diet compositions have been reported previously (De Souza et al. 2007).
Intraperitoneal glucose (ipGTT) and insulin tolerance test (ipITT)
For ipGTT, blood glucose levels (time 0) were measured in overnight fasted mice using a glucose analyzer (Accu-Chek Advantage, Roche Diagnostic, Switzerland). A glucose load of 2 g/kg body weight was then administered by ip injection and additional blood samples were collected at 15, 30, 60, 120 and 180 min. For ipITT, fed mice were injected with 1.25 U/kg body weight of human insulin (Biohulin® R, Biobrás, Brazil). Blood samples were collected before insulin injection (time 0) and at the times 15, 30, 60 and 90 min after insulin administration for glucose analysis.
General nutritional parameters
Body weights were measured during 19 weeks of diet and Tau treatments. Food and water intake were monitored during the first 3 months of the experimental period. Food efficiency was also measured by the ratio of the subtraction of final body weight from initial body weight divided by the total food intake in the period and multiplying by 100 (Thomson et al. 1994). At the end of the diet and supplementation treatments, the final body weight and nasoanal length were measured in all groups for calculation of the Lee index [from the ratio of body weight (g)1/3/nasoanal length (cm) × 1,000] used as a predictor of obesity in rodents (Bernardis and Patterson 1968). In addition, fasted and fed mice were decapitated, their blood collected and plasma was stored at −20 °C. Total plasma protein and plasma albumin were measured using standard commercial kits, according to the manufacturer’s instructions (Laborlab, Guarulhos, SP, Brazil). Plasma glucose was also analyzed and insulin was measured by radioimmunoassay (RIA; as previously reported by Ribeiro et al. 2010). Total cholesterol (CHOL) and triglycerides (TG) were measured using standard commercial kits, according to the manufacturer’s instructions (Boehringer Mannhein®, Germany; Merck®, Germany).
Tau plasma levels
Plasma-free amino acids were extracted using 80 % ethanol containing 0.1 M HCl. The mixture was sonicated for 10 min and further homogenized for 1 h, followed by centrifugation at 21,000g for 15 min. The supernatant was filtered through a 0.22-mm membrane; 40 μL of the sample was derivatized with phenylisothiocyanate (PTC) [waters pico-tag for free amino acids (WAT0 10954 Ver4)], and 20 μL of the PTC derivative was separated by chromatography using a Luna C-18 5 μ, 250 × 4.6-mm column (00G-4252-EQ; Phenomenex, Torrance, CA, USA), at 50 °C, in an HPLC system (SCL-10avp, CTO10avp, SPDm10avp; Shimadzu Scientific Instruments, Columbia, MD, USA) with CLASS-VP 6.12 software. An amino acid standard solution was derivatized and analyzed together with the samples and methionine sulfone was used as an internal control.
Islet isolation and insulin secretion
Islets were isolated by collagenase digestion of the pancreas. For static incubations, groups of four islets were first incubated for 30 min at 37 °C in Krebs-Ringer bicarbonate (KRB) buffer with 5.6 mM glucose and 3 g of BSA/L, and equilibrated with a mixture of 95 % O2/5 % CO2 to give pH 7.4. This medium was then replaced with fresh buffer and the islets incubated for 1 h in the presence of 2.8; 8.3 and 16.7 mM glucose or with 30 mM K+. Aliquots of the supernatant at the end of the incubation period were kept at –20 °C for posterior insulin measurement by RIA. For islet insulin content, groups of four islets were collected and transferred to tubes of 1.5 mL. Deionized water (1 mL) was added to the samples, followed by disruption of the pancreatic cells using a Polytron PT 1200 C homogenizer (Brinkmann Instruments, NY, USA) and the islet insulin content was also measured by RIA.
Pancreas morphometry and immunohistochemistry
For morphometric analysis, pancreases from both groups of mice were removed, weighed and fixed for 16 h in Bouin’s solution. After fixation, each pancreas was cut into five pieces of similar sizes. All pancreas fragments were embedded in paraffin and three fragments were selected (Inuwa and El Mardi 2005). From each fragment block, exhaustive 5-μm serial sections were obtained (every 20th section) and randomly selected for insulin and glucagon immunoperoxidase reaction. For immunohistochemistry, paraffin was removed; the sections were rehydrated and washed with 0.05 M Tris–saline buffer (TBS) pH 7.4, and incubated with TBS containing 0.3 % H2O2 for endogenous peroxidase activity blockade and permeabilized for 1 h with TTBS (0.1 % Tween 20 and 5 g/% of fat free milk in TBS). The sections were incubated with a polyclonal guinea pig anti-insulin (1:100; Dako North America, Inc., CA, USA) or rabbit anti-glucagon (1:50; Dako North America, Inc., CA, USA) antibody at 4 °C overnight and, after this period, incubated with rabbit anti-guinea pig IgG or goat anti-rabbit conjugated antibody with HRP for 1 h and 30 min. The positive insulin or glucagon cells were detected with diaminobenzidine (DAB; Sigma-Aldrich Chemicals, St Louis, MO, USA) solution (10 % DAB and 0.2 % H2O2 in TBS). Finally, the sections were quickly stained with Ehrlich’s hematoxylin and mounted for microscopy observation (Carvalho et al. 2006). All islets present in the sections were covered systematically by capturing images with a digital camera (Nikon FDX-35) coupled to a Nikon Eclipse E800 microscope. The islet, β-cell, α-cell and section areas were analyzed using the free software, Image Tool (http://ddsdx.uthscsa.edu/dig/itdesc.html). The islet and β-cell mass were calculated by multiplying the pancreas weight by the total islet or β-cell area per pancreas section.
Western blotting
For the evaluation of phospho (p)-Akt/Akt ratio, mice were anesthetized with a mixture of ketamine (Vetbrands®, Paulínia, SP, Brazil) and xylazine (Rompun, Bayer®, São Paulo, SP, Brazil), and subsequently received an ip injection of insulin (100 μL; 1.10−6 M) or 0.9 % saline (100 μL). After 5 min, fragments of the liver and the gastrocnemius muscle were excised and immediately solubilized in homogenization buffer at 4 °C (containing: 100 mM Tris pH 7.5, 10 mM sodium pyrophosphate, 100 mM sodium fluoride, 10 mM EDTA, 10 mM sodium vanadate, 2 mM phenylmethylsulfonyl fluoride and 1 % Triton-X 100) using a Polytron PTA 20S generator (model PT 10/35; Brinkmann Instruments, Westbury, NY) (Araujo et al. 2007). The extracts were then centrifuged at 12,600g at 4 °C for 30 min to remove insoluble material. The protein concentration in the supernatants was assayed using the Bradford dye method (Bradford, 1974), using BSA as a standard curve and Bradford reagent (Bio-Agency Lab., São Paulo, SP, Brazil). For SDS gel electrophoresis and Western blot analysis, the samples were homogenized with a loading buffer containing DTT. After heating at 95 °C for 5 min, the proteins were separated by electrophoresis (100 μg protein/lane, 8 % gels) and after that transferred to nitrocellulose membranes. The membranes were subsequently blotted with specific rabbit polyclonal antibodies to the Akt1/2/3 or pAkt Ser4731/2/3 (1:1,000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). Visualization of specific protein bands was done by incubating the membranes with goat anti-rabbit secondary antibody (1:10,000; Zymed Laboratories, Inc., San Francisco, CA, USA), followed by exposure to X-ray film. The band intensities were quantified by optical densitometry using the free software, Image Tool (http://ddsdx.uthscsa.edu/dig/itdesc.html).
Statistical analysis
Results are presented as mean values ± SEM for the number of determinations (n) indicated. When working with islets, n refers to the number of experiments performed with groups of four islets each. The data were analyzed by two-way ANOVA (for mean comparisons between diet and supplementation variables) and two-way ANOVA with repeated measures (for glucose and insulin tolerance experiments, and Akt phosphorylation followed by insulin administration) with Bonferroni post hoc test and were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA, USA). The level of significance was set at P < 0.05.
Results
Mice features
Figure 1 shows that body weight (BW) in HFD and HTAU mice was significantly higher even at 6 weeks after the onset of the diet, when compared with CTL mice. The total body weight expressed by the area under the curve (AUC) during the experimental period was also higher in HFD and HTAU mice compared with CTL (Fig. 1b; P < 0.001). Food and water intakes as well as food efficiency were similar between all groups (data not shown). Also, at the end of experimental period, mice submitted to HFD showed increased final BW, retroperitoneal fat pads and Lee index compared with CTL mice (P < 0.02 and P < 0.0001, respectively; Table 1). In addition, liver and heart weights were higher in the HFD group (3.5 ± 0.2 g and 181 ± 5 mg, respectively) compared with the CTL group (2.4 ± 0.1 g and 153 ± 4 mg, respectively; P < 0.0001). Tau supplementation did not modify the alterations in BW, fat accumulation and organ weight in HTAU mice (3.5 ± 0.2 g and 177 ± 5 mg, for liver and spleen, respectively) (Table 1). Fasted and fed HFD mice were hyperglycemic and hyperinsulinemic compared with CTL (P < 0.0001; Table 2). Fed CHOL plasma levels were also higher in HFD group, whereas TG, albumin and total protein concentrations were similar between the HFD and CTL groups. Under fasting and fed conditions, the HTAU group showed improved plasma glucose and insulin concentrations compared with the HFD group (Table 2). In addition, the supplementation methodology applied in our study efficiently increased Tau plasma levels in HTAU and CTAU groups, when compared with their respective controls (P < 0.01 and P < 0.0001, respectively; Table 2).
a Body weight during the feeding period was measured weekly in mice fed on HFD or CTL diet without or with Tau supplementation. b Mean ± SEM of AUC of body weight registered during the 19 weeks of diets and Tau treatments obtained from 10 to 12 mice for each group. Asterisk significant difference between HFD and HTAU groups compared to controls. The letters over the bars represent significant differences (P < 0.05)
Glucose tolerance and insulin sensitiveness
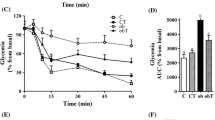
At the end of the treatment period, mice were submitted to an ipGTT and ipITT. Figure 2a shows that after a glucose load, plasma glucose concentration reached maximal levels at 60 min in all groups. Higher plasma glucose levels were observed in HFD compared to the CTL group at all times of the test (Fig. 2a), whereas HTAU mice showed lower glycemia at 120 and 180 min compared with HFD mice (P < 0.0001). The AUC during the ipGTT in HFD was higher than that of the CTL group (P < 0.0001; Fig. 2b). This impaired glucose tolerance was in accordance with a lower insulin action, demonstrated by a delay in the glucose disappearance rate in HFD compared with CTL mice during ipITT (Fig. 2c). The AUC during ipITT was also higher in HFD than CTL group (P < 0.001; Fig. 2d). Tau supplementation enhanced glucose tolerance and insulin sensitivity with glucose levels during ipGTT and ipITT that fell between those observed for CTL and HFD mice (Fig. 2b, d).
Changes in plasma glucose levels during ipGTT (a) and ipITT (c) in CTL, CTAU, HFD and HTAU mice. Total glucose plasma concentration during the ipGTT (b) and ipITT (d) expressed by AUC. Data are mean values ± SEM obtained from 13 to 19 mice for each group. The different letters in each glycemia point of ipITT and over the bars represent significant differences. Asterisk HFD and HTAU were different from CTL and CTAU. Hash indicates difference between the HTAU and HFD group, P < 0.05
For demonstrated effective Akt phosphorylation and activation after insulin administration, the ratio of band densitometry of pAkt is divided by total Akt content (Gomez-Perez et al. 2011), since this result excludes any pAkt increase due to alterations in Akt total amount. It is necessary to state that, in our study, Akt protein levels were similar in the liver and gastrocnemius muscle of all groups. Insulin administration efficiently increased pAkt expression (Fig. 3). Liver pAkt/Akt ratio in response to insulin administration was similar between HFD and CTL groups, whereas HTAU showed 25 and 30 % higher pAkt/Akt ratio in the liver of CTL and HFD mice, respectively (P < 0.001; Fig. 3a). No difference between pAkt/Akt ratio was noticed in the gastrocnemius muscles for any of the groups (Fig. 3b).
pAkt Ser4731/2/3 /Akt1/2/3 protein expression in CTL, CTAU, HFD and HTAU mice. Mice of both experimental groups were anesthetized and received an ip injection of 0.9 % saline (100 μL) (−) or insulin (100 μL; 1.10−6 M) (+). After 5 min, fragments were obtained from liver (a) and gastrocnemius muscle (b) and used for immunoblotting experiments. The bars represent mean values ± SEM of the percentage from the ratio of densitometry values of pAkt divided by total Akt protein content in the liver (a) and gastrocnemius muscle (b) from CTL (n = 8), CTAU (n = 7), HFD (n = 8) and HTAU (n = 8) mice treated with saline (−) or insulin (+). Different letters over the bars indicate significant differences, P < 0.05
Insulin secretion and islet insulin content
Figure 3a shows glucose-induced insulin secretion in isolated islets from all groups of mice. At stimulatory glucose concentrations (8.3 and 16.7 mM), insulin secretion was higher in HFD compared with CTL islets (P < 0.0001; Fig. 4a). HFD islets also secreted more insulin at 2.8 mM glucose plus 30 mM K+ (P < 0.05; Fig. 4b). Isolated islets from HTAU showed a similar glucose-induced insulin secretion compared with islets isolated from CTL mice (Fig. 4a); however, and differently than that observed for glucose, at 30 mM K+, a similar insulin secretion was registered in HTAU and HFD islets (Fig. 4b). Total insulin content in HFD was 78 % higher than that of CTL islets (192 ± 22 and 108 ± 9 ng/islet, respectively; P < 0.0001), whereas in HTAU islets the insulin content was similar to that of the controls (HTAU 130 ± 11 and CTAU 103 ± 8 ng/islet).
a Glucose and b K+-induced insulin secretion in isolated islets from CTL, CTAU, HFD and HTAU mice. For static insulin secretion, groups of four islets were incubated for 1 h with 2.8, 8.3, 16.7 mM glucose (G) or 2.8 mM glucose plus 30 mM K+. Data are mean values ± SEM obtained from 30 to 34 groups of islets. Different letters over the bars indicate significant differences, P < 0.05
Pancreatic islet morphology and morphometry
Table 3 shows pancreatic morphometry of all groups of mice. Pancreas weight was similar between HFD and CTL mice but higher in HTAU compared with CTL (P < 0.001). Histological analysis revealed that pancreatic islets from HFD were hypertrophic with increased β-cell area compared with the CTL group (P < 0.001; Fig. 6), without alterations in the α-cell area (Table 3). In addition, islet and β-cell mass were higher in HFD compared with the CTL group (P < 0.05). The alterations in islet and β-cell areas observed in the HFD were partially prevented in the HTAU group (Table 3). However, the β-cell and islet mass in the pancreas of HTAU was similar to that of CTL mice (Fig. 5). The number of islets per pancreatic section was similar between the CTL, HFD and HTAU groups, whereas in the CTAU group, the amount of islets per section was significantly lower than for the HFD and HTAU groups (P < 0.05 and P < 0.001, respectively). The morphology and cytoarchitecture of the islets were not altered by HFD or Tau treatment (Fig. 6).
β-cell (a) and islet mass (b) of the endocrine pancreas from CTL, CTAU, HFD and HTAU mice. The islet and β-cell mass were calculated by multiplying the pancreas weight by the total β-cell or islet area per pancreas section. Data are mean values ± SEM obtained from 18 to 26 pancreas sections analyzed. Different letters over the bars indicate significant differences, P < 0.05
Discussion
In our study, and in agreement with previous reports (Ahren and Pacini 2002; De Souza et al. 2007; Tsuboyama-Kasaoka et al. 2006; Winzell et al. 2007; Araujo et al. 2007), HFD mice presented higher body weight, increased fat pad depots, hyperglycemia, hyperinsulinemia, glucose intolerance and insulin resistance. Furthermore, isolated islets from HFD mice secreted more insulin associated with islet and β-cell hyperplasia and hypertrophy. These data confirm that HFD was effective in inducing obesity and a T2DM phenotype. In addition, we demonstrated, for the first time, that Tau supplementation prevented pancreatic β-cell morphologic and functional compensatory adaptations in HFD, accompanied by improvements in glucose homeostasis and insulin action.
Several reports have shown that decreased Tau plasma concentrations disrupt glucose homeostasis (Anuradha and Balakrishnan 1999; Colivicchi et al. 2004; Tsuboyama-Kasaoka et al. 2006), whereas restoration of Tau plasma levels ameliorated glucose control and insulin sensitivity (Anuradha and Balakrishnan 1999; Nakaya et al. 2000; Nandhini and Anuradha 2002; Tsuboyama-Kasaoka et al. 2006; Loizzo et al. 2007; Carneiro et al. 2009; Ribeiro et al. 2009). Tau enhances insulin’s actions probably by acting on the insulin receptor (IR) (Maturo and Kulakowski 1988); we demonstrated that an ip injection of Tau increased IR phosphorylation in the liver and muscle with a similar level to that induced by insulin (Carneiro et al. 2009).
Here, although HFD mice did not present any alteration in Tau plasma levels, the HTAU group showed enhanced Tau plasma concentrations (Table 2) and we also observed increased Akt phosphorylation in the liver of HTAU mice after an ip administration of insulin (Fig. 3a). It was also reported that Tau increased Akt phosphorylation and promoted cardiomyocyte protection against doxorubicin-induced cardiac oxidative stress (Das et al. 2011). By enhancing phosphoinositide 3-kinase (PI3K)/Akt activation, Tau also prevented liver insulin resistance induced by a 48-h intralipid plus heparin infusion (Wu et al. 2010). As such, the increased pAkt/Akt ratio in the liver of HTAU mice may be responsible for the improvement of insulinemia and glycemia in this group (Table 2). This assumption is based on the fact that Akt regulates liver glucose storage via phosphorylation and inactivation of the GSK3 (glycogen synthase kinase 3). In addition, Akt inactivates transcription factors such as Foxo1 (forkhead transcription factors) and PGC-1α (peroxisome proliferator-activated receptor-coactivator 1γ) that regulate the expression of genes involved in gluconeogenesis and fatty acid oxidation (Whiteman et al. 2002; Li et al. 2007).
Our data demonstrated that Tau enhanced insulin signaling and preserved β-cell secretory function, since HTAU released insulin in a similar manner to that of CTL islets in the presence of glucose (Fig. 4a). In addition, insulinemia in HTAU was significantly lower than in HFD mice (Table 2).
We have shown that Tau supplementation improved β-cell responsiveness to glucose in normal and prediabetic rodents, regulating islet Ca2+ handling and expression of β-cell proteins involved in the stimulus/secretion coupling (Ribeiro et al. 2009; Ribeiro et al. 2010; Batista et al. 2012). However, the mechanism by which Tau prevents β-cell dysfunction in obesity has been less explored. It has been demonstrated that reduced insulin secretion in obese subjects, submitted to a 48-h intralipid infusion, was prevented by Tau treatment (Xiao et al. 2008). Oprescu et al. (2007) observed that oleate infusion decreased C peptide plasma concentrations in rats. They also showed a normal β-cell secretory response in isolated islets incubated with oleate plus Tau. In addition, INS1 832/13 cells, incubated with high glucose concentrations, together with Tau, showed reduced β-cell autophagy (Kaniuk et al. 2007). It has been suggested that the potential preventive effect of Tau in β-cell dysfunction, in the presence of glucose or fatty acids, is due to the antioxidant properties of the amino acid (Kaniuk et al. 2007; Oprescu et al. 2007; Xiao et al. 2008). Considering that Tau interacts with IR (Maturo and Kulakowski 1988; Carneiro et al. 2009), associated with the fact that the pAkt/Akt ratio was higher in the liver of HTAU mice (Fig. 3a), we suggest that Tau-induced preservation of β-cell function may also be due to an enhancement in the autocrine insulin signaling in these cells.
The inhibitory autocrine action of insulin is via PI3K (Zawalich and Zawalich 2000; Zawalich et al. 2002). Insulin also regulates its own gene transcription (Persaud et al. 2008) and, via interaction with IR and the insulin like-growth factor receptor, activates Akt and ERK1/2, which are involved in β-cell proliferation and apoptosis (Elghazi et al. 2006; Lawrence et al. 2008). Thus, the increased Tau concentration in the islet microenvironment, induced by supplementation, together with the insulin secreted by the β-cells may account for the prevention of islet insulin hypersecretion and contribute to the maintenance of normal islet and β-cell mass in HTAU mice (Figs. 4a, 5).
At the present time, we do not have a clear explanation as to why obesity was not prevented in HTAU mice, since several reports have shown that Tau supplementation exerts a negative effect upon fat accumulation in different rodent models of obesity (Tsuboyama-Kasaoka et al. 2006; Nardelli et al. 2011). We can only speculate that Tau effects and sensitivity differ between species and mice strain used.
In conclusion, our study shows that Tau supplementation ameliorates glucose homeostasis in mice submitted to HFD and prevents β-cell hypersecretion, as well as β-cell and islet mass compensatory alterations induced by the insulin-resistant state. These effects may be due, at least in part, to enhancing insulin signaling via phosphorylation of Akt.
References
Ahren B, Pacini G (2002) Insufficient islet compensation to insulin resistance vs. reduced glucose effectiveness in glucose-intolerant mice. Am J Physiol Endocrinol Metab 283(4):E738–E744
Anuradha CV, Balakrishnan SD (1999) Taurine attenuates hypertension and improves insulin sensitivity in the fructose-fed rat, an animal model of insulin resistance. Can J Physiol Pharmacol 77(10):749–754
Arany E, Strutt B, Romanus P, Remacle C, Reusens B, Hill DJ (2004) Taurine supplement in early life altered islet morphology, decreased insulitis and delayed the onset of diabetes in non-obese diabetic mice. Diabetologia 47(10):1831–1837
Araujo EP, De Souza CT, Ueno M, Cintra DE, Bertolo MB, Carvalheira JB, Saad MJ, Velloso LA (2007) Infliximab restores glucose homeostasis in an animal model of diet-induced obesity and diabetes. Endocrinology 148(12):5991–5997
Batista TM, Ribeiro RA, Amaral AG, de Oliveira CA, Boschero AC, Carneiro EM (2012) Taurine supplementation restores glucose and carbachol-induced insulin secretion in islets from low-protein diet rats: involvement of Ach-M3R, Synt 1 and SNAP-25 proteins. J Nutr Biochem 23(3): 306–312
Bernardis LL, Patterson BD (1968) Correlation between ‘Lee index’ and carcass fat content in weanling and adult female rats with hypothalamic lesions. J Endocrinol 40(4):527–528
Carneiro EM, Latorraca MQ, Araujo E, Beltra M, Oliveras MJ, Navarro M, Berna G, Bedoya FJ, Velloso LA, Soria B, Martin F (2009) Taurine supplementation modulates glucose homeostasis and islet function. J Nutr Biochem 20(7):503–511
Carvalho CP, Martins JC, da Cunha DA, Boschero AC, Collares-Buzato CB (2006) Histomorphology and ultrastructure of pancreatic islet tissue during in vivo maturation of rat pancreas. Ann Anat 188(3):221–234
Chang KJ (2000) Effect of taurine and beta-alanine on morphological changes of pancreas in streptozotocin-induced rats. Adv Exp Med Biol 483:571–577
Cnop M, Welsh N, Jonas JC, Jorns A, Lenzen S, Eizirik DL (2005) Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54(Suppl 2):S97–S107
Colivicchi MA, Raimondi L, Bianchi L, Tipton KF, Pirisino R, Della Corte L (2004) Taurine prevents streptozotocin impairment of hormone-stimulated glucose uptake in rat adipocytes. Eur J Pharmacol 495(2–3):209–215
Das J, Ghosh J, Manna P, Sil PC (2011) Taurine suppresses doxorubicin-triggered oxidative stress and cardiac apoptosis in rat via up-regulation of PI3-K/Akt and inhibition of p53, p38-JNK. Biochem Pharmacol 81(7):891–909
De Souza CT, Araujo EP, Stoppiglia LF, Pauli JR, Ropelle E, Rocco SA, Marin RM, Franchini KG, Carvalheira JB, Saad MJ, Boschero AC, Carneiro EM, Velloso LA (2007) Inhibition of UCP2 expression reverses diet-induced diabetes mellitus by effects on both insulin secretion and action. FASEB J 21(4):1153–1163
Di Leo MA, Santini SA, Silveri NG, Giardina B, Franconi F, Ghirlanda G (2004) Long-term taurine supplementation reduces mortality rate in streptozotocin-induced diabetic rats. Amino Acids 27(2):187–191
Elghazi L, Balcazar N, Bernal-Mizrachi E (2006) Emerging role of protein kinase B/Akt signaling in pancreatic beta-cell mass and function. Int J Biochem Cell Biol 38(2):157–163
Franconi F, Bennardini F, Mattana A, Miceli M, Ciuti M, Mian M, Gironi A, Anichini R, Seghieri G (1995) Plasma and platelet taurine are reduced in subjects with insulin-dependent diabetes mellitus: effects of taurine supplementation. Am J Clin Nutr 61(5):1115–1119
Gomez-Perez Y, Gianotti M, Proenza AM, Llado I (2011) Age-related decline of skeletal muscle insulin sensitivity in rats: effect of sex and muscle type. Rejuvenation Res 14(2):153–161
Hansen SH (2001) The role of taurine in diabetes and the development of diabetic complications. Diabetes Metab Res Rev 17(5):330–346
Inuwa IM, El Mardi AS (2005) Correlation between volume fraction and volume-weighted mean volume, and between total number and total mass of islets in post-weaning and young Wistar rats. J Anat 206(2):185–192
Kahn SE, Prigeon RL, Schwartz RS, Fujimoto WY, Knopp RH, Brunzell JD, Porte D Jr (2001) Obesity, body fat distribution, insulin sensitivity and islet beta-cell function as explanations for metabolic diversity. J Nutr 131(2):354S–360S
Kaniuk NA, Kiraly M, Bates H, Vranic M, Volchuk A, Brumell JH (2007) Ubiquitinated-protein aggregates form in pancreatic beta-cells during diabetes-induced oxidative stress and are regulated by autophagy. Diabetes 56(4):930–939
Lawrence M, Shao C, Duan L, McGlynn K, Cobb MH (2008) The protein kinases ERK1/2 and their roles in pancreatic beta cells. Acta Physiol (Oxford) 192(1):11–17
Li X, Monks B, Ge Q, Birnbaum MJ (2007) Akt/PKB regulates hepatic metabolism by directly inhibiting PGC-1alpha transcription coactivator. Nature 447(7147):1012–1016
Loizzo A, Carta S, Bennardini F, Coinu R, Loizzo S, Guarino I, Seghieri G, Ghirlanda G, Franconi F (2007) Neonatal taurine administration modifies metabolic programming in male mice. Early Hum Dev 83(10):693–696
Maturo J, Kulakowski EC (1988) Taurine binding to the purified insulin receptor. Biochem Pharmacol 37(19):3755–3760
Nakaya Y, Minami A, Harada N, Sakamoto S, Niwa Y, Ohnaka M (2000) Taurine improves insulin sensitivity in the Otsuka Long-Evans Tokushima Fatty rat, a model of spontaneous type 2 diabetes. Am J Clin Nutr 71(1):54–58
Nandhini AT, Anuradha CV (2002) Taurine modulates kallikrein activity and glucose metabolism in insulin resistant rats. Amino Acids 22(1):27–38
Nardelli TR, Ribeiro RA, Balbo SL, Vanzela EC, Carneiro EM, Boschero AC, Bonfleur ML (2011) Taurine prevents fat deposition and ameliorates plasma lipid profile in monosodium glutamate-obese rats. Amino Acids 41(4):901–908
Oprescu AI, Bikopoulos G, Naassan A, Allister EM, Tang C, Park E, Uchino H, Lewis GF, Fantus IG, Rozakis-Adcock M, Wheeler MB, Giacca A (2007) Free fatty acid-induced reduction in glucose-stimulated insulin secretion: evidence for a role of oxidative stress in vitro and in vivo. Diabetes 56(12):2927–2937
Persaud SJ, Muller D, Jones PM (2008) Insulin signalling in islets. Biochem Soc Trans 36(Pt 3):290–293
Ribeiro RA, Bonfleur ML, Amaral AG, Vanzela EC, Rocco SA, Boschero AC, Carneiro EM (2009) Taurine supplementation enhances nutrient-induced insulin secretion in pancreatic mice islets. Diabetes Metab Res Rev 25(4):370–379
Ribeiro RA, Vanzela EC, Oliveira CA, Bonfleur ML, Boschero AC, Carneiro EM (2010) Taurine supplementation: involvement of cholinergic/phospholipase C and protein kinase A pathways in potentiation of insulin secretion and Ca2+ handling in mouse pancreatic islets. Br J Nutr 104(8):1148–1155
Thomson JE, Jones EE, Eisen EJ (1994) Effect of spray-dried porcine plasma protein on feed intake, growth rate, and efficiency of gain in mice. J Anim Sci 72(10):2690–2695
Tsuboyama-Kasaoka N, Shozawa C, Sano K, Kamei Y, Kasaoka S, Hosokawa Y, Ezaki O (2006) Taurine (2-aminoethanesulfonic acid) deficiency creates a vicious circle promoting obesity. Endocrinology 147(7):3276–3284
Whiteman EL, Cho H, Birnbaum MJ (2002) Role of Akt/protein kinase B in metabolism. Trends Endocrinol Metab 13(10):444–451
Winzell MS, Magnusson C, Ahren B (2007) Temporal and dietary fat content-dependent islet adaptation to high-fat feeding-induced glucose intolerance in mice. Metabolism 56(1):122–128
Wu N, Lu Y, He B, Zhang Y, Lin J, Zhao S, Zhang W, Li Y, Han P (2010) Taurine prevents free fatty acid-induced hepatic insulin resistance in association with inhibiting JNK1 activation and improving insulin signaling in vivo. Diabetes Res Clin Pract 90(3):288–296
Xiao C, Giacca A, Lewis GF (2008) Oral taurine but not N-acetylcysteine ameliorates NEFA-induced impairment in insulin sensitivity and beta cell function in obese and overweight, non-diabetic men. Diabetologia 51(1):139–146
Zawalich WS, Zawalich KC (2000) A link between insulin resistance and hyperinsulinemia: inhibitors of phosphatidylinositol 3-kinase augment glucose-induced insulin secretion from islets of lean, but not obese, rats. Endocrinology 141(9):3287–3295
Zawalich WS, Tesz GJ, Zawalich KC (2002) Inhibitors of phosphatidylinositol 3-kinase amplify insulin release from islets of lean but not obese mice. J Endocrinol 174(2):247–258
Acknowledgments
This study was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ E-26/111.914/2011) and Conselho Nacional para o Desenvolvimento Científico e Tecnológico (CNPq). We are grateful to LD Teixeira for animal care, E.C. Vanzela and J.C. Souza for technical assistance, T.M. Batista for help in data analysis and interpretation and Nicola Conran for editing the English. We also thank E.M. Risso and Prof. Dr. J.A. Farfán from the Laboratório de Fontes Protéicas (Faculdade de Engenharia de Alimentos, UNICAMP) for plasma Tau concentration determination.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ribeiro, R.A., Santos-Silva, J.C., Vettorazzi, J.F. et al. Taurine supplementation prevents morpho-physiological alterations in high-fat diet mice pancreatic β-cells. Amino Acids 43, 1791–1801 (2012). https://doi.org/10.1007/s00726-012-1263-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-012-1263-5