Abstract
Background:
Two brown-like adipocytes, including classical brown adipocytes from brown adipose tissues and beige cells from white adipose tissues, regulate thermogenesis. The developmental and functional induction of brown-like cells provides a defense against obesity and associated metabolic diseases. Our previous study suggests dietary luteolin can improve diet-induced obesity and insulin resistance in mice. Here we further elucidated the action of the natural flavonoid on energy expenditure and adaptive thermogenesis.
Methods:
Five-week-old male C57BL/6 mice were fed low-fat diet (LFD), high-fat diet (HFD) and HFD supplemented with 0.01% luteolin. After 12 weeks, their energy expenditure were detected using a combined indirect calorimetry system. Moreover, thermogenic program and associated molecular regulators were assessed in adipose tissues. In another independent study, even-aged mice were fed LFD and luteolin-containing LFD for 12 weeks, and their energy expenditure and thermogenic program were also investigated. Finally, differentiated primary brown and subcutaneous adipocytes were used to identify the critical participation of AMPK/PGC1α signaling in luteolin-regulated browning and thermogenesis.
Results:
In mice fed either HFD or LFD, dietary luteolin supplement increased oxygen consumption, carbon dioxide production and respiratory exchange ratio. The enhancement in energy expenditure was accompanied by the upregulation of thermogenic genes in brown and subcutaneous adipose tissues. Meanwhile, several important AMPK/PGC1α signaling molecules were activated by dietary luteolin in the tissues. Further, luteolin treatment directly elevated thermogenic gene expressions and activated AMPK/PGC1α signaling in differentiated primary brown and subcutaneous adipocytes, whereas AMPK inhibitor Compound C reversed the efficiencies.
Conclusions:
Dietary luteolin activated browning and thermogenesis through an AMPK/PGC1α pathway-mediated mechanism.
Similar content being viewed by others
Introduction
In mammals, energy expenditure primarily adopts three basic forms, including basal metabolism, adaptive thermogenesis and physical activity. When total energy intake chronically exceeds total body energy expenditure, it will result in the development of obesity and metabolic syndromes.1 Adipose tissues have been regarded as master regulators in energy balance and nutritional homeostasis. In rodents, white adipocytes store excess energy in the form of triglycerides. Differently, two types of uncoupling protein 1 (UCP1)-positive adipocytes, that is, classic brown adipocytes from brown adipose tissues (BATs) and beige adipocytes from white adipose tissues (WATs), specialize in thermogenesis.2, 3, 4 In adult human, the existence of functional genuine brown fat have been identified in the supraclavicular and spinal regions.5, 6 The pharmacologic or nutritional enhancement in the development and activity of brown or beige fat might produce a beneficial anti-obesity and anti-diabetic action.2, 4, 7
Luteolin is a natural flavonoid and is abundant in many edible and medicinal plants such as pepper, celery, thyme, peppermint and honeysuckle.8 Recently, we9 and other investigators10 reported that dietary luteolin ameliorated diet-induced obesity and insulin resistance in mice. Meanwhile, adipose tissue weights were reduced in high-fat diet (HFD)-fed mice and the mice did not have alterations in energy intake.9, 10 Moreover, it has been showed that luteolin could inhibit adipogenic differentiation and lipid accumulation in 3T3-L1 (refs 11, 12) or primary adipose cells.13 However, it remains unclear whether luteolin regulates adipocyte browning and affects energy expenditure and adaptive thermogenesis in mice.
PGC1α is a pivotal regulator of mitochondrial biogenesis, oxidative metabolism and thermogenesis gene expression in brown and beige adipocytes.2, 14 AMPK and SIRT1, two important nutrient and energy sensors,15, 16 solely or/and collaboratively increase PGC1α expression17 and phosphorylation.18 Thus, as an energy-sensitive pathway, AMPK/PGC1α signaling dominantly regulates the differentiation and function in brown and beige fat. Notably, luteolin can inhibit adipogenic differentiation in adipocytes through activating AMPK and SIRT1.19 Therefore, the functional relation between luteolin and AMPK/PGC1α signaling in adipocyte browning and thermogenesis should be explored.
In this study, we investigated the effects of dietary luteolin on energy expenditure in HFD-fed and low-fat diet (LFD)-fed mice, respectively. The alterations of thermogenic genes, browning markers and AMPK/PGC1α molecules were detected in their adipose tissues, including BAT, epididymal adipose tissue (EAT) and subcutaneous adipose tissue (SAT). Further, the action of luteolin treatment on thermogenic program and AMPK/PGC1α signaling were identified in differentiated primary brown and subcutaneous adipocytes.
Materials and methods
Experimental animals and metabolic parameter measures
Male C57BL/6 mice were purchased from Vital River Laboratory Animal Technology Co. Ltd (Beijing, China) at 4 weeks old. Mice were housed in ventilated cages within a pathogen-free barrier facility that was maintained at 22±2 °C with a 12-h light/12-h dark cycle and free to access autoclaved water and irradiated food throughout the feeding study and metabolic parameters measurement. All protocols were conducted with approval of the Hefei University of Technology Standing Committee on Animals. After adapting for 1 week, mice were randomly divided into three dietary groups and fed on LFD (D12450B diet containing 3.85 kcal g−1 and 10% fat; n=12), HFD (D12451 diet containing 4.73 kcal g−1 and 45% fat; n=12) and HFD supplemented with 0.01% luteolin (high performance liquid chromatography⩾98.0%, Huayi Biotechnology, Shanghai, China; n=12), respectively. Their body weights and food intakes were weekly measured. Energy intake was described as kcal per gram body weight weekly (kcal per week per gram body weight). After 12 weeks, mice were acclimated to a combined indirect calorimetry system (TSE Systems GmbH, Bad Homburg, Germany) for 2 days. Oxygen consumption (VO2), carbon dioxide production (VCO2) and respiratory exchange ratio (VCO2/VO2) were continuously monitored during the next 24 h. At the end of the experiments, mice were killed by exposing to CO2. The adipose tissues were rapidly isolated and weighed on ice and then were snap frozen in liquid nitrogen and stored at −80 °C. In another independent study, to assess the effects of luteolin on energy expenditure in LFD-fed mice, some 5-week-old mice were fed LFD or LFD supplemented with 0.01% luteolin for 12 weeks, respectively. Their body weights, food intakes and metabolic parameters were monitored, and their tissues were collected as described above. Blinding was not used in this study.
Glucose tolerance tests and insulin tolerance tests
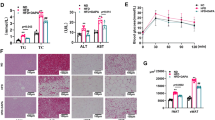
At 17 weeks old, insulin tolerance tests and glucose tolerance tests were performed after overnight fasting. For glucose tolerance tests, mice were injected glucose intraperitoneally (1 g per kg body weight, Sigma-Aldrich, Saint Louis, MO, USA). For insulin tolerance tests, mice were injected insulin intraperitoneally (1.5 IU per kg bodyweight, WanBang BioPharma, Xuzhou, China). The glucose levels were measured by a blood glucose meter (Omnitest Plus, B. BRAUN, Melsungen, Germany) at 0, 15, 30, 45, 60, 90 and 120 min after injection and the blood was collected from the tail vein.
Body temperature
Core body temperatures were rectally measured at room temperature at 0900 h using a themocoupler (Physitemp, Clifton, NJ, USA).
Histology, immunohistochemistry and western blotting
Adipose tissues were fixed in 4% formalin, embedded in paraffin and serially sliced into 5-μm thickness. Hematoxylin–eosin staining was routinely performed on adipose tissue sections. Using anti-mouse UCP1 polyclonal antibody (1:500, Abcam, Cambridge, MA, USA), immunohistochemistry for UCP1 protein was performed on deparaffinized sections. The UCP1-positive areas in five random fields in each section were determined by detecting the staining intensity with Image-Pro Plus Version 6.0 (Media Cybernetics, Bethesda, MD, USA) and data were presented as positive area percent. For western blotting, tissue proteins were extracted and equal amounts of proteins were separated with SDS-polyacrylamide gel electrophoresis. Immunoblotting analysis was performed as previous described.20 Primary and secondary antibodies for western blotting are listed in Supplementary Table 1.
Total RNA isolation and quantitative real-time PCR
Total RNA was extracted from adipose tissues using RNAiso Plus reagent (TAKARA, Dalian, China) and then reverse transcripted to cDNA using Oligo d(T)18 (TAKARA) and M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol. Real-time PCR was analyzed using SYBR green dye (TAKARA) in a Bio-Rad MyiQ2 Real-time PCR System (Bio-Rad, Hercules, CA, USA) as pervious described.20 All primer sequences used in this study are listed in Supplementary Table 2.
Cell culture
Primary brown and subcutaneous pre-adipocytes were fractionated according to the published methods.21, 22 Briefly, the interscapular BATs were isolated from newborn mice at postnatal day 2–3 and SATs were collected from 8-week-old mice. Adipose tissues were dissected, minced and digested with collagenase II (Sigma-Aldrich) for 45 min at 37 °C. The digested tissues were filtered through a 100-μm mesh filter and centrifuged at 200 g for 10 min at 4 °C. The cell pellets were suspended with RBC lysis buffer for 5 min. After centrifugation, the fractionated pre-adipocytes were cultured in Dulbecco’s modified Eagle’s medium (Gibco, Auckland, New Zealand) supplemented with 10% FBS (Gibco). Adipocyte differentiation was induced in pre-adipocyte cultures containing 0.5 mM isobutylmethylxanthine (Sigma-Aldrich), 125 nM indomethacin (Sigma-Aldrich), 1 μM dexamethasone (Sigma-Aldrich), 850 nM insulin (Sigma-Aldrich), 1 nM 3,3′,5-triiodo-L-thyronine (T3, Sigma-Aldrich) and 1 μM rosiglitazone (Sigma-Aldrich) for 48 h. Next, the cells were maintained in medium with 850 nM insulin, 1 nM T3 and 1 μM rosiglitazone for 6 days.23 After differentiation, the cells were pre-incubated with 20 μM Compound C (Sigma-Aldrich) or vehicle for 2 h. Next, the cells were treated with 100 nM luteolin or 2 mM 5-aminoimidazole-4-carboxamide-1-β4-ribofuranoside (AICAR, Sigma-Aldrich) for 24 h.
Statistical analysis
In vivo data were obtained from one experiment, with 12 mice in each group. The in vitro results were analyzed in triplicates and four parallels were used in each experiment. All data were presented as mean±s.e.m. The comparisons between two animal groups were assessed by non-parametric Mann–Whitney test, owing to our small sample sizes and data abnormal distribution. Student’s t-test was used to evaluate the difference among various cell assay groups. SPSS 16.0 (SPSS Inc., Chicago, IL, USA) was used as statistical software and a P-value <0.05 was considered statistically significant.
Results
Dietary luteolin increased energy expenditure in HFD-fed mice
Five-week-old male C57BL/6 mice were fed LFD, HFD and HFD supplemented with 0.01% luteolin, respectively. After 12 weeks, HFD led to higher body weight gain (Supplementary Figure S1A) and ratio of fat mass to body weight (Supplementary Figure S1B) in mice than LFD. Moreover, HFD-fed mice had lower glucose tolerance (Supplementary Figure S1C) and insulin sensitivity (Supplementary Figure S1D) than LFD-fed mice. Supporting our pervious study,9 dietary luteolin obviously protected mice against HFD-induced body weight gain (Supplementary Figure S1A), fat accumulation (Supplementary Figure S1B) and insulin resistance (Supplementary Figures S1C and D). However, it did not affect their energy intake (Supplementary Figure S1E) and body temperature (Supplementary Figure S1F).
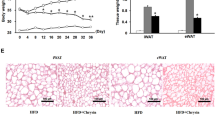
Along with lower body weight, mice fed LFD and luteolin-containing HFD had higher O2 consumption (Figures 1a and b) and CO2 production (Figures 1c and d) during both light and dark period than mice fed only HFD. In addition, their respiratory exchange ratio (Figures 1e and f) were also significantly higher, suggesting these lean mice tended to use preferentially carbohydrate substrates.24 The results demonstrate that dietary luteolin potently elevates energy expenditures in HFD-fed mice.
Dietary luteolin increased energy expenditure in HFD-fed mice. Five-week-old male C57BL/6 mice were fed LFD, HFD and HFD supplemented with 0.01% luteolin (HFD+0.01% LU) for 12 weeks, respectively. At 17 weeks old, their metabolic parameters were measured. (a) Oxygen consumption (VO2), (c) carbon dioxide production (VCO2) and (e) respiratory exchange ratio (RER, VCO2/VO2) during a 24-h light and dark cycle, and their average for each group in the light cycle or dark cycle (b for a, d for c and f for e). Non-parametric Mann–Whitney test. **P<0.01 and ***P<0.001. n=12 per group. All data are mean±s.e.m.
Dietary luteolin promoted thermogenic program in BAT and SAT in HFD-fed mice
BATs are specialized adipose tissues, which use the actions of UCP1 to transform stored chemical energy into heat energy and protect mice against overfeeding or cold.7 Compared with mice fed only HFD, mice fed LFD and luteolin-containing HFD possessed denser small brown adipocytes with multilocular and cytoplasmic staining lipid droplets in BATs (Figure 2a). In addition, their BAT UCP1-positive areas were strikingly higher (Figures 2a and b). Consistent with the results, both UCP1 protein (Figures 2c and d) and transcript (Figure 2e) levels were also increased in their BATs. Further, we detected some important thermogenic genes in BATs. Dietary luteolin upregulated the mRNA expressions of Pgc1α, PPARα, Cidea and Sirt1, but did not affect those of Prdm16 and Elovl3 (Figure 2e). These results suggest that dietary luteolin activates BAT thermogenic program.
Dietary luteolin induced BAT and SAT thermogenic program in HFD-fed mice. (a) Representative hematoxylin and eosin staining and UCP1 staining for BAT sections and (b) quantification of UCP1-positive area; scale bars in (a), 100 μm in length (original magnification, × 200). (c) Immunoblot analysis for UCP1 and (d) quantification of UCP1 to β-actin in BAT. (e) Real-time PCR quantitative mRNA expressions of thermogenic genes in BAT. (f) Representative hematoxylin and eosin staining and UCP1 staining for SAT sections and (g) quantification of UCP1-positive area; scale bars in (f), 100 μm in length (original magnification, × 200). (h) Immunoblot analysis for UCP1 and (i) quantification of UCP1 to β-actin in SAT. Real-time PCR analysis for (j) thermogenic genes and (k) beige cell markers in SAT. β-Actin was used as reference gene in real-time PCR analysis. Non-parametric Mann–Whitney test. *P<0.05, **P<0.01 and ***P<0.001. n=8 per group. All data in b, d, e, g and i–k are mean±s.e.m.
Besides classical brown adipocytes from BATs, beige adipocytes from WATs are also UCP1 positive and can be induced to express thermogenic genes and drive respiration and energy expenditure.3 Thus, we further investigated the effects of dietary luteolin on thermogenic program in WATs, including SATs and EATs. Similar to their BATs, SATs of mice fed luteolin-containing HFD also possessed more brown-like adipocytes (Figure 2f) and higher UCP1 levels (Figures 2f–j) than those of mice fed only HFD. Correspondingly, dietary luteolin elevated the expressions of a series of thermogenic genes (Figure 2j) and beige cell-selective markers (Figure 2k). However, the morphological (Supplementary Figure S2A) and expressional (Supplementary Figures S2B and C) alterations did not occur in EAT in mice fed luteolin-containing HFD. Therefore, we conclude that luteolin also increases thermogenic program and induces adipocyte browning in SATs.
Dietary luteolin inherently increased energy expenditure in LFD-fed mice
As luteolin reduces body weight gain of HFD-fed mice, the promotion in mouse energy expenditure may only be a result of their body weight reduction. To avoid the probability, the effects of luteolin on metabolic parameters were investigated in LFD-fed mice. As expected, diet supplement of luteolin for 12 weeks did not affect the body weights, energy intakes, fat mass, body temperatures and blood insulin and glucose levels of the LFD-fed mice (Supplementary Figure S3). However, energy expenditure was strikingly elevated in mice fed luteolin-containing LFD, including increased O2 consumption during light and dark period (Figures 3a and b), and CO2 production (Figures 3c and d) and respiratory exchange ratio (Figures 3e and f) during light period. These results demonstrate that luteolin inherently enhances energy expenditure in LFD-fed mice.
Dietary luteolin inherently increased energy expenditure in LFD-fed mice. Five-week-old male C57BL/6 mice were fed LFD and LFD supplemented with 0.01% luteolin (LFD+0.01% LU) for 12 weeks, respectively. At 17 weeks old, their metabolic parameters were measured. (a) Oxygen consumption (VO2), (c) carbon dioxide production (VCO2) and (e) respiratory exchange ratio (RER, VCO2/VO2) during a 24-h light and dark cycle and their average for each group in the light cycle or dark cycle (b for a, d for c and f for e). Non-parametric Mann–Whitney test. *P<0.05, **P<0.01 and ***P<0.001. n=12 per group. All data are mean±s.e.m.
Dietary luteolin also promoted thermogenic program in BATs and SATs in LFD-fed mice
Although histological morphology of BATs (Figure 4a) was unchanged in mice fed luteolin-containing LFD, their UCP1 mRNA (Figure 4b) and protein (Figures 4c and d) expressions in BATs were remarkably elevated compared with those of mice fed only LFD. Consistently, dietary luteolin also induced the expressions of thermogenic gene Pgc1α, PPARα and Sirt1 in BATs (Figure 4b). In addition, similar histological (Figure 4e) and thermogenic gene expression (Figures 4f–h) phenomena also occurs in their SATs, although dietary luteolin still did not affect thermogenic program in their EATs (Supplementary Figure S4). Therefore, dietary luteolin also promotes thermogenic program in BATs and SATs in LFD-fed mice.
Dietary luteolin promoted thermogenic program in BAT and SAT in LFD-fed mice. (a) Representative hematoxylin and eosin staining and UCP1 staining for BAT sections; scale bars in (a), 100 μm in length (original magnification, × 200). (b) Real-time PCR quantitative mRNA expressions of thermogenic genes in BAT. (c) Immunoblot analysis for UCP1 and (d) quantification of UCP1 to β-actin in BAT. (e) Representative hematoxylin and eosin staining and UCP1 staining for SAT sections; scale bars in (e), 100 μm in length (original magnification, × 200). (f) Real-time PCR quantitative mRNA expressions of thermogenic genes and beige cell markers in SAT. (g) Immunoblot analysis for UCP1 and (h) quantification of UCP1 to β-actin in SAT. β-Actin was used as reference gene in real-time PCR analysis. Non-parametric Mann–Whitney test. *P<0.05 and **P<0.01. n=8 per group. All data in b, d, f and h are mean±s.e.m.
Dietary luteolin activated AMPK/PGC1α signaling in BATs and SATs
PGC1α is a critical transcriptional cofactor inducing UCP1 expression and regulating mitochondrial biogenesis and oxidative metabolism in brown-like adipocytes2, 7 and can be regulated by AMPK and SIRT1.14, 15, 16, 17, 18 To explore the mechanisms of dietary luteolin augmenting energy expenditure and adaptive thermogenesis in HFD- and LFD-fed mice, we further detected several important AMPK/PGC1α pathway molecules in their BATs and SATs. Consistent with increased Pgc1α and Sirt1 transcript levels in BATs (Figures 2e and 4b) and SATs (Figures 2j and 4f), dietary luteolin also enhanced their protein levels in BATs and SATs (Figures 5a–d). Similarly, associated phosphorylated AMPKα (Figures 5e and f) and ACC (Figures 5g and h) levels were also strikingly strengthened in BATs and SATs.
Dietary luteolin activated AMPK/PGC1α signaling in BAT and SAT. Immunoblot for (a) PGC1α, (c) SIRT1, (e) total and phosphorylated AMPKα (pAMPKα) and (g) total and phosphorylated ACC (pACC) in BAT and SAT. Quantification of protein expressions was described as the ratio of (b) PGC1α or (d) SIRT1 to β-actin. Quantification of phosphorylated protein levels was described as (f) pAMPKα to total AMPKα or (h) pACC to total ACC. Non-parametric Mann–Whitney test. *P<0.05 and **P<0.01. n=8 per group. All data in b, d, f and h are mean±s.e.m.
Luteolin treatment facilitated browning in differentiated primary brown and subcutaneous adipocytes through regulating AMPK/PGC1α pathway
In order to affirm a potential participation of AMPK/PGC1α pathway in luteolin-regulated browning, the fractionated and differentiated primary brown and subcutaneous adipocytes (Supplementary Figure S5) were treated with luteolin or AMPK activator AICAR. Five to 100 nM luteolin elevated the mRNA expression of Ucp1 in the cells in a dose-dependent manner (Supplementary Figure S6); therefore, 100 nM of luteolin was used in the further cell experiments. Similar to AICAR, luteolin treatment significantly increased a series of thermogenic gene expressions in differentiated primary brown (Figure 6a) and subcutaneous (Figure 6b) adipocytes. Correspondingly, the protein levels of UCP1, PGC1α and SIRT1, and the phosphorylation levels of AMPKα and ACC were also augmented by luteolin or AICAR treatment (Figures 6c–f). Moreover, luteolin also induced the expressions of beige cell-selective markers in differentiated primary subcutaneous adipocytes (Figure 6b). As a selective AMPK inhibitor, Compound C blocked all above-mentioned actions of luteolin and AICAR (Figure 6).
Luteolin treatment facilitated browning in differentiated primary brown and subcutaneous adipocytes through regulating AMPK/PGC1α pathway. Real-time PCR analysis for (a) thermogenic gene expressions in differentiated primary brown adipocytes and (b) thermogenic gene and beige cell marker expressions in differentiated primary subcutaneous adipocytes. β-Actin was used as reference gene in real-time PCR analysis. (c) Immunoblot for UCP1 and AMPK/PGC1α pathway proteins and (d) quantification of UCP1 to β-actin, PGC1α to β-actin, SIRT1 to β-actin, pAMPKα to total AMPKα and pACC to total ACC in differentiated primary brown adipocytes. (e) Immunoblot for UCP1 and AMPK/PGC1α pathway proteins and (f) quantification of UCP1 to β-actin, PGC1α to β-actin, SIRT1 to β-actin, pAMPKα to total AMPKα and pACC to total ACC in differentiated primary subcutaneous adipocytes. C.c., Compound C. Student’s t-test. *P<0.05, **P<0.01 and ***P<0.001. n=4 per group. All data in a, b, d and f are mean±s.e.m.
Discussion
The balance between food intake and energy expenditure can mainly determine animal body weight and adiposity.1 In mice, both classic brown adipocytes and beige adipocytes increase energy expenditure through UCP1-mediated thermogenesis. Thus, the pharmacologic or nutritional strategies for promoting adipocyte browning and thermogenesis will help to control body weight homeostasis.2, 4, 7 In this study, dietary luteolin obviously elevates energy expenditure in both HFD-fed and LFD-fed mice. Meanwhile, dietary luteolin also strengthens brown adipocyte activity and beige adipocyte formation and associated thermogenic program. Furthermore, in vivo and in vitro studies show that luteolin activates AMPK/PGC1α pathway, thereby enhancing browning and thermogenesis in BATs and SATs.
Luteolin, as a natural flavonoid, possesses sufficient anti-obesity and anti-diabetic activities. We previously reported that dietary luteolin could ameliorate diet-induced obesity and insulin resistance,9 and a recent report confirmed our results.10 However, a detailed molecular and cellular mechanism about luteolin reducing body weight gain is not fully understood. To identify the effects of luteolin on adipocyte browning and thermogenesis, as well as associated molecular regulators, we designed this study. In the above-mentioned two reports, diet supplement of 0.002–0.01% luteolin can effectively reduce HFD-induced body weight gain in a dose-dependent manner.9, 10 Therefore, we choose 0.01% luteolin as the dose of diet supplement in this study.
Using a combined indirect calorimetry system, we detected mouse energy expenditure. In HFD-fed mice, along with an expectant improvement in obesity and insulin resistance (Supplementary Figure S1), dietary luteolin significantly elevated their metabolic rates during both light and dark period (Figure 1). Moreover, mice fed luteolin-containing LFD also had a higher metabolic level than mice fed only LFD, although their body weights were unaffected by dietary luteolin. In addition, in the two dietary interventions, the supplements of luteolin did not influence mouse food intake (Supplementary Figures S1E and S3B). As obesity is caused by total energy intake chronically exceeding total energy consumption, the results suggest dietary luteolin inherently elevates energy expenditure and may hereby protect mice from HFD-induced obesity and insulin resistance.
Adipose tissue has been regarded as a main regulator of energy balance and nutritional homeostasis, and BAT is a specialized tissue to use UCP1 and dissipate stored chemical energy in the form of heat.7 Given the abilities of brown adipocytes in burning fat and producing heat, targeting to brown adipocyte activation may offer a viable approach to counteract obesity.4 Notably, dietary luteolin enhanced BAT thermogenic program in either HFD-fed (Figures 2a–e) or LFD-fed (Figures 4a–d) mice, suggesting luteolin can strengthen the functional activities of classic brown adipocytes.
As another type of UCP1-positive adipocytes, beige or brite (brown-in-white) cells reside sporadically within WATs and have a basal level of UCP1 at ambient temperature.3 In response to specific stimuli, such as cold exposure or β3 adrenergic receptor agonists, the quiescent beige cells turn on a robust program of respiration and energy expenditure.25, 26 However, not all WATs equally retain the inducible activity of browning and thermogenesis. UCP1-positive multilocular adipocyte is barely detected in EATs.3 Compared with EATs, SATs possess higher level of UCP1 and other thermogenic proteins, and they are more sensitive to browning-associated stimuli.3, 27 In this study, both HFD and LFD supplement of luteolin promoted browning and theromgenic program in SATs (Figures 2f–k and 4e–h) rather than in EATs (Supplementary Figures S2 and S4). The results show that luteolin is an efficacy stimulator for SAT browning and powerfully induces thermogenic abilities of beige adipocytes. Although thermogenic program was increased both in BATs and SATs after luteolin treatment, there were no detectable differences in body temperatures among all four groups. Maybe, the induction of UCP1 and browning in adipose tissue was not enough to cause a measurable body temperature elevation. Indeed, a published report showed that LFD-fed and HFD-fed mice had no differences in core body temperatures at ambient temperature.28 On the other hand, besides adaptive thermogenesis, body temperature also depended on circadian phase, environmental temperature and physical activity.29 Maybe, the factors buffered the effects of luteolin on body temperature, a hypothesis requires further investigation.
The differentiation and function of classical and inducible brown-like adipocytes are regulated by a complex network of hormones and signaling pathways. Much of the specialized function is controlled by some transcriptional cofactors, which can bind and activate associated transcription factors.2, 7 Here, our results showed that BAT and SAT transcriptional cofactor PGC1α were upregulated by dietary luteolin in either HFD-fed (Figures 2e and j, and 5a and 5b) or LFD-fed (Figures 4bf and 4f, and 5a and 5b) mice. Similar phenomena also occurred in differentiated primary brown and subcutaneous adipocytes (Figure 6). Conversely, another co-regulator PRDM16 was not affected by dietary luteolin in the above-mentioned adipose tissues (Figures 2e and 2j, and 4b and 4f). In the two transcriptional cofactors, PGC1α is a critical regulator of thermogenic function but not a promotor of brown-like adipocyte differentiation,7, 30 whereas PRDM16 is important in brown adipocytes development.7, 31 Therefore, our results implied that luteolin treatment might promote existed brown-like adipocyte thermogenesis rather than their progenitor differentiation, a hypothesis that merits further investigation.
AMPK, a key regulator of energy metabolism, can increase PGC1α expression and activity.17, 18 Moreover, AMPK can also enhance SIRT1 activity to induce PGC1α deacetylation and activation.14, 16 Thus, PGC1α, SIRT1 and AMPK forms a metabolism-sensing network to control energy expenditure.32 As luteolin upregulated PGC1α in the thermogenesis-associated adipose tissues (Figures 2e and 2j, 4b and 4f, and 5a and 5b) and adipocytes (Figure 6), it should also activate AMPK/PGC1α pathway in the tissues and cells. Our further results supported this hypothesis. First, dietary luteolin increased SIRT1 expressions (Figures 2e and j, 4b and 4f, and 5a and 5b) as well as phosphorylated AMPKα and ACC levels (Figures 5e–h) in BATs and SATs. Second, luteolin treatment elevated the expressions of thermogenic genes and the activities of AMPK/PGC1α signaling molecules in differentiated primary brown and subcutaneous adipocytes, which fully mimicked the actions of AMPK activator AICAR (Figure 6). Finally, AMPK inhibitor Compound C could reverse the efficiencies of luteolin and AICAR (Figure 6). Altogether, our results indicate that luteolin induces adipocyte browning and thermogenesis through activating AMPK/PGC1α signaling, although the involvement of other PGC1α modulators such as RIP140 (ref. 33) and Retinoblastoma protein34 in luteolin-induced browning and thermogenesis remains unclear.
In summary, we here illustrate that luteolin inherently increases energy expenditure in both HFD-fed and LFD-fed mice. Furthermore, luteolin promotes thermogenic program in their BATs and SATs, and induces white-to-brown fat transition in their SATs. Finally, AMPK/PGC1α pathway is identified as a critical molecular signaling to regulate luteolin-induced thermogensis and browning in differentiated primary adipocytes. All the results reveal that luteolin protects mice from diet-induced obesity and insulin resistance through activating AMPK/PGC1α-mediated energy expenditure.
References
Spiegelman BM, Flier JS . Obesity and the regulation of energy balance. Cell 2001; 104: 531–543.
Rosen ED, Spiegelman BM . What we talk about when we talk about fat. Cell 2014; 156: 20–44.
Wu J, Bostrom P, Sparks LM, Ye L, Choi JH, Giang AH et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012; 150: 366–376.
Harms M, Seale P . Brown and beige fat: development, function and therapeutic potential. Nat Med 2013; 19: 1252–1263.
Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med 2009; 360: 1509–1517.
Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T et al. Functional brown adipose tissue in healthy adults. N Engl J Med 2009; 360: 1518–1525.
Kajimura S, Saito M . A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol 2014; 76: 225–249.
Lopez-Lazaro M . Distribution and biological activities of the flavonoid luteolin. Mini Rev Med Chem 2009; 9: 31–59.
Xu N, Zhang L, Dong J, Zhang X, Chen YG, Bao B et al. Low-dose diet supplement of a natural flavonoid, luteolin, ameliorates diet-induced obesity and insulin resistance in mice. Mol Nutr Food Res 2014; 58: 1258–1268.
Kwon EY, Jung UJ, Park T, Yun JW, Choi MS . Luteolin attenuates hepatic steatosis and insulin resistance through the interplay between the liver and adipose tissue in diet-induced obese mice. Diabetes 2015; 64: 1658–1669.
Puhl AC, Bernardes A, Silveira RL, Yuan J, Campos JL, Saidemberg DM et al. Mode of peroxisome proliferator-activated receptor gamma activation by luteolin. Mol Pharmacol 2012; 81: 788–799.
Park HS, Kim SH, Kim YS, Ryu SY, Hwang JT, Yang HJ et al. Luteolin inhibits adipogenic differentiation by regulating PPARgamma activation. Biofactors 2009; 35: 373–379.
Ding L, Jin D, Chen X . Luteolin enhances insulin sensitivity via activation of PPARgamma transcriptional activity in adipocytes. J Nutr Biochem 2010; 21: 941–947.
Fernandez-Marcos PJ, Auwerx J . Regulation of PGC-1alpha, a nodal regulator of mitochondrial biogenesis. Am J Clin Nutr 2011; 93: 884S–890S.
Hardie DG, Ross FA, Hawley SA . AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 2012; 13: 251–262.
Canto C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 2009; 458: 1056–1060.
Suwa M, Nakano H, Kumagai S . Effects of chronic AICAR treatment on fiber composition, enzyme activity, UCP3, and PGC-1 in rat muscles. J Appl Physiol 2003; 95: 960–968.
Jager S, Handschin C, St-Pierre J, Spiegelman BM . AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA 2007; 104: 12017–12022.
Xiao N, Mei F, Sun Y, Pan G, Liu B, Liu K . Quercetin, luteolin, and epigallocatechin gallate promote glucose disposal in adipocytes with regulation of AMP-activated kinase and/or sirtuin 1 activity. Planta Med 2014; 80: 993–1000.
Dong J, Zhang X, Zhang L, Bian HX, Xu N, Bao B et al. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: a mechanism including AMPKalpha1/SIRT1. J Lipid Res 2014; 55: 363–374.
Tseng YH, Kriauciunas KM, Kokkotou E, Kahn CR . Differential roles of insulin receptor substrates in brown adipocyte differentiation. Mol Cell Biol 2004; 24: 1918–1929.
Klein J, Fasshauer M, Ito M, Lowell BB, Benito M, Kahn CR . beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J Biol Chem 1999; 274: 34795–34802.
Zhang Z, Zhang H, Li B, Meng X, Wang J, Zhang Y et al. Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun 2014; 5: 5493.
McDonald ME, Li C, Bian H, Smith BD, Layne MD, Farmer SR . Myocardin-related transcription factor A regulates conversion of progenitors to beige adipocytes. Cell 2015; 160: 105–118.
Rosenwald M, Perdikari A, Rulicke T, Wolfrum C . Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol 2013; 15: 659–667.
Lee YH, Petkova AP, Mottillo EP, Granneman JG . In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab 2012; 15: 480–491.
Cousin B, Cinti S, Morroni M, Raimbault S, Ricquier D, Penicaud L et al. Occurrence of brown adipocytes in rat white adipose tissue: molecular and morphological characterization. J Cell Sci 1992; 103: 931–942.
Okla M, Wang W, Kang I, Pashaj A, Carr T, Chung S . Activation of Toll-like receptor 4 (TLR4) attenuates adaptive thermogenesis via endoplasmic reticulum stress. J Biol Chem 2015; 290: 26476–26490.
Mekjavic IB, Eiken O . Contribution of thermal and nonthermal factors to the regulation of body temperature in humans. J Appl Physiol 2006; 100: 2065–2072.
Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM . Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab 2006; 3: 333–341.
Kajimura S, Seale P, Tomaru T, Erdjument-Bromage H, Cooper MP, Ruas JL et al. Regulation of the brown and white fat gene programs through a PRDM16/CtBP transcriptional complex. Genes Dev 2008; 22: 1397–1409.
Canto C, Auwerx J . PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol 2009; 20: 98–105.
Hallberg M, Morganstein DL, Kiskinis E, Shah K, Kralli A, Dilworth SM et al. A functional interaction between RIP140 and PGC-1alpha regulates the expression of the lipid droplet protein CIDEA. Mol Cell Biol 2008; 28: 6785–6795.
Hansen JB, Jorgensen C, Petersen RK, Hallenborg P, De Matteis R, Boye HA et al. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc Natl Acad Sci USA 2004; 101: 4112–4117.
Acknowledgements
This work was supported by projects from National Natural Science Foundation of China (Grant Numbers 311171315 and 31471320 to JL, and 31401204 to BB); The Fundamental Research Funds for the Central Universities, China (Grant Number 2014HGCH0005 to JL); Anhui Science and Technology Research Projects of China (Grant Number 1401b042018 to JL); and the Anhui Provincial Natural Science Foundation (Grant Number 1408085QC48 to BB).
Author contributions
XZ conceived and designed the experiments, performed the study and wrote the manuscript. Q-XZ, XW and LZ participated in the study and contributed to data analysis. WQ and BB contributed to data analysis and revised manuscript. JL reviewed/approved the research protocol, edited the manuscript and has taken full responsibility for the work as a whole, including the study design, access to data and the decision to submit and publish the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on International Journal of Obesity website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhang, X., Zhang, QX., Wang, X. et al. Dietary luteolin activates browning and thermogenesis in mice through an AMPK/PGC1α pathway-mediated mechanism. Int J Obes 40, 1841–1849 (2016). https://doi.org/10.1038/ijo.2016.108
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ijo.2016.108
- Springer Nature Limited
This article is cited by
-
Pyrazolone derivative C29 protects against HFD-induced obesity in mice via activation of AMPK in adipose tissue
Acta Pharmacologica Sinica (2021)
-
Polyphenols and their anti-obesity role mediated by the gut microbiota: a comprehensive review
Reviews in Endocrine and Metabolic Disorders (2021)
-
Natural Bioactive Compounds as Potential Browning Agents in White Adipose Tissue
Pharmaceutical Research (2021)
-
Neuronal cell-based high-throughput screen for enhancers of mitochondrial function reveals luteolin as a modulator of mitochondria-endoplasmic reticulum coupling
BMC Biology (2021)
-
Dietary and policy priorities to reduce the global crises of obesity and diabetes
Nature Food (2020)