Abstract
LY-404,039 is an orthosteric agonist of metabotropic glutamate 2 and 3 receptors (mGluR2/3) that may harbour additional agonist effect at dopamine D2 receptors. LY-404,039 and its pro-drug, LY-2140023, have previously entered clinical trials as treatment options for schizophrenia. They could therefore be repurposed, if proven efficacious, for other conditions, notably Parkinson’s disease (PD). We have previously shown that the mGluR2/3 orthosteric agonist LY-354,740 alleviated L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesia and psychosis-like behaviours (PLBs) in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned marmoset. Unlike LY-404,039, LY-354,740 does not stimulate dopamine D2 receptors, suggesting that LY-404,039 may elicit broader therapeutic effects in PD. Here, we sought to investigate the effect of this possible additional dopamine D2-agonist action of LY-404,039 by assessing its efficacy on dyskinesia, PLBs and parkinsonism in the MPTP-lesioned marmoset. We first determined the pharmacokinetic profile of LY-404,039 in the marmoset, in order to select doses resulting in plasma concentrations known to be well tolerated in the clinic. Marmosets were then injected L-DOPA with either vehicle or LY-404,039 (0.1, 0.3, 1 and 10 mg/kg). The addition of LY-404,039 10 mg/kg to L-DOPA resulted in a significant reduction of global dyskinesia (by 55%, P < 0.01) and PLBs (by 50%, P < 0.05), as well as reduction of global parkinsonism (by 47%, P < 0.05). Our results provide additional support of the efficacy of mGluR2/3 orthosteric stimulation at alleviating dyskinesia, PLBs and parkinsonism. Because LY-404,039 has already been tested in clinical trials, it could be repurposed for indications related to PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic administration of the dopamine precursor L-3,4-dyhydroxyphenylalanine (L-DOPA), the most effective treatment of Parkinson’s disease (PD), ultimately leads to motor complications such as L-DOPA-induced dyskinesia (Dawson and Dawson 2003). Ninety-five percent of patients eventually suffer from dyskinesia after 15 years of L-DOPA therapy (Hely et al. 2005). On top of the motor complications, 50% of patients with advanced PD also suffer symptoms of psychosis (Hely et al. 2005). Amantadine, which is thought to act primarily through antagonism of N-methyl-D-aspartate (NMDA) glutamate receptors, is the only US FDA-approved treatment for dyskinesia (Rascol et al. 2021). However, development of tolerance (Thomas et al. 2004) and psychiatric side effects such as confusion and hallucinations (Postma and Van Tilburg 1975) limit the use of amantadine. Due to these limitations of amantadine, the discovery of novel treatments to alleviate dyskinesia is crucial.
Metabotropic glutamate 2 receptors (mGluR2) are densely expressed at the pre-synaptic terminals in the striatum, where they modulate glutamatergic transmission (Picconi et al. 2002). Dyskinetic symptoms from chronic L-DOPA administration are thought to result from hyperactive glutamatergic transmission in the synaptic cleft of neurons in the striatum (Cenci 2014; Huot et al. 2013). Enhanced glutamatergic transmission triggers excessive activation of ionotropic glutamate receptors, notably through hyperactivation of post-synaptic glutamate receptors such as NMDA and mGluR5 (Reiner and Levitz 2018). mGluR2/3 create a negative feedback mechanism to regulate excessive glutamate release and maintain homeostasis in the synaptic cleft (Gregory and Goudet 2021). Dyskinesia expression could therefore be diminished by reducing glutamate levels, notably via mGluR2/3 activation. mGluR2/3 are also connected to other neuropsychiatric disorders such as anxiety disorders, depression, mood disorders, addiction and psychotic disorders (Conn and Jones 2009).
We have previously shown that mGluR2 activation, via both orthosteric stimulation (Frouni et al. 2019) and positive allosteric modulation (Frouni et al. 2021; Nuara et al. 2020; Sid-Otmane et al. 2020), reduces dyskinesia in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned marmoset, a non-human primate with high predictive value of the success of drugs in clinical settings (Beaudry and Huot 2020; Veyres et al. 2018). Here, we have expanded our study of mGluR2 receptor activation with the mGluR2/3 orthosteric agonist (OA) LY-404,039 (pomaglumetad) on the severity of dyskinesia. We concurrently assessed the effects of LY-404,039 on psychosis-like behaviours (PLBs) and parkinsonian disability. LY-404,039 is a potent mGluR2/3 orthosteric agonist (Rorick-Kehn et al. 2007). Because of poor oral bioavailability (Annes et al. 2015), a pro-drug (LY-2140023 [pomaglumetad methionyl]) was developed and tested in the clinic (Downing et al. 2014; Patil et al. 2007) and has well established safety, tolerability and pharmacokinetic (PK) profiles in human, suggesting that it could be repurposed for the treatment of PD. An additional interest of LY-404,039 in the specific context of PD stems from a possible interaction with dopamine D2 receptors. It has been shown that LY-404,039 displays significant affinity for the dopamine D2 receptor, with a dissociation constant at D2High of 8.2–12.6 nM (Seeman 2013; Seeman and Guan 2009). This is lower than the dissociation constants of 92–149 nM for human mGluR2/3, which suggests that LY-404,039 will bind to D2 at clinical doses. This potential agonist action at dopamine D2 receptors suggests that it may have an anti-parkinsonian effect, although the D2 agonist effect remains unclear and controversial.
Methods
Animals
Twelve common marmosets (Callithrix jacchus; 300–450 g; McGill University breeding colony) were used in the experiments detailed below, 6 for the PK experiments and 6 for the behavioural studies, with an equal number of female and male animals in all settings. Animals were aged between 2 and 6 years old at the time of experiments and were housed in groups of 2 under conditions of controlled temperature (24 ± 1 °C), humidity (50 ± 5%) and light (12-h light/dark cycle, on 07:15 a.m.). They had unlimited access to water, with food (Mazuri® marmoset jelly, boiled eggs, boiled pasta, nuts, legumes) and fresh fruits served twice daily. Cages were enriched with primate toys and perches. Animals were acclimatised to handling, sub-cutaneous (s.c.) injections, as well as transfers to observation cages prior to the experiments. Marmosets were cared for in accordance with a protocol approved by McGill University and the Montreal Neurological Institute-Hospital (The Neuro) Animal Care Committees, both in accordance with the regulations of the Canadian Council on Animal Care.
The marmosets were previously used in other studies. Marmosets utilised in the PK studies were given a 30-day washout period before the start of the current experiments, during which they were not administered any drug or chemical substance. MPTP-lesioned marmosets had also been employed in previous studies. Prior to the experiments reported here, they were allowed a 30-day washout period, to ensure complete washout of previous treatments. During this washout, they were only administered L-DOPA/benserazide on a daily basis to maintain the dyskinesia and PLB phenotypes stable. No animals were excluded for any behavioural reasons.
Pharmacokinetic profile of LY-404,039
As we have previously reported (Gaudette et al. 2017, 2018; Kwan et al. 2021), we used a sparse sampling technique to collect a minimal volume of blood from marmosets (Tse and Nedelman 1996). Following administration of LY-404,039 (0.3 mg/kg s.c.; MilliporeSigma, Oakville, ON, Canada), blood samples were collected at 10 time points, baseline, 5 min, 10 min, 15 min, 30 min, 1 h, 2 h, 4 h, 6 h and 8 h. Additional samples were collected at 30 min, 1 h and 2 h from marmosets after s.c. administration LY-404,039 0.1, 0.3 and 1 mg/kg. Plasma was isolated by centrifugation and stored at − 80° until analysis. Levels of LY-404,039 were determined by high-performance liquid chromatography and tandem mass spectrometry (Kang et al. 2022). Plasma PK parameters were determined from the mean concentration value at each time point by a non-compartmental analysis method using PKSolver (Rowland and TN 1995; Zhang et al. 2010). Area under the curve (AUC) was calculated using the linear trapezoidal rule. AUC0-t, AUC0-∞, maximal plasma concentration (Cmax), time to Cmax (Tmax), elimination rate constant (λz), terminal half-life (T1/2), relative clearance (CL/F), relative volume of distribution (Vz/F) and mean residence time (MRT) were all calculated.
Induction of parkinsonism, dyskinesia and psychosis-like behaviours
Six marmosets were rendered parkinsonian by daily injections of MPTP hydrochloride (2 mg/kg, s.c., MilliporeSigma) over 5 days. Animals were given a month recovery period for development and stabilisation of parkinsonian symptoms. Animals were then orally administered L-DOPA/benserazide (15/3.75 mg/kg, MilliporeSigma) once daily for a minimum of 30 days, a treatment schedule that was shown to elicit stable dyskinetic and psychotic phenotypes (Hamadjida et al. 2018a, 2018b, 2017; Hamadjida et al. 2018c, d) and lead to clinically relevant plasma levels of L-DOPA (Huot et al. 2012b).
Assessment of parkinsonism, dyskinesia and PLBs
On days of assessment, marmosets were administered LY-404,039 (0.1, 0.3, 1, 10 mg/kg s.c.) or vehicle (0.9% NaCl) in combination with L-DOPA (15/3.75 mg/kg s.c., MilliporeSigma). Drug administration followed a randomised schedule according to a within-subject design that ensured all animals received all treatments. Following administration of treatment, each marmoset was placed in an individual observation cage (36 × 33 × 22 in) that contained water, food and a wooden perch and left undisturbed for 6 h. Treatments were separated by minimum of 72 h for complete drug clearance. Behaviours were recorded via webcam. Dyskinesia, PLBs and parkinsonism were all scored post hoc using previously validated scales (Fox et al. 2010; Huot et al. 2012a, 2011, 2014; Visanji et al. 2006) by a single experienced rater blinded to the treatment, to minimise inter-individual variability in the scoring of each animal. Over the course of a 6-h observation period, behaviours were examined for 5 min every 10 min. Parkinsonian disability was assessed for range of movement, bradykinesia, posture and attention/alertness. Range of movement was scored on a scale from 0 to 9, where 0 = running, jumping and use of limbs for different activities and 9 = no movement. Bradykinesia was scored from 0 to 3, where 0 = normal initiation and speed of movement and 3 = prolonged freezing, akinesia and immobile. Postural abnormality was scored 0 or 1, where 0 = normal balance with upright body posture and head is held up and 1 = impaired balance, prone body posture with head down. Attention/alertness was scored 0 or 1, where 0 = normal head checking and movement of neck is smooth in different directions and in small movements and 1 = less or no head checking, and head is in one position for more than 50% of the time. Global parkinsonian disability score was calculated as a combination of the behaviours mentioned above for each observation period using this formula: (range of movement × 1) + (bradykinesia × 3) + (posture × 9) + (alertness × 9), with 36 as the highest possible parkinsonian disability score per 5-min period. Dyskinesia rating evaluated chorea and dystonia, which were both scored on a scale from 0 to 4, where 0 = absent, 1 = mild, present less than 70% of the observation period and animal can eat and perform normal activity, 2 = moderate, 3 = marked and 4 = severe, present more than 70% of the observation period and animal is unable to perform normal activity. The PLB rating scale measured each of hyperkinesia (0-4), hallucinatory-like behaviour (0-4), repetitive grooming (0-4) and stereotypies (0-4); the PLB score attributed during any observation period was the most severe of these 4 behaviours. On the PLB rating scale, 0 = absent and 4 = present for more than 30% of the observation period and disabling.
Statistical analysis
Time courses of parkinsonian disability, dyskinesia and PLB scores are presented as the median. The AUC of the time courses are graphed as the mean ± SEM (referred to as “global” scores hereafter) and was analysed using one-way analysis of variance (ANOVA) followed by Tukey’s post test. Parkinsonian disability, dyskinesia and PLB scores at peak dose (90–150 min after administration) are graphed as the median with individual values and were analysed using Friedman followed by Dunn’s post test. Statistical analyses were performed with GraphPad Prism 9.4.1 (GraphPad Software Inc., San Diego, CA, USA).
Results
LY-404,039 was well tolerated by marmosets, regardless of the dose administered. We did not notice any adverse effect. The original data files are available as supplementary materials.
Pharmacokinetic profile of LY-404,039
Plasma PK parameters of LY-404,039 in the marmoset are shown in Table 1. After s.c. administration of LY-404,039 at 0.3 mg/kg, Cmax was 731.7 ng/mL, Tmax was observed at 1 h and a T1/2 was 32 min. Additionally, Cmax of 211.6 ng/mL and 2625.4 ng/mL was observed following s.c. administration at doses of 0.1 and 1 mg/kg, respectively, suggesting concentration-time profiles appear linear.
Effect of LY-404,039 on dyskinesia
As presented in Fig. 1A and B, LY-404,039 significantly diminished global dyskinesia severity (F(4,25) = 4.223, P < 0.01, one-way ANOVA). Specifically, the addition of LY-404,039 1 and 10 mg/kg to L-DOPA alleviated global dyskinesia when compared to vehicle, by ≈44% (P < 0.05, Tukey’s post test) and ≈55% (P < 0.01, Tukey’s post test), respectively.
Effect of LY-404,039 on L-DOPA-induced dyskinesia in the MPTP-lesioned marmoset. A Time course of dyskinesia over the 6-h observation period. Each time point represents the median cumulated dyskinesia scores for every 5-min observation period during the preceding 30 min. The maximal dyskinesia score at any time point is 12. B Area under the curve of dyskinesia time course. LY-404,039 1 and 10 mg/kg significantly reduced the global dyskinesia, by ≈44% and ≈55%, respectively. C Dyskinesia severity at peak dose (90-150 min after treatment administration). LY-404,039 1 and 10 mg/kg reduced the severity of peak dose dyskinesia by ≈45% and ≈55%, respectively. The maximal dyskinesia score at any time point is 24. Data are presented as the median (A), the mean ± SEM (B) and the median with individual values (C). *: P < 0.05; **: P < 0.01; ***: P < 0.001
As shown in Fig. 1C, LY-404,039 also diminished the severity of peak dose dyskinesia (Friedman statistic (FS) = 17.41, P < 0.01); LY-404,039 1 and 10 mg/kg significantly reduced peak dyskinesia severity, by ≈45% (P < 0.05, Dunn’s post test) and ≈55% (P < 0.01, Dunn’s post test), respectively, when compared to vehicle treatment.
Effect of LY-404,039 on PLBs
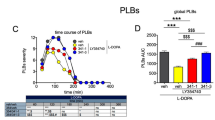
Figure 2A shows the time course of PLBs over the 6-h observation period and Fig. 2B shows the AUC of the time course. We found that LY-404,039 significantly diminished global PLBs severity (F(4,25) = 3.273, P < 0.05, one-way ANOVA). Specifically, when added to L-DOPA, LY-404,039 10 mg/kg alleviated global PLBs when compared to vehicle, by ≈50% (P < 0.05, Tukey’s post test).
Effect of LY-404,039 on L-DOPA-induced PLBs in the MPTP-lesioned marmoset. A Time course of PLBs over the 6-h observation period. Each time point represents the median cumulated PLB scores for every 5-min observation period during the preceding 30 min. The maximal PLB score at any time point is 12. B Area under the curve of PLBs time course. LY-404,039 10 mg/kg significantly reduced global PLBs, by ≈ 50%. C PLBs severity at peak dose (90-150 min after treatment administration). LY-404,039 1 and 10 mg/kg reduced the severity of peak dose PLBs, by ≈38% and ≈53%, respectively. The maximal PLB score at any time point is 24. Data are presented as the median (A), the mean ± SEM (B) and the median with individual values (C). *: P < 0.05; **: P < 0.01
LY-404,039 also reduced the severity of peak dose PLBs (FS = 16.73, P < 0.01, Fig. 2C). Thus, LY-404,039 1 and 10 mg/kg significantly alleviated peak PLBs severity by ≈38% (P < 0.05, Dunn’s post hoc test) and ≈53% (P < 0.01, Dunn’s post hoc test), respectively, when compared to vehicle treatment.
Effect of LY-404,039 on parkinsonism
In Fig. 3A and B, we show that LY-404,039 had a significant effect on global parkinsonism severity (F(4,25) = 3.274, P < 0.05, one-way ANOVA). Thus, combining LY-404,039 10 mg/kg with L-DOPA diminished global parkinsonian disability when compared to vehicle, by ≈47% (P < 0.05, Tukey’s post test, Fig. 3B).
Effect of LY-404,039 on parkinsonian disability in the MPTP-lesioned marmoset. A Time course of parkinsonism over the 6-h observation period. Each time point represents the median cumulated parkinsonism scores for every 5-min observation period during the preceding 30 min. The maximal parkinsonism score at any time point is 108. B Area under the curve of parkinsonism time course. LY-404,039 10 mg/kg significantly enhanced the anti-parkinsonian action of L-DOPA. Administration of LY-404,039 10 mg/kg parkinsonism reduced global parkinsonian disability by ≈47% when compared to L-DOPA alone. Data are presented as the median (A) and the mean ± SEM (B). *: P < 0.05; **: P < 0.01
Discussion
In the experiments reported here, we have demonstrated the effects of LY-404,039 as an adjunct to L-DOPA. LY-404,039 significantly attenuated the severity of dyskinesia and PLBs, while enhancing the anti-parkinsonian benefits of L-DOPA. The result of this study provides further support for acute mGluR2/3 orthosteric activation as an effective therapeutic strategy to reduce motor and non-motor complications in PD. LY-404,039 10 mg/kg consistently elicited the most significant reduction in dyskinesia, PLBs and parkinsonism, while the dose of 1 mg/kg also displayed anti-dyskinetic efficacy. Whereas it cannot be ruled out that greater effects on each of dyskinesia, PLBs and parkinsonism might have been obtained had we administered higher doses of LY-404,039, we ultimately elected not to do so, as such doses would have led to plasma exposure greater than that documented to be well tolerated in human (Annes et al. 2015; Patil et al. 2007; Rorick-Kehn et al. 2007; Mehta et al. 2018). It should be noted that U-shaped anti-dyskinetic dose-response curves were observed with mGluR2 activators in the 6-hydroxydopamine (6-OHDA)-lesioned rat (Frouni et al. 2019; Hamadjida et al. 2020), but not in the MPTP-lesioned marmoset (Frouni et al. 2019, 2021; Sid-Otmane et al. 2020), including here.
Although the specific mechanism(s) and cellular populations underlying the therapeutic effect of mGluR2/3 activation remain poorly characterised, reduction of glutamatergic neurotransmission and restoration of glutamatergic balance in the striatum may play an important role (Fabbrini et al., 2007; Muguruza et al. 2016). This is due to the fact that overactive glutamatergic transmission due to increased glutamatergic levels in the striatum is a defining characteristic of dyskinesia (Cenci and Konradi 2010). The brain regions at which mGluR2/3 are expressed also support this possibility, as they are strategically located pre-synaptically at the cortico-striatal pathway in the basal ganglia. mGluR2/3 agonists decrease pre-synaptic glutamate release and blunt the hyper-glutamatergic condition that is associated with the dyskinetic state. Specifically, it is believed that mGluR2/3 stimulation activates Gαi/o protein, which in turn inhibits adenylate cyclase (AC) that converts ATP to cAMP (Li et al. 2015). The resulting lower cAMP level limits the activity of various ion channels and members of serine/threonine-specific protein kinase A (PKA) family (Anwyl, 1999), which in turn diminishes pre-synaptic glutamate release. While mechanistically different, amantadine achieves a similar effect by antagonising glutamate binding to NMDA receptors to mitigate the over-activity of the direct striatal output pathway in dyskinesia (Sharma et al. 2018).
As LY-404,039 interacts in a virtually equipotent manner with both mGluR2 and mGluR3, the individual roles of each receptor in the anti-dyskinetic mechanism of mGluR2/3 agonists remain undetermined. However, we would suggest that the compound acted primarily through activation of mGluR2 rather than mGluR3. Indeed, there is a cross-talk between mGluR3 and mGluR5, resulting in an increase of mGluR5 downstream signalling following mGluR3 activation (Di Menna et al. 2018). As mGluR5 negative allosteric modulation is considered a potential anti-dyskinetic strategy (Bezard et al. 2014; Tison et al. 2016), any activating action at mGluR3 might result in a worsening of dyskinesia severity, thereby countering the anti-dyskinetic benefits conferred by mGluR2 activation.
Regarding the anti-psychotic effects, we would propose that mGluR2 activation within the infero-temporal cortex may be the primary mechanism through which LY-404,039 diminished L-DOPA-induced PLBs. Thus, antagonism of serotonin (5-HT) type 2A receptors (5-HT2AR) with pimavanserin (Cummings et al. 2014) alleviated PD psychosis in a randomised controlled clinical trial and pimavanserin is now used in the USA for the treatment of PD psychosis. 5-HT2AR and mGluR2 receptors form functional heterodimers, in which 5-HT2AR antagonism and mGluR2 stimulation produce similar downstream signalling effects (Fribourg et al. 2011), which could theoretically underlie the anti-psychotic action of LY-404,039.
In addition, we found that LY-404,039 significantly augmented the anti-parkinsonian action of L-DOPA. Whereas no head-to-head comparison was performed in the current series of experiments, this enhancement appears to be of greater magnitude when compared to our previous work in the same animal model with other mGluR2/3 activators such as OA LY-354,740 (Frouni et al. 2019) and the positive allosteric modulator (PAM) LY-487,379 (Sid-Otmane et al. 2020). For instance, LY-354,740 enhanced the anti-parkinsonian action of L-DOPA by 17% (Frouni et al. 2019), while the additional anti-parkinsonian benefit obtained with LY-487,379 was 15% (Sid-Otmane et al. 2020). It is noteworthy that the degree to which LY-404,039 enhanced the anti-parkinsonian action of L-DOPA is almost threefold greater than that of LY-354,740 and LY-487,379 at 47%. We propose two possible explanations for these differences. First, it is possible that the once described possible dopamine D2-agonist effect of LY-404,039 may have played a role in this extra anti-parkinsonian effect (Seeman 2013; Seeman and Guan 2009). Second, we previously tested the mGluR2 PAM CBiPES in the MPTP-lesioned marmoset (Frouni et al. 2021) and discovered a 43% enhancement of L-DOPA anti-parkinsonian action. CBiPES is structurally derived from LY-487,379 but has improved PK properties and improved brain distribution (Johnson et al. 2005). Although we have not determined brain concentrations of LY-404,039 in the marmoset, it is possible that better PK/pharmacodynamic properties of the compound may explain its seemingly greater anti-parkinsonian effect, when compared to LY-354,740 and LY-487,379. On a similar note, it would be interesting to eventually assess the anti-parkinsonian action of LY-404,039 as monotherapy, especially considering its purported interaction with dopamine D2 receptors.
LY-404,039 and its prodrug LY-2140023, which enhances the oral bioavailability of LY-404,039, have already undergone clinical trials for the treatment of schizophrenia (Adams et al. 2013; Liu et al. 2012; Mehta et al. 2018; Patil et al. 2007). In one such trial, LY-2140023 (or olanzapine as an active control) was administered to patients with schizophrenia (Patil et al., 2007). They reported that the treatment was not only safe and well-tolerated, but the patients showed statistically significant improvements in both positive and negative symptoms of schizophrenia compared to placebo, demonstrating its anti-psychotic properties. In subsequent trials, patients treated with LY-2140023 did not significantly improve compared to placebo (Adams et al. 2013). In addition, LY-2140023 was tested in a magnetic resonance imaging study (Mehta et al. 2018), as well as a pharmacogenetic study (Liu et al. 2012). LY-404,039 and LY-2140023 have therefore entered several clinical trials, during which they were safe and well tolerated.
In summary, our results provide additional evidence that orthosteric stimulation of mGluR2/3 receptors may be an effective approach for simultaneously reducing dyskinesia and psychosis, while providing additional anti-parkinsonian effect, when combined with L-DOPA. In the case of LY-404,039, a possible agonistic effect at dopamine D2 receptors might make this molecule especially suited as an adjunct therapeutic in PD. Moreover, as mentioned above, LY-404,039 is essentially ready for clinical testing in PD patients, as it has previously undergone studies in the clinic for psychiatric conditions.
Data availability
Data are available as supplementary materials.
References
Adams DH, Kinon BJ, Baygani S, Millen BA, Velona I, Kollack-Walker S, Walling DP (2013) A long-term, phase 2, multicenter, randomized, open-label, comparative safety study of pomaglumetad methionil (LY2140023 monohydrate) versus atypical antipsychotic standard of care in patients with schizophrenia. BMC Psychiatry 13:143. https://doi.org/10.1186/1471-244X-13-143
Annes WF, Long A, Witcher JW, Ayan-Oshodi MA, Knadler MP, Zhang W, Mitchell MI, Cornelissen K, Hall SD (2015) Relative contributions of presystemic and systemic peptidases to oral exposure of a novel metabotropic glutamate 2/3 receptor agonist (LY404039) after oral administration of prodrug pomaglumetad methionil (LY2140023). J Pharm Sci 104(1):207–214. https://doi.org/10.1002/jps.24226
Anwyl R (1999) Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Rev 29(1):83–120. https://doi.org/10.1016/S0165-0173(98)00050-2
Beaudry F, Huot P (2020) The MPTP-lesioned marmoset model of Parkinson’s disease: proposed efficacy thresholds that may potentially predict successful clinical trial results. J Neural Transm (vienna) 127(10):1343–1358. https://doi.org/10.1007/s00702-020-02247-2
Bezard E, Pioli EY, Li Q, Girard F, Mutel V, Keywood C, Tison F, Rascol O, Poli SM (2014) The mGluR5 negative allosteric modulator dipraglurant reduces dyskinesia in the MPTP macaque model. Mov Disord 29(8):1074–1079. https://doi.org/10.1002/mds.25920
Cenci MA (2014) Glutamatergic pathways as a target for the treatment of dyskinesias in Parkinson’s disease. Biochem Soc Trans 42(2):600–604. https://doi.org/10.1042/BST20140006
Cenci MA, Konradi C (2010) Maladaptive striatal plasticity in L-DOPA-induced dyskinesia. Prog Brain Res 183:209–233. https://doi.org/10.1016/S0079-6123(10)83011-0
Conn PJ, Jones CK (2009) Promise of mGluR2/3 activators in psychiatry. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 34 (1):248-249. https://doi.org/10.1038/npp.2008.156
Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, Dhall R, Ballard C (2014) Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 383(9916):533–540. https://doi.org/10.1016/S0140-6736(13)62106-6
Dawson TM, Dawson VL (2003) Molecular pathways of neurodegeneration in Parkinson’s disease. Science 302(5646):819–822. https://doi.org/10.1126/science.1087753
Di Menna L, Joffe ME, Iacovelli L, Orlando R, Lindsley CW, Mairesse J, Gressèns P, Cannella M, Caraci F, Copani A, Bruno V, Battaglia G, Conn PJ, Nicoletti F (2018) Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology 128:301–313. https://doi.org/10.1016/j.neuropharm.2017.10.026
Downing AM, Kinon BJ, Millen BA, Zhang L, Liu L, Morozova MA, Brenner R, Rayle T, Nisenbaum L, Zhao F, Gomez J (2014) A double-bind, placebo-controlled comparator study of LY2140023 monohydrate in patients with schizophrenia. BMC Psychiatry 14(1):351. https://doi.org/10.1186/s12888-014-0351-3
Fabbrini G, Brotchie, JM, Grandas F, Nomoto M,Goetz CG (2007) Levodopa-induced dyskinesias. Mov Disord 22(10):1379-1389. https://doi.org/10.1002/mds.21475
Fox SH, Visanji N, Reyes G, Huot P, Gomez-Ramirez J, Johnston T, Brotchie JM (2010) Neuropsychiatric behaviors in the MPTP marmoset model of Parkinson’s disease. Can J Neurol Sci 37(1):86–95
Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adney SK, Hatcher C, Eltit JM, Ruta JD, Albizu L, Li Z, Umali A, Shim J, Fabiato A, MacKerell AD Jr, Brezina V, Sealfon SC, Filizola M, Gonzalez-Maeso J, Logothetis DE (2011) Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell 147(5):1011–1023. https://doi.org/10.1016/j.cell.2011.09.055
Frouni I, Hamadjida A, Kwan C, Bedard D, Nafade V, Gaudette F, Nuara SG, Gourdon JC, Beaudry F, Huot P (2019) Activation of mGlu2/3 receptors, a novel therapeutic approach to alleviate dyskinesia and psychosis in experimental parkinsonism. Neuropharmacology 158:107725. https://doi.org/10.1016/j.neuropharm.2019.107725
Frouni I, Kwan C, Nuara SG, Belliveau S, Kang W, Hamadjida A, Bedard D, Gourdon JC, Huot P (2021) Effect of the mGlu2 positive allosteric modulator CBiPES on dyskinesia, psychosis-like behaviours and parkinsonism in the MPTP-lesioned marmoset. J Neural Transm (vienna) 128(1):73–81. https://doi.org/10.1007/s00702-020-02287-8
Gaudette F, Hamadjida A, Bedard D, Nuara SG, Beaudry F, Huot P (2017) Development and validation of a high-performance liquid chromatography-tandem mass spectrometry method to quantify LY-354,740 in rat and marmoset plasma. J Chromatogr B Analyt Technol Biomed Life Sci 1061–1062:392–398. https://doi.org/10.1016/j.jchromb.2017.07.007
Gaudette F, Hamadjida A, Bedard D, Nuara SG, Gourdon JC, Michaud V, Beaudry F, Huot P (2018) Development of a selective and sensitive high-performance liquid chromatography-tandem mass spectrometry assay to support pharmacokinetic studies of LY-487,379 in rat and marmoset. J Chromatogr B Analyt Technol Biomed Life Sci 1093–1094:1–7. https://doi.org/10.1016/j.jchromb.2018.06.036
Gregory KJ, Goudet C (2021) International Union of Basic and Clinical Pharmacology. CXI. Pharmacology, signaling, and physiology of metabotropic glutamate receptors. Pharmacol Rev 73(1):521–569. https://doi.org/10.1124/pr.119.019133
Hamadjida A, Nuara SG, Bedard D, Frouni I, Kwan C, Gourdon JC, Huot P (2018a) Nefazodone reduces dyskinesia, but not psychosis-like behaviours, in the parkinsonian marmoset. Naunyn Schmiedebergs Arch Pharmacol 391(12):1339–1345. https://doi.org/10.1007/s00210-018-1549-6
Hamadjida A, Nuara SG, Bedard D, Gaudette F, Beaudry F, Gourdon JC, Huot P (2018b) The highly selective 5-HT2A antagonist EMD-281,014 reduces dyskinesia and psychosis in the l-DOPA-treated parkinsonian marmoset. Neuropharmacology 139:61–67. https://doi.org/10.1016/j.neuropharm.2018.06.038
Hamadjida A, Nuara SG, Gourdon JC, Huot P (2018c) The effect of mianserin on the severity of psychosis and dyskinesia in the parkinsonian marmoset. Prog Neuropsychopharmacol Biol Psychiatry 81:367–371. https://doi.org/10.1016/j.pnpbp.2017.09.001
Hamadjida A, Nuara SG, Gourdon JC, Huot P (2018d) Trazodone alleviates both dyskinesia and psychosis in the parkinsonian marmoset model of Parkinson’s disease. J Neural Transm (vienna) 125(9):1355–1360. https://doi.org/10.1007/s00702-017-1830-8
Hamadjida A, Nuara SG, Veyres N, Frouni I, Kwan C, Sid-Otmane L, Harraka MJ, Gourdon JC, Huot P (2017) The effect of mirtazapine on dopaminergic psychosis and dyskinesia in the parkinsonian marmoset. Psychopharmacology 234(6):905–911. https://doi.org/10.1007/s00213-017-4530-z
Hamadjida A, Sid-Otmane L, Kwan C, Frouni I, Nafade V, Bedard D, Gagnon D, Wallman MJ, Rouillard C, Parent A, Parent M, Huot P (2020) The highly selective mGlu2 receptor positive allosteric modulator LY-487,379 alleviates l-DOPA-induced dyskinesia in the 6-OHDA-lesioned rat model of Parkinson’s disease. Eur J Neurosci 51(12):2412–2422. https://doi.org/10.1111/ejn.14679
Hely MA, Morris JG, Reid WG, Trafficante R (2005) Sydney Multicenter Study of Parkinson’s disease: non-L-dopa-responsive problems dominate at 15 years. Mov Disord 20(2):190–199. https://doi.org/10.1002/mds.20324
Huot P, Johnston TH, Gandy MN, Reyes MG, Fox SH, Piggott MJ, Brotchie JM (2012a) The monoamine re-uptake inhibitor UWA-101 improves motor fluctuations in the MPTP-lesioned common marmoset. PLoS ONE 7(9):e45587. https://doi.org/10.1371/journal.pone.0045587
Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM (2012b) L-DOPA pharmacokinetics in the MPTP-lesioned macaque model of Parkinson’s disease. Neuropharmacology 63(5):829–836. https://doi.org/10.1016/j.neuropharm.2012.06.012
Huot P, Johnston TH, Koprich JB, Fox SH, Brotchie JM (2013) The pharmacology of L-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol Rev 65(1):171–222. https://doi.org/10.1124/pr.111.005678
Huot P, Johnston TH, Lewis KD, Koprich JB, Reyes MG, Fox SH, Piggott MJ, Brotchie JM (2011) Characterization of 3,4-methylenedioxymethamphetamine (MDMA) enantiomers in vitro and in the MPTP-lesioned primate: R-MDMA reduces severity of dyskinesia, whereas S-MDMA extends duration of ON-time. J Neurosci 31(19):7190-7198. 31/19/7190 [pii]. https://doi.org/10.1523/JNEUROSCI.1171-11.2011
Huot P, Johnston TH, Lewis KD, Koprich JB, Reyes MG, Fox SH, Piggott MJ, Brotchie JM (2014) UWA-121, a mixed dopamine and serotonin re-uptake inhibitor, enhances L-DOPA anti-parkinsonian action without worsening dyskinesia or psychosis-like behaviours in the MPTP-lesioned common marmoset. Neuropharmacology 82:76–87. https://doi.org/10.1016/j.neuropharm.2014.01.012
Johnson MP, Barda D, Britton TC, Emkey R, Hornback WJ, Jagdmann GE, McKinzie DL, Nisenbaum ES, Tizzano JP, Schoepp DD (2005) Metabotropic glutamate 2 receptor potentiators: receptor modulation, frequency-dependent synaptic activity, and efficacy in preclinical anxiety and psychosis model(s). Psychopharmacology 179(1):271–283. https://doi.org/10.1007/s00213-004-2099-9
Kang W, Gaudette F, Bedard D, Beaudry F, Huot P (2022) Quantitative determination of LY-404,039, a metabotropic glutamate 2/3 receptor agonist, in rat plasma using chemical derivatization and HPLC-MRM/MS. Biomed Chromatogr e5423. https://doi.org/10.1002/bmc.5423
Kwan C, Nuara SG, Bedard D, Gaudette F, Gourdon JC, Beaudry F, Huot P (2021) Selective blockade of the 5-HT3 receptor acutely alleviates dyskinesia and psychosis in the parkinsonian marmoset. Neuropharmacology 182:108386. https://doi.org/10.1016/j.neuropharm.2020.108386
Li ML, Hu XQ, Li F, Gao WJ (2015) Perspectives on the mGluR2/3 agonists as a therapeutic target for schizophrenia: still promising or a dead end? Prog Neuropsychopharmacol Biol Psychiatry 60:66–76. https://doi.org/10.1016/j.pnpbp.2015.02.012
Liu W, Downing AC, Munsie LM, Chen P, Reed MR, Ruble CL, Landschulz KT, Kinon BJ, Nisenbaum LK (2012) Pharmacogenetic analysis of the mGlu2/3 agonist LY2140023 monohydrate in the treatment of schizophrenia. Pharmacogenomics J 12(3):246–254. https://doi.org/10.1038/tpj.2010.90
Mehta MA, Schmechtig A, Kotoula V, McColm J, Jackson K, Brittain C, Tauscher-Wisniewski S, Kinon BJ, Morrison PD, Pollak T, Mant T, Williams SCR, Schwarz AJ (2018) Group II metabotropic glutamate receptor agonist prodrugs LY2979165 and LY2140023 attenuate the functional imaging response to ketamine in healthy subjects. Psychopharmacology 235(7):1875–1886. https://doi.org/10.1007/s00213-018-4877-9
Muguruza C, Meana JJ, Callado LF (2016) Group II metabotropic glutamate receptors as targets for novel antipsychotic drugs. Front Pharmacol 7:130. https://doi.org/10.3389/fphar.2016.00130
Nuara SG, Hamadjida A, Gourdon JC, Huot P (2020) The mGlu2/3 antagonist LY-341,495 reverses the anti-dyskinetic and anti-psychotic effects of the mGlu2 activators LY-487,379 and LY-354,740 in the MPTP-lesioned marmoset. J Neural Transm (vienna) 127(7):1013–1021. https://doi.org/10.1007/s00702-020-02196-w
Patil ST, Zhang L, Martenyi F, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD (2007) Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized phase 2 clinical trial. Nat Med 13(9):1102–1107. https://doi.org/10.1038/nm1632
Picconi B, Pisani A, Centonze D, Battaglia G, Storto M, Nicoletti F, Bernardi G, Calabresi P (2002) Striatal metabotropic glutamate receptor function following experimental parkinsonism and chronic levodopa treatment. Brain 125(Pt 12):2635–2645
Postma JU, Van Tilburg W (1975) Visual hallucinations and delirium during treatment with amantadine (Symmetrel). J Am Geriatr Soc 23(5):212–215
Rascol O, Fabbri M, Poewe W (2021) Amantadine in the treatment of Parkinson’s disease and other movement disorders. Lancet Neurol 20(12):1048–1056. https://doi.org/10.1016/S1474-4422(21)00249-0
Reiner A, Levitz J (2018) Glutamatergic signaling in the central nervous system: ionotropic and metabotropic receptors in concert. Neuron 98:1080–1098. https://doi.org/10.1016/j.neuron.2018.05.018
Rorick-Kehn LM, Johnson BG, Burkey JL, Wright RA, Calligaro DO, Marek GJ, Nisenbaum ES, Catlow JT, Kingston AE, Giera DD, Herin MF, Monn JA, McKinzie DL, Schoepp DD (2007) Pharmacological and pharmacokinetic properties of a structurally novel, potent, and selective metabotropic glutamate 2/3 receptor agonist: in vitro characterization of agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]-hexane-4,6-dicarboxylic acid (LY404039). J Pharmacol Exp Ther 321(1):308–317. https://doi.org/10.1124/jpet.106.110809
Rowland M, TN T (1995) Clinical pharmacokinetics: concepts and application. Lippincott Williams and Wilkins., Philadelphia (PA)
Seeman P (2013) An agonist at glutamate and dopamine D2 receptors, LY404039. Neuropharmacology 66:87–88. https://doi.org/10.1016/j.neuropharm.2012.07.001
Seeman P, Guan HC (2009) Glutamate agonist LY404,039 for treating schizophrenia has affinity for the dopamine D2(High) receptor. Synapse 63(10):935–939. https://doi.org/10.1002/syn.20704
Sharma VD, Lyons KE, Pahwa R (2018) Amantadine extended-release capsules for levodopa-induced dyskinesia in patients with Parkinson’s disease. Ther Clin Risk Manag 14:665–673. https://doi.org/10.2147/TCRM.S144481
Sid-Otmane L, Hamadjida A, Nuara SG, Bedard D, Gaudette F, Gourdon JC, Michaud V, Beaudry F, Panisset M, Huot P (2020) Selective metabotropic glutamate receptor 2 positive allosteric modulation alleviates L-DOPA-induced psychosis-like behaviours and dyskinesia in the MPTP-lesioned marmoset. Eur J Pharmacol 873:172957. https://doi.org/10.1016/j.ejphar.2020.172957
Thomas A, Iacono D, Luciano AL, Armellino K, Di Iorio A, Onofrj M (2004) Duration of amantadine benefit on dyskinesia of severe Parkinson’s disease. J Neurol Neurosurg Psychiatry 75(1):141–143
Tison F, Keywood C, Wakefield M, Durif F, Corvol JC, Eggert K, Lew M, Isaacson S, Bezard E, Poli SM, Goetz CG, Trenkwalder C, Rascol O (2016) A phase 2A trial of the novel mGluR5-negative allosteric modulator dipraglurant for levodopa-induced dyskinesia in Parkinson’s disease. Mov Disord 31(9):1373–1380. https://doi.org/10.1002/mds.26659
Tse FL, Nedelman JR (1996) Serial versus sparse sampling in toxicokinetic studies. Pharm Res 13(7):1105–1108
Veyres N, Hamadjida A, Huot P (2018) Predictive value of parkinsonian primates in pharmacologic studies: a comparison between the macaque, marmoset, and squirrel monkey. J Pharmacol Exp Ther 365(2):379–397. https://doi.org/10.1124/jpet.117.247171
Visanji NP, Gomez-Ramirez J, Johnston TH, Pires D, Voon V, Brotchie JM, Fox SH (2006) Pharmacological characterization of psychosis-like behavior in the MPTP-lesioned nonhuman primate model of Parkinson’s disease. Mov Disord 21(11):1879–1891. https://doi.org/10.1002/mds.21073
Zhang Y, Huo M, Zhou J, Xie S (2010) PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 99(3):306–314. https://doi.org/10.1016/j.cmpb.2010.01.007
Acknowledgements
IF holds a scholarship from Parkinson Canada. CK holds a scholarship from Fonds de Recherche Québec—Santé. PH has received research support from Parkinson Canada, Parkinson Québec, Fonds de Recherche Québec—Santé, the Weston Brain Institute, the Michael J Fox Foundation for Parkinson’s Research, the Natural Sciences and Engineering Research Council of Canada, Healthy Brains for Healthy Lives and Biogen Inc. FB is the holder of the Canada Research Chair in metrology of bioactive molecule and target discovery.
Funding
PH has research support from Parkinson Canada, Fonds de Recherche Québec—Santé, the Weston Brain Institute, the Michael J Fox Foundation for Parkinson’s Research, the Natural Sciences and Engineering Research Council of Canada and Healthy Brains for Healthy Lives. FB is the holder of the Canada Research Chair in metrology of bioactive molecule and target discovery.
Author information
Authors and Affiliations
Contributions
PH conceived research. CK, AH, JCG, FG, FB and PH organised experiments. WK, SGN, DB, IF and FG conducted experiments. WK and IF wrote the manuscript. All authors read and approved the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Competing interests
PH has received payments from Neurodiem, AbbVie, adMare BioInnovations, Sanford Burnham Prebys, Sunovion and Throughline Strategy.
Ethics approval
Experiments were approved by McGill University and the Montreal Neurological Institute Animal Care Committees, which are in accordance with the regulations defined by the Canadian Council on Animal Care (Animal Use Protocol 2017–7922).
Conflict of interest
PH has received payments from Neurodiem, AbbVie, adMare BioInnovations, Sanford Burnham Prebys, Sunovion and Throughline Strategy.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, W., Nuara, S.G., Bédard, D. et al. The mGluR2/3 orthosteric agonist LY-404,039 reduces dyskinesia, psychosis-like behaviours and parkinsonism in the MPTP-lesioned marmoset. Naunyn-Schmiedeberg's Arch Pharmacol 396, 2347–2355 (2023). https://doi.org/10.1007/s00210-023-02587-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-023-02587-2