Abstract
We have recently shown that activation of metabotropic glutamate 2 (mGlu2) receptors through positive allosteric modulation and orthosteric stimulation is a novel approach to reduce L-3,4-dihydroxyphenylalanine (l-DOPA)-induced dyskinesia and dopaminergic psychosis in Parkinson’s disease (PD). We have obtained these benefits with the mGlu2-positive allosteric modulator (PAM) LY-487,379 and the mGlu2/3 orthosteric agonist (OA) LY-354,740 in experiments conducted in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned marmoset. Here, we sought to pharmacologically characterise the anti-dyskinetic and anti-psychotic effects of LY-487,379 and LY-354,740, by assessing whether their benefits would be reversed by the mGlu2/3 orthosteric antagonist LY-341,495. Six MPTP-lesioned marmosets exhibiting stable dyskinesia and psychosis-like behaviours (PLBs) entered the experiments. In the first series of experiments, animals were injected l-DOPA in combination with either vehicle, LY-487,379 (10 mg/kg), LY-341,495 (1 mg/kg) or LY-487,379/LY-341,495. In the second series of experiments, marmosets were injected l-DOPA in combination with either vehicle, LY-354,740 (1 mg/kg), LY-341,495 (1 mg/kg) or LY-354,740/LY-341495. As we previously demonstrated, both LY-487,379 and LY-354,740 alleviated dyskinesia (by 44% and 47%, both P < 0.001) and PLBs (by 44% and 39%, P < 0.01 and P < 0.001) when compared to vehicle treatment. When LY-487,379 and LY-354,740 were administered concurrently with LY-341,495, the anti-dyskinetic and anti-psychotic benefits were abolished. When administered with l-DOPA in the absence of LY-487,379 and LY-354,740, LY-341,495 did not worsen dyskinesia or PLBs and did not hamper l-DOPA anti-parkinsonian action. Our results indicate that the anti-dyskinetic and anti-psychotic effects of mGlu2-positive allosteric modulation and mGlu2/3 orthosteric stimulation are reversed by mGlu2/3 orthosteric blockade.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In advanced Parkinson’s disease (PD), many patients are afflicted by motor complications, which encompass both motor fluctuations and dyskinesia, as well as by psychotic manifestations (Hely et al. 2005). Treatment options for motor complications and psychosis are limited, they are not effective for all patients, and they may lead to adverse effects. For instance, the anti-dyskinetic agent amantadine (Hauser et al. 2017; Pahwa and Hauser 2017; Pahwa et al. 2017) may trigger hallucinations (Postma and Van Tilburg 1975), while entacapone, which is used to confer further anti-parkinsonian benefit when administered with L-3,4-dihydroxyphenylalanine (l-DOPA), may exacerbate dyskinesia (Mizuno et al. 2007). On the other hand, the reduction of psychosis conferred by pimavanserin was only 13%, compared to 6% by placebo, in a Phase III trial (Cummings et al. 2014).
In recent experiments performed in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned primate with the positive allosteric modulator (PAM) LY-487,379 (Sid-Otmane et al. 2020) and the orthosteric agonist (OA) LY-354,740 (Frouni et al. 2019), we have shown that metabotropic glutamate 2 (mGlu2) receptor activation may alleviate both dyskinesia and psychosis-like behaviours (PLBs) and enhance the anti-parkinsonian action of l-DOPA.
Here, we sought explore of the mechanism underlying the anti-dyskinetic, anti-psychotic, and anti-parkinsonian effects of LY-487,379 and LY-354,740. To do so, we have administered each compound in combination with the mGlu2/3 orthosteric antagonist LY-341,495 (Kingston et al. 1998) and assessed the resulting effects on l-DOPA-induced dyskinesia, PLBs, and l-DOPA anti-parkinsonian action in the MPTP-lesioned marmoset, which has high predictivity of the efficacy of drugs in clinical trials (Veyres et al. 2018).
Materials and methods
Animals
Six (3 males and 3 females) common marmosets (Callithrix jacchus; McGill University breeding colony) weighing 300–450 g and aged between 3 and 7 years were used in the experiments.
Marmosets were pair-housed under conditions of controlled temperature (24 ± 1 °C), humidity (50 ± 5%), and a 12 h light/dark cycle (07:15 lights on). They had unlimited access to water, with fresh fruits and food (Mazuri® marmoset jelly) served twice daily. Their housing cages were enriched with primate toys and perches. Prior to the start of studies, animals were acclimatised to handling, sub-cutaneous (s.c.) injections, as well as to transfers to observation cages for behavioural experiments. Animals were cared for in accordance with a protocol approved by the Montreal Neurological Institute and McGill University Animal Care Committees, both in accordance with regulations defined by the Canadian Council on Animal Care.
Induction of parkinsonism, dyskinesia, and PLBs
Parkinsonism was induced by injections of MPTP hydrochloride (MilliporeSigma, Oakville, ON, Canada) 2 mg/kg s.c. once daily or every other day for 5 days, tailored to the animals’ reaction to MPTP, as detailed in (Hamadjida et al. 2018b). The MPTP administration phase was followed by a 6-week recovery period to allow the development of stable parkinsonism (Hamadjida et al. 2018b, c, d; Kwan et al. 2019).
Dyskinesia and PLBs were induced by once daily oral administration of l-DOPA/benserazide (henceforth referred to as l-DOPA, 15/3.75 mg/kg; MilliporeSigma) for a minimum of 30 days. This treatment regimen was previously demonstrated to elicit stable l-DOPA-induced dyskinesia and PLBs (Hamadjida et al. 2017, 2018a, b, c, d). Moreover, such doses of l-DOPA were shown to lead to plasma exposure comparable to that achieved with l-DOPA in the clinic (Huot et al. 2012b; Zhang et al. 2003).
Behavioural experiments
LY-487,379, LY-354,740, and LY-341,495 were purchased from Cedarlane Laboratories (Burlington, ON, Canada). LY-487,379 was dissolved in 25% DMSO in 0.9% NaCl, while LY-354,740 and LY-341,495 were dissolved in 0.9% NaCl.
Experiments were divided in two series. The first series investigated the effect of LY-341,495 on the benefits conferred by LY-487,379, by adding the following treatments to l-DOPA: vehicle/vehicle, LY-487,379/vehicle, vehicle/LY-341,495, and LY-487,379/LY-341,495. After a 1-month washout period, the second set of experiments assessed the effect of LY-341,495 on the therapeutic actions of LY-354,740. The following treatments were administered in combination with l-DOPA: vehicle/vehicle, LY-354,740/vehicle, vehicle/LY-341,495, and LY-354,740/LY-341,495.
On experimental days, marmosets were injected with a therapeutic dose of l-DOPA (15/3.75 mg/kg) in combination with the treatments described above. The doses of LY-487,379 (10 mg/kg) and LY-354,740 (1 mg/kg) were selected from prior pharmacokinetic (Gaudette et al. 2017, 2018) and behavioural (Frouni et al. 2019; Sid-Otmane et al. 2020) experiments that we conducted in the marmoset with these 2 molecules. The dose of LY-341,495 (1 mg/kg) was selected based on the rat literature (Tizzano et al. 2002; Okamura et al. 2003).
Drugs were injected s.c. and administration schedules of both series of experiments were randomised according to a Latin square design. After administration of a given treatment, each animal was placed individually into an observation cage (36 × 33 × 22 in) containing food, water, and a wooden perch, and left undisturbed for the 6-h duration of the experiment. At least 72 h were left between each treatment in any marmoset. Behaviours were recorded via webcam for post hoc analysis by an experienced movement disorder neurologist blinded to the treatment given.
Evaluation of parkinsonism, dyskinesia, and PLBs
The scales used for assessment of parkinsonism (Huot et al. 2011, 2012a, 2014), dyskinesia (Huot et al. 2011, 2012a, 2014), and PLBs (Fox et al. 2010, 2006; Visanji et al. 2006) have been extensively used and detailed previously. On each of these scales, the higher the score, the greater the disability.
The parkinsonism scale comprises measures of range of movement (0–9), bradykinesia (0–3), posture (0–1), and attention/alertness (0–1). For each observation period, a global parkinsonian disability score is calculated as a combination of the behaviours mentioned above, equally weighted, according to the following formula: (range of movement × 1) + (bradykinesia × 3) + (posture × 9) + (alertness × 9). The maximal parkinsonian disability score per observation period is 36.
The dyskinesia rating scale evaluates each of chorea (0–4) and dystonia (0–4), and the score attributed during any observation period is the most severe dyskinesia observed, either chorea or dystonia. The PLBs rating scale assesses each of hyperkinesia (0–4), hallucinatory-like behaviour (0–4), repetitive grooming (0–4) and stereotypies (0–4); the PLBs score attributed during any observation period being the most severe between these four behaviours.
Parkinsonism, dyskinesia, and PLBs scores were assessed for 5 min every 10 min and were cumulated for each 30 min across the entire 6 h of observation.
Statistical analysis
Time courses of dyskinesia, PLBs, and parkinsonism are presented as the median, and were analysed by computing the area under the curve (AUC), after which parametric non-repeated-measures one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test was performed. For all experiments, statistical significance was set to P < 0.05. Statistical analyses were computed using GraphPad Prism 8.4.0 (GraphPad Software Inc, La Jolla, CA, USA).
Results
Treatments were well tolerated by the animals, no sedation was noted.
LY-341,495 reverses the anti-dyskinetic and anti-psychotic benefits, but not the anti-parkinsonian effect, of LY-487,379
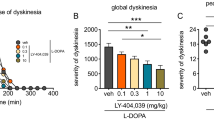
Dyskinesia
Figure 1a illustrates the dyskinesia time course following treatment administration, while Fig. 1b depicts the AUC of the time course. As expected, LY-487,379 significantly reduced global dyskinesia severity, but the benefit was lost after concurrent administration of LY-487,379/LY-341,495, while LY-341,495 alone did not worsen dyskinesia, when compared to vehicle treatment (F(3,20) = 12.07, P < 0.001, one-way ANOVA). LY-487,379 diminished dyskinesia severity by 44% (P < 0.001, Tukey’s post hoc test). This anti-dyskinetic effect was no longer present when LY-341,495 was added to LY-487,379 (P < 0.01 when LY-487,379 is compared to LY-487,379/LY-341,495 and P > 0.05 when LY-487,379/LY-341,495 is compared to vehicle, Tukey’s post hoc test), while dyskinesia severity after administration of l-DOPA/LY-341,495 was not different to that elicited by l-DOPA alone, but was significantly more severe than after the addition of LY-487,379 to l-DOPA (P < 0.001 when LY-487,379 is compared to LY-341,495, Tukey’s post hoc test).
a Time course of dyskinesia in MPTP-lesioned marmosets treated with l-DOPA in combination with vehicle, LY-487,379 10 mg/kg, LY-341,495 1 mg/kg or combination thereof. Each time point represents the cumulated dyskinesia scores for every 5 min observation period during the preceding 30 min. The maximal dyskinesia score at any time point is 12. b AUC of the time course of dyskinesia. c Time course of PLBs in MPTP-lesioned marmosets treated with l-DOPA in combination with vehicle, LY-487,379 10 mg/kg, LY-341,495 1 mg/kg or combination thereof. Each time point represents the cumulated PLBs scores for every 5 min observation period during the preceding 30 min. The maximal PLBs score at any time point is 12. d AUC of the time course of PLBs. e Time course of parkinsonism in MPTP-lesioned marmosets treated with l-DOPA in combination with vehicle, LY-487,379 10 mg/kg, LY-341,495 1 mg/kg or combination thereof. Each time point represents the cumulated parkinsonian disability scores for every 5 min observation period during the preceding 30 min. The maximal parkinsonian disability score at any time point is 108. f AUC of the time course of parkinsonism. In a, c, and e, data are presented as the median; in b, d and f, data are presented as the mean with the SEM and the individual values. **P < 0.01; ***P < 0.001
PLBs
Figure 1c illustrates the PLBs time course following treatment administration, while Fig. 1d depicts the AUC of the time course. LY-487,379 significantly reduced global PLBs severity, but the benefit was no longer present after concurrent administration of LY-487,379/LY-341,495, while LY-341,495 alone did not worsen PLBs, when compared to vehicle treatment (F(3,20) = 5.68, P < 0.01, one-way ANOVA). LY-487,379 diminished PLBs severity by 44% (P < 0.01, Tukey’s post hoc test). This anti-psychotic effect was not encountered when LY-341,495 was added to LY-487,379 (P > 0.05 when LY-487,379/LY-341,495 was compared to vehicle, Tukey’s post hoc test). PLBs severity after administration of l-DOPA/LY-341,495 was not different to that elicited by l-DOPA alone (P > 0.05, Tukey’s post hoc test).
Parkinsonism
Figure 1e illustrates the parkinsonian disability time course following treatment administration, while Fig. 1f depicts the AUC of the time course. As in our previous studies, LY-487,379 significantly reduced global parkinsonism (F(3,20) = 4.98, P < 0.01, one-way ANOVA). LY-487,379 enhanced l-DOPA anti-parkinsonian action by reducing parkinsonism by 46% (P < 0.01, Tukey’s post hoc test). While LY-341,495 did not significantly enhance l-DOPA anti-parkinsonian action, a non-significant 33% reduction of parkinsonism was obtained (P = 0.07 when l-DOPA/vehicle is compared to l-DOPA/LY-341,495). Similarly, a non-significant 35% reduction of parkinsonism was obtained when LY-487,379/LY-341,495 was added to l-DOPA (P = 0.06 when compared to l-DOPA/vehicle).
LY-341,495 reverses the anti-dyskinetic and anti-psychotic benefits of LY-354,740
Dyskinesia
Figure 2a illustrates the dyskinesia time course following treatment administration, while Fig. 2b depicts the AUC of the time course. As expected, LY-354–740 significantly reduced global dyskinesia severity, but the benefit was lost after concurrent administration of LY-354,740/LY-341,495, while LY-341,495 alone did not worsen dyskinesia, when compared to vehicle treatment (F(3,20) = 16.04, P < 0.001, one-way ANOVA). LY-354,740 diminished dyskinesia severity by 47% (P < 0.001, Tukey’s post hoc test). This anti-dyskinetic effect was no longer present when LY-341,495 was added to LY-354,740 (P < 0.05 when LY-354,740 is compared to LY-354,740/LY-341,495 and P > 0.05 when LY-354,740/LY-341,495 is compared to vehicle, Tukey’s post hoc test), while dyskinesia severity after administration of l-DOPA/LY-341,495 was not different to that elicited by l-DOPA alone, but was significantly more severe than after the addition of LY-354,740 to l-DOPA (P < 0.001 when LY-354,740 is compared to LY-341,495, Tukey’s post hoc test).
a Time course of dyskinesia in MPTP-lesioned marmosets treated with l-DOPA in combination with vehicle, LY-354,740 1 mg/kg, LY-341,495 1 mg/kg, or combination thereof. Each time point represents the cumulated dyskinesia scores for every 5 min observation period during the preceding 30 min. The maximal dyskinesia score at any time point is 12. b AUC of the time course of dyskinesia. c Time course of PLBs in MPTP-lesioned marmosets treated with l-DOPA in combination with vehicle, LY-354,740 1 mg/kg, LY-341,495 1 mg/kg, or combination thereof. Each time point represents the cumulated PLBs scores for every 5 min observation period during the preceding 30 min. The maximal PLBs score at any time point is 12. d AUC of the time course of PLBs. e Time course of parkinsonism in MPTP-lesioned marmosets treated with l-DOPA in combination with vehicle, LY-354,740 1 mg/kg, LY-341,495 1 mg/kg or combination thereof. Each time point represents the cumulated parkinsonian disability scores for every 5 min observation period during the preceding 30 min. The maximal parkinsonian disability score at any time point is 108. f AUC of the time course of parkinsonism. In a, c, and e, data are presented as the median; in b, d, and f, data are presented as the mean with the SEM and the individual values. *: P < 0.05; ***: P < 0.001
PLBs
Figure 2c illustrates the PLBs time course following treatment administration, while Fig. 2d depicts the AUC of the time course. LY-354,740 significantly reduced global PLBs severity, but the benefit was no longer present after simultaneous administration of LY-354,740/LY-341,495, while LY-341,495 alone did not worsen PLBs, when compared to vehicle treatment (F(3,20) = 11.57, P < 0.001, one-way ANOVA). LY-354,740 diminished PLBs severity by 39% (P < 0.001, Tukey’s post hoc test). This anti-psychotic effect was lost when LY-341,495 was added to LY-354,740 (P < 0.001 when LY-354,740 was compared to LY-341,495, P < 0.05 when LY-354,740 was compared to LY-354,740/LY-341,495 and P > 0.05 when LY-354,740/LY-341,495 was compared to vehicle, Tukey’s post hoc test). PLBs’ severity after administration of l-DOPA/LY-341,495 was not different to that elicited by l-DOPA alone (P > 0.05, Tukey’s post hoc test).
Parkinsonism
Figure 2e illustrates the parkinsonian disability time course following treatment administration, while Fig. 2f depicts the AUC of the time course. When added to l-DOPA, LY-354,740, LY-341,495, or LY-354,740/LY-341,495 did not have any effect on global parkinsonism (F(3,20) = 1.38, P > 0.05, one-way ANOVA).
Discussion
Here, we have found that LY-341,495 reverses the anti-dyskinetic and anti-psychotic, but not the anti-parkinsonian, effects conferred by LY-487,379, while it abolished the anti-dyskinetic and anti-psychotic benefits elicited by LY-354,740. Of note, LY-341,495, when added to l-DOPA without concurrent administration of LY-487,379 or LY-354,740, did not exacerbate dyskinesia or PLBs.
These results are in agreement with the previous studies that we performed, in which we showed that both LY-487,379 (Sid-Otmane et al. 2020) and LY-354,740 (Frouni et al. 2019) reduce l-DOPA-induced dyskinesia and PLBs and may also enhance l-DOPA anti-parkinsonian action. Moreover, the experiments reported here provide mechanistic insights into the actions of LY-487,379 and LY-354,740.
LY-487,379 is a highly selective PAM that appears to bind solely to mGlu2 receptors (Pinkerton et al. 2004; Schaffhauser et al. 2003), which makes mGlu2-positive allosteric modulation its putative mechanism of action, although it has not been ruled out that other mechanisms might underlie its action in vivo in the marmoset, notably because its metabolism in the primate has not been characterised and it is unknown whether active metabolites with off-target affinity might play a role in its actions. In contrast, LY-354,740 is an mGlu2/3 OA, with approximately fivefold greater affinity at mGlu2 than mGlu3 receptors (Monn et al. 1997; Schoepp et al. 1997), making it difficult to attribute any beneficial effect to an mGlu2-selective mechanism of action.
LY-341,495 is a selective mGlu2/3 orthosteric antagonist with similar affinity for both mGlu2 and mGlu3 receptors (Kingston et al. 1998). The reversal of the anti-dyskinetic and anti-psychotic benefits conferred by LY-487,379 when LY-341,495 was added to LY-487,379 provides pharmacological support to the hypothesis that positive allosteric modulation of mGlu2 receptors underlies the actions of LY-487,379. Indeed, we do not believe that orthosteric blockade of mGlu3 receptors by LY-341,495 contributed to the reversal of the anti-dyskinetic effects that we encountered here, although the argument for psychosis is not as straightforward.
Pertaining to dyskinesia, a recent study discovered that mGlu3 activation amplifies mGlu5 receptor signalling (Di Menna et al. 2018). Because mGlu5 negative allosteric modulation has been investigated pre-clinically and clinically as an anti-dyskinetic approach in PD (Johnston et al. 2010; Tison et al. 2016), blockade of mGlu3 receptors might reduce signalling at mGlu5 receptors, which should reduce dyskinesia; in our current experiments, this would have translated by a greater reduction of dyskinesia upon administration of the combination LY-487,379/LY-341,495. Here, that the anti-dyskinetic benefit conferred by LY-487,379 was lost after LY-341,495 was added points towards an mGlu2-preferential mechanism of action for dyskinesia.
In the case of PD psychosis, an mGlu2-mechanistic claim is more difficult to make based on the results of the current study. Indeed, mGlu5 activation is regarded as a promising anti-psychotic strategy (Conde-Ceide et al. 2015; Rook et al. 2015). Based upon the interaction between mGlu3 and mGlu5 receptors discussed in the paragraph above, antagonising mGlu3 receptors might have exacerbated PD psychosis; as such, the reversal of the anti-psychotic effect of LY-487,379 when it was combined with LY-341,495 might be due to the actions of LY-341,495 at both mGlu2 and mGlu3 receptors, which limit the conclusions that can be drawn. Further studies that would employ selective mGlu2 or mGlu3 negative allosteric modulators are warranted. Molecules with such selectivity, e.g., the mGlu3 receptor negative modulators such as ML289 (Sheffler et al. 2012) or ML337 (Wenthur et al. 2013), or the mixed mGlu2 agonist and mGlu3 antagonist LY-395,756 (Li et al. 2015), might help to provide further pharmacological evidence that an mGlu2-selective mechanism underlies the actions of LY-487,379 and LY-354,740 in experimental parkinsonism.
An unexpected finding of our experiments was the apparent, albeit non-significant, anti-parkinsonian effect of LY-341,495. It is noteworthy that this possible anti-parkinsonian effect was obtained only in one of the two sets of experiments (those with LY-487,379), in which it failed to reach statistical significance, despite a trend. Whether significant differences would have been encountered with a different molecule or a greater number of animals remains unclear. Indeed, when administered alone, LY-341,495 enhanced the anti-parkinsonian action of l-DOPA and did not reverse the extra anti-parkinsonian benefit achieved with LY-487,379. While requiring careful appraisal, our results, nevertheless, suggest that mGlu3 blockade may elicit an anti-parkinsonian effect when administered with l-DOPA. Further studies are needed to determine the potential mechanisms and brain areas that may underlie the anti-parkinsonian effect of antagonising mGlu3 receptors.
In summary, the experiments reported here have shown that the mGlu2/3 orthosteric antagonist LY-341,495 reverses the anti-dyskinetic and anti-psychotic effects achieved upon positive allosteric modulation of mGlu2 receptors (LY-487,379 experiments) and orthosteric stimulation of mGlu2/3 receptors (LY-354,740 experiments). While our results provide mechanistic insights into the actions of LY-487,379 and LY-354,740, further studies are needed, with more selective molecules, especially selective mGlu2 orthosteric antagonists or negative allosteric modulators, to achieve a better comprehension of the pharmacology underlying the possible beneficial effects of mGlu2-positive allosteric modulation and orthosteric stimulation in PD.
References
Conde-Ceide S, Martinez-Viturro CM, Alcazar J, Garcia-Barrantes PM, Lavreysen H, Mackie C, Vinson PN, Rook JM, Bridges TM, Daniels JS, Megens A, Langlois X, Drinkenburg WH, Ahnaou A, Niswender CM, Jones CK, Macdonald GJ, Steckler T, Conn PJ, Stauffer SR, Bartolome-Nebreda JM, Lindsley CW (2015) Discovery of VU0409551/JNJ-46778212: an mGlu5 positive allosteric modulator clinical candidate targeting schizophrenia. ACS Med Chem Lett 6(6):716–720. https://doi.org/10.1021/acsmedchemlett.5b00181
Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, Dhall R, Ballard C (2014) Pimavanserin for patients with Parkinson's disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 383(9916):533–540. https://doi.org/10.1016/S0140-6736(13)62106-6
Di Menna L, Joffe ME, Iacovelli L, Orlando R, Lindsley CW, Mairesse J, Gressèns P, Cannella M, Caraci F, Copani A (2018) Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology 128:301–313
Fox SH, Visanji N, Reyes G, Huot P, Gomez-Ramirez J, Johnston T, Brotchie JM (2010) Neuropsychiatric behaviors in the MPTP marmoset model of Parkinson's disease. Can J Neurol Sci 37(1):86–95
Fox SH, Visanji NP, Johnston TH, Gomez-Ramirez J, Voon V, Brotchie JM (2006) Dopamine receptor agonists and levodopa and inducing psychosis-like behavior in the MPTP primate model of Parkinson disease. Arch Neurol 63(9):1343–1344. https://doi.org/10.1001/archneur.63.9.1343
Frouni I, Hamadjida A, Kwan C, Bédard D, Nafade V, Gaudette F, Nuara SG, Gourdon JC, Beaudry F, Huot P (2019) Activation of mGlu2/3 receptors, a novel therapeutic approach to alleviate dyskinesia and psychosis in experimental parkinsonism. Neuropharmacology 158:107725
Gaudette F, Hamadjida A, Bedard D, Nuara SG, Beaudry F, Huot P (2017) Development and validation of a high-performance liquid chromatography-tandem mass spectrometry method to quantify LY-354,740 in rat and marmoset plasma. J Chromatogr B Anal Technol Biomed Life Sci 1061–1062:392–398. https://doi.org/10.1016/j.jchromb.2017.07.007
Gaudette F, Hamadjida A, Bedard D, Nuara SG, Gourdon JC, Michaud V, Beaudry F, Huot P (2018) Development of a selective and sensitive high-performance liquid chromatography-tandem mass spectrometry assay to support pharmacokinetic studies of LY-487,379 in rat and marmoset. J Chromatogr B Anal Technol Biomed Life Sci 1093–1094:1–7. https://doi.org/10.1016/j.jchromb.2018.06.036
Hamadjida A, Nuara SG, Bedard D, Frouni I, Kwan C, Gourdon JC, Huot P (2018a) Nefazodone reduces dyskinesia, but not psychosis-like behaviours, in the parkinsonian marmoset. Naunyn Schmiedebergs Arch Pharmacol 391(12):1339–1345. https://doi.org/10.1007/s00210-018-1549-6
Hamadjida A, Nuara SG, Bedard D, Gaudette F, Beaudry F, Gourdon JC, Huot P (2018b) The highly selective 5-HT2A antagonist EMD-281,014 reduces dyskinesia and psychosis in the l-DOPA-treated parkinsonian marmoset. Neuropharmacology 139:61–67. https://doi.org/10.1016/j.neuropharm.2018.06.038
Hamadjida A, Nuara SG, Gourdon JC, Huot P (2018c) The effect of mianserin on the severity of psychosis and dyskinesia in the parkinsonian marmoset. Prog Neuropsychopharmacol Biol Psychiatry 81:367–371. https://doi.org/10.1016/j.pnpbp.2017.09.001
Hamadjida A, Nuara SG, Gourdon JC, Huot P (2018d) Trazodone alleviates both dyskinesia and psychosis in the parkinsonian marmoset model of Parkinson's disease. J Neural Transm (Vienna) 125(9):1355–1360. https://doi.org/10.1007/s00702-017-1830-8
Hamadjida A, Nuara SG, Veyres N, Frouni I, Kwan C, Sid-Otmane L, Harraka MJ, Gourdon JC, Huot P (2017) The effect of mirtazapine on dopaminergic psychosis and dyskinesia in the parkinsonian marmoset. Psychopharmacology 234(6):905–911. https://doi.org/10.1007/s00213-017-4530-z
Hauser RA, Pahwa R, Tanner CM, Oertel W, Isaacson SH, Johnson R, Felt L, Stempien MJ (2017) ADS-5102 (Amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson's Disease (EASE LID 2 Study): interim results of an open-label safety study. J Parkinsons Dis 7(3):511–522. https://doi.org/10.3233/JPD-171134
Hely MA, Morris JG, Reid WG, Trafficante R (2005) Sydney multicenter study of Parkinson's disease: non-l-Dopa-responsive problems dominate at 15 years. Mov Disord 20(2):190–199. https://doi.org/10.1002/mds.20324
Huot P, Johnston TH, Gandy MN, Reyes MG, Fox SH, Piggott MJ, Brotchie JM (2012a) The monoamine re-uptake inhibitor UWA-101 improves motor fluctuations in the MPTP-lesioned common marmoset. PLoS ONE 7(9):e45587. https://doi.org/10.1371/journal.pone.0045587
Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM (2012b) l-DOPA pharmacokinetics in the MPTP-lesioned macaque model of Parkinson's disease. Neuropharmacology 63(5):829–836. https://doi.org/10.1016/j.neuropharm.2012.06.012
Huot P, Johnston TH, Lewis KD, Koprich JB, Reyes MG, Fox SH, Piggott MJ, Brotchie JM (2011) Characterization of 3,4-methylenedioxymethamphetamine (MDMA) enantiomers in vitro and in the MPTP-lesioned primate: R-MDMA reduces severity of dyskinesia, whereas S-MDMA extends duration of ON-time. J Neurosci 31(19):7190–7198. https://doi.org/10.1523/JNEUROSCI.1171-11.2011
Huot P, Johnston TH, Lewis KD, Koprich JB, Reyes MG, Fox SH, Piggott MJ, Brotchie JM (2014) UWA-121, a mixed dopamine and serotonin re-uptake inhibitor, enhances l-DOPA anti-parkinsonian action without worsening dyskinesia or psychosis-like behaviours in the MPTP-lesioned common marmoset. Neuropharmacology 82:76–87. https://doi.org/10.1016/j.neuropharm.2014.01.012
Johnston TH, Fox SH, McIldowie MJ, Piggott MJ, Brotchie JM (2010) Reduction of l-DOPA-induced dyskinesia by the selective metabotropic glutamate receptor 5 antagonist 3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson's disease. J Pharmacol Exp Ther 333(3):865–873. https://doi.org/10.1124/jpet.110.166629
Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD (1998) LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology 37(1):1–12. https://doi.org/10.1016/s0028-3908(97)00191-3
Kwan C, Frouni I, Bedard D, Nuara SG, Gourdon JC, Hamadjida A, Huot P (2019) 5-HT2A blockade for dyskinesia and psychosis in Parkinson's disease: is there a limit to the efficacy of this approach? A study in the MPTP-lesioned marmoset and a literature mini-review. Exp Brain Res 237(2):435–442. https://doi.org/10.1007/s00221-018-5434-9
Li ML, Yang SS, Xing B, Ferguson BR, Gulchina Y, Li YC, Li F, Hu XQ, Gao WJ (2015) LY395756, an mGluR2 agonist and mGluR3 antagonist, enhances NMDA receptor expression and function in the normal adult rat prefrontal cortex, but fails to improve working memory and reverse MK801-induced working memory impairment. Exp Neurol 273:190–201. https://doi.org/10.1016/j.expneurol.2015.08.019
Mizuno Y, Kanazawa I, Kuno S, Yanagisawa N, Yamamoto M, Kondo T (2007) Placebo-controlled, double-blind dose-finding study of entacapone in fluctuating parkinsonian patients. Mov Disord 22(1):75–80. https://doi.org/10.1002/mds.21218
Monn JA, Valli MJ, Massey SM, Wright RA, Salhoff CR, Johnson BG, Howe T, Alt CA, Rhodes GA, Robey RL, Griffey KR, Tizzano JP, Kallman MJ, Helton DR, Schoepp DD (1997) Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J Med Chem 40(4):528–537. https://doi.org/10.1021/jm9606756
Okamura N, Hashimoto K, Shimizu E, Koike K, Ohgake S, Koizumi H, Kumakiri C, Komatsu N, Iyo M (2003) Protective effect of LY379268, a selective group II metabotropic glutamate receptor agonist, on dizocilpine-induced neuropathological changes in rat retrosplenial cortex. Brain Res 992(1):114–119. https://doi.org/10.1016/j.brainres.2003.08.045
Pahwa R, Hauser RA (2017) ADS-5102 (Amantadine) extended release for levodopa-induced dyskinesia. JAMA Neurol 74(12):1507–1508. https://doi.org/10.1001/jamaneurol.2017.3205
Pahwa R, Tanner CM, Hauser RA, Isaacson SH, Nausieda PA, Truong DD, Agarwal P, Hull KL, Lyons KE, Johnson R, Stempien MJ (2017) ADS-5102 (Amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson Disease (EASE LID Study): a randomized clinical trial. JAMA Neurol 74(8):941–949. https://doi.org/10.1001/jamaneurol.2017.0943
Pinkerton AB, Cube RV, Hutchinson JH, James JK, Gardner MF, Schaffhauser H, Rowe BA, Daggett LP, Vernier JM (2004) Allosteric potentiators of the metabotropic glutamate receptor 2 (mGlu2). Part 2: 4-thiopyridyl acetophenones as non-tetrazole containing mGlu2 receptor potentiators. Bioorg Med Chem Lett 14(23):5867–5872. https://doi.org/10.1016/j.bmcl.2004.09.028
Postma JU, Van Tilburg W (1975) Visual hallucinations and delirium during treatment with amantadine (Symmetrel). J Am Geriatr Soc 23(5):212–215
Rook JM, Xiang Z, Lv X, Ghoshal A, Dickerson JW, Bridges TM, Johnson KA, Foster DJ, Gregory KJ, Vinson PN, Thompson AD, Byun N, Collier RL, Bubser M, Nedelcovych MT, Gould RW, Stauffer SR, Daniels JS, Niswender CM, Lavreysen H, Mackie C, Conde-Ceide S, Alcazar J, Bartolome-Nebreda JM, Macdonald GJ, Talpos JC, Steckler T, Jones CK, Lindsley CW, Conn PJ (2015) Biased mGlu5-positive allosteric modulators provide in vivo efficacy without potentiating mGlu5 modulation of NMDAR currents. Neuron 86(4):1029–1040. https://doi.org/10.1016/j.neuron.2015.03.063
Schaffhauser H, Rowe BA, Morales S, Chavez-Noriega LE, Yin R, Jachec C, Rao SP, Bain G, Pinkerton AB, Vernier JM, Bristow LJ, Varney MA, Daggett LP (2003) Pharmacological characterization and identification of amino acids involved in the positive modulation of metabotropic glutamate receptor subtype 2. Mol Pharmacol 64(4):798–810. https://doi.org/10.1124/mol.64.4.798
Schoepp DD, Johnson BG, Wright RA, Salhoff CR, Mayne NG, Wu S, Cockerman SL, Burnett JP, Belegaje R, Bleakman D, Monn JA (1997) LY354740 is a potent and highly selective group II metabotropic glutamate receptor agonist in cells expressing human glutamate receptors. Neuropharmacology 36(1):1–11. https://doi.org/10.1016/s0028-3908(96)00160-8
Sheffler DJ, Wenthur CJ, Bruner JA, Carrington SJ, Vinson PN, Gogi KK, Blobaum AL, Morrison RD, Vamos M, Cosford ND, Stauffer SR, Daniels JS, Niswender CM, Conn PJ, Lindsley CW (2012) Development of a novel, CNS-penetrant, metabotropic glutamate receptor 3 (mGlu3) NAM probe (ML289) derived from a closely related mGlu5 PAM. Bioorg Med Chem Lett 22(12):3921–3925. https://doi.org/10.1016/j.bmcl.2012.04.112
Sid-Otmane L, Hamadjida A, Nuara SG, Bedard D, Gaudette F, Gourdon JC, Michaud V, Beaudry F, Panisset M, Huot P (2020) Selective metabotropic glutamate receptor 2 positive allosteric modulation alleviates l-DOPA-induced psychosis-like behaviours and dyskinesia in the MPTP-lesioned marmoset. Eur J Pharmacol 873:172957. https://doi.org/10.1016/j.ejphar.2020.172957
Tison F, Keywood C, Wakefield M, Durif F, Corvol JC, Eggert K, Lew M, Isaacson S, Bezard E, Poli SM (2016) A Phase 2A trial of the novel mGluR5-negative allosteric modulator dipraglurant for levodopa-induced dyskinesia in Parkinson's Disease. Mov Disord 31(9):1373–1380
Tizzano JP, Griffey KI, Schoepp DD (2002) The anxiolytic action of mGlu2/3 receptor agonist, LY354740, in the fear-potentiated startle model in rats is mechanistically distinct from diazepam. Pharmacol Biochem Behav 73(2):367–374. https://doi.org/10.1016/s0091-3057(02)00850-x
Veyres N, Hamadjida A, Huot P (2018) Predictive value of parkinsonian primates in pharmacologic studies: a comparison between the macaque, marmoset, and squirrel monkey. J Pharmacol Exp Ther 365(2):379–397. https://doi.org/10.1124/jpet.117.247171
Visanji NP, Gomez-Ramirez J, Johnston TH, Pires D, Voon V, Brotchie JM, Fox SH (2006) Pharmacological characterization of psychosis-like behavior in the MPTP-lesioned nonhuman primate model of Parkinson's disease. Mov Disord 21(11):1879–1891. https://doi.org/10.1002/mds.21073
Wenthur CJ, Morrison R, Felts AS, Smith KA, Engers JL, Byers FW, Daniels JS, Emmitte KA, Conn PJ, Lindsley CW (2013) Discovery of (R)-(2-fluoro-4-((-4-methoxyphenyl)ethynyl)phenyl) (3-hydroxypiperidin-1-yl)methanone (ML337), an mGlu3 selective and CNS penetrant negative allosteric modulator (NAM). J Med Chem 56(12):5208–5212. https://doi.org/10.1021/jm400439t
Zhang J, Qu FR, Nakatsuka A, Nomura T, Nagai M, Nomoto M (2003) Pharmacokinetics of l-Dopa in plasma and extracellular fluid of striatum in common marmosets. Brain Res 993(1–2):54–58
Funding
PH has received research support from Parkinson Canada, Fonds de Recherche Québec–Santé, the Weston Brain Institute (Grant No. TR150146), the Michael J Fox Foundation for Parkinson’s Research, and the Natural Sciences and Engineering Research Council of Canada and Healthy Brains for Healthy Lives.
Author information
Authors and Affiliations
Contributions
AH, SGN, JCG, and PH conceived and designed research. AH and SN conducted experiments. PH analysed data. PH wrote the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Experiments were approved by McGill University and the Montreal Neurological Institute Animal Care Committees, which are in accordance with the regulations defined by the Canadian Council on Animal Care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nuara, S.G., Hamadjida, A., Gourdon, J.C. et al. The mGlu2/3 antagonist LY-341,495 reverses the anti-dyskinetic and anti-psychotic effects of the mGlu2 activators LY-487,379 and LY-354,740 in the MPTP-lesioned marmoset. J Neural Transm 127, 1013–1021 (2020). https://doi.org/10.1007/s00702-020-02196-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-020-02196-w