Abstract
Trazodone is a clinically available anti-depressant that exhibits affinity for serotonin 1A and 2A receptors, as well as for alpha-adrenoceptors, suggesting that it may be useful to treat l-3,4-dihydroxyphenylalanine (l-DOPA)-induced dyskinesia and psychosis that are encountered in advanced Parkinson’s disease (PD). Here, we investigated the anti-dyskinetic and anti-psychotic effects of trazodone in the parkinsonian non-human primate. 6 common marmosets were rendered parkinsonian by administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). Following repeated administration of l-DOPA to induce stable dyskinesia and psychosis-like behaviours (PLBs), trazodone (0.1, 1 and 10 mg/kg) or vehicle was administered in combination with l-DOPA and its effects on dyskinesia, PLBs and parkinsonism were determined. The addition of trazodone 10 mg/kg to l-DOPA reduced peak dose dyskinesia by ≈ 39% (P < 0.01) and peak dose PLBs by ≈ 17% (P < 0.01). However, parkinsonian disability was significantly worsened by trazodone 10 mg/kg (P < 0.05) and duration of anti-parkinsonian action was diminished by ≈ 21% (P < 0.05). Our results suggest that trazodone may be effective in alleviating l-DOPA-induced dyskinesia and psychosis in PD, but its deleterious effect on motor function is a concern and may limit its tolerability and usefulness in clinical settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Psychosis and dyskinesia hinder the quality of life of as many as 50–95% of patients with Parkinson’s disease (PD) 15 years after treatment with l-3,4-dihydroxyphenylalanine (l-DOPA) has been commenced (Hely et al. 2005). Treatment options for both conditions are few; in fact, only clozapine was shown to alleviate each of psychosis (French Clozapine Parkinson Study Group 1999; Parkinson Study Group 1999) and dyskinesia (Durif et al. 2004) in randomised, double-blind, placebo-controlled clinical trials. Despite its effectiveness in clinical studies, clinicians are often reluctant to prescribe clozapine, because of the risk of agranulocytosis associated with its use (Alvir et al. 1993).
However, looking at the pharmacological profile of clozapine may be informative when it comes to selecting drugs with potential anti-dyskinetic and anti-psychotic action in PD. Clozapine is not selective and binds to a wealth of receptors, including dopamine D4, serotonin (5-HT) 1A, 2A receptors, as well as alpha (α)-adrenoceptors (Ashby and Wang 1996), which are considered, individually, as potential targets for l-DOPA-induced dyskinesia (Huot et al. 2012b; Lewitt et al. 2012; Svenningsson et al. 2015; Vanover et al. 2008) and PD psychosis (Cummings et al. 2014). Clozapine lack of selectivity makes it virtually impossible to pinpoint a specific mechanism by which it alleviates dyskinesia and psychosis, but it raises the interesting possibility that drugs with affinity for several targets, including those cited above with which clozapine interact, could potentially be beneficial in alleviating l-DOPA-induced dyskinesia and PD psychosis.
Trazodone is a clinically available anti-depressant which, as clozapine, displays high affinity for 5-HT1A, 5-HT2A and α-adrenoceptors (Owens et al. 1997). Because of its affinity for each of these targets, we hypothesised that trazodone would effectively alleviate both dyskinesia and psychosis in PD. Here, we have tested our hypothesis in the gold-standard animal model of PD, the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned non-human primate. We also assessed the effect of trazodone on l-DOPA anti-parkinsonian action.
Materials and methods
Induction of parkinsonism, dyskinesia and psychosis-like behaviours in the common marmoset
Six common marmosets (Callithrix jacchus; WorldWide Primates, USA; 3 females and 3 males), weighing 300–450 g, were housed in pairs under conditions of controlled temperature (24 ± 1 °C), humidity (50%) and a 12-h light/dark cycle (07:15 lights on). Animals were cared for in accordance with a protocol approved by McGill University Animal Care Committee in accordance with the regulations defined by the Canadian Council on Animal Care. Marmosets had unrestricted access to water, food, nuts and fresh fruits. Home cages were enriched with primate toys, nest boxes and perches. Prior to the start of studies, animals were acclimatised to handling, administration of subcutaneous (s.c.) treatments, as well as transfer to observation cages for behavioural assessment.
Parkinsonism was induced by s.c. injections of MPTP hydrochloride (2 mg/kg daily or every other day, for 5 days, tailored to the animals’ reaction to MPTP; Sigma-Aldrich, Canada). MPTP administration was followed by a 6-week recovery period to allow parkinsonian symptoms to develop and stabilise. Treatment-related complications, i.e. dyskinesia and psychosis-like behaviours (PLBs), were elicited by treatment with oral l-DOPA/benserazide (henceforth referred to as l-DOPA, 15/3.75 mg/kg; Sigma-Aldrich, Canada) once daily for at least 30 days. This treatment paradigm has been previously demonstrated to produce a stable model of l-DOPA-induced dyskinesia and PLBs (Hamadjida et al. 2017, 2018; Huot et al. 2012a, 2014; Johnston et al. 2013).
Administration of trazodone, in combination with l-DOPA, to parkinsonian marmosets
On experimental days, at 08:00, marmosets were injected with l-DOPA 15/3.75 mg/kg s.c. (Sigma-Aldrich, Canada) in combination with either vehicle (0.9% NaCl) or trazodone (0.1, 1 and 10 mg/kg s.c.; Cedarlane Laboratories, Canada). Doses of trazodone were selected based on rat studies (Crissman and O’Donnell 2002; Mason et al. 1987; Rudissaar et al. 2001).
Drug administration schedule was randomised according to a Latin square design in which all treatments were administered to each animal, in a random order. After treatment administration, each marmoset was placed individually into an observation cage (36 × 33 × 22 in) containing food, water and a wooden perch, and left undisturbed for the 6 h duration of the experiment. Behaviour was recorded via webcam and analysed post hoc by a movement disorder neurologist blinded to the treatment. At least 48 h were left between each experiment in any animal.
Behavioural analysis
The scales used for assessment of behaviour were detailed previously (Fox et al. 2010; Huot et al. 2011; Johnston et al. 2012). Parkinsonian disability was rated for 5 min every 10 min using a parkinsonian disability scale encompassing measures of range of movement, bradykinesia, posture, and attention/alertness. Range of movement was rated on a 0–9 scale: 0 = running, jumping between roof, walls, perch, using limbs through a wide range of activity; 9 = no movement. Bradykinesia was rated from 0 to 3: 0 = normal initiation and speed of movement; 3 = prolonged freezing, akinesia, inability to move. Postural abnormalities were rated 0 or 1: 0 = normal balance, upright posture, head held up; 1 = impaired balance, crouched posture, head down. Attention/alertness was rated 0 or 1; 0 = normal head checking movements, movement of neck in variable directions, smooth, small movements; 1 = reduced or absent head checking, head in one position for more than 50% of observation period. The score attributed to each of the behaviours assessed was the most prevalent during the 5-min observation period. A global parkinsonian disability score was calculated as a combination of the aforementioned behaviours, equally weighted, according to the following formula: (range of movement × 1) + (bradykinesia × 3) + (posture × 9) + (alertness × 9). The maximal parkinsonian disability score per 5 min observation period was 36.
l-DOPA-induced dyskinesia and PLBs were also assessed. Both dyskinesia and PLBs were rated from 0 to 4. For dyskinesia, 0 = absent, while 4 = severe, continuous, replacing normal activity, present more than 70% of the observation period. In each 5-min period of assessment, choreiform and dystonic dyskinesias were graded separately and the dyskinesia score given reflected the most disabling dyskinesia observed. The following PLBs were assessed: hyperkinesia, response to non-apparent stimuli (hallucinatory-like behaviour), repetitive grooming and stereotypies. Each of these was rated from 0 to 4: 0 = absent; 4 = severe, at times interfering with normal activity, present more than 30% of the observation period. For every 5-min period of assessment, the PLBs score attributed was the most disabling of any of the four sub-scores observed. Several articles assessing PLBs in the MPTP-lesioned marmoset have been published (Fox et al. 2006, 2010; Hamadjida et al. 2017, 2018; Huot et al. 2011, 2012a; Visanji et al. 2006) and the scale used here to rate behaviours was detailed and validated in Fox et al. (2010). Peak dose dyskinesia/PLBs correspond to 60–150 min after treatment administration.
Dyskinesia, PLBs and parkinsonian disability scores were cumulated for each hour across the entire 6 h of observation. Duration of anti-parkinsonian benefit, on-time, was defined as the number of minutes for which bradykinesia score was 0.
Statistical analysis
Categorical, discontinuous scores for parkinsonian disability, dyskinesia and PLBs severity are presented as the median with individual values and were analysed using non-parametric Friedman followed by Dunn’s post hoc tests. On-time data are presented as the mean ± standard error (SEM) and were analysed by one-way repeated measures analysis of variance (RM ANOVA) followed by Tukey’s post hoc tests. Statistical significance was assigned when P < 0.05. Statistical analyses were computed using GraphPad Prism 6.0 h (GraphPad Software Inc, La Jolla, USA).
Results
Trazodone appeared to be poorly tolerated by animals, especially at 10 mg/kg, where it seemed to have a sedative effect on marmosets. Female and male animals showed similar reactions to the effects of trazodone.
Trazodone reduces the severity of l-DOPA-induced dyskinesia
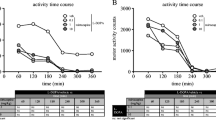
As illustrated in Fig. 1a, adding trazodone to l-DOPA resulted in a significant reduction of dyskinesia severity in the first 2 h following treatment administration [Friedman statistic (FS) = 17.00, P < 0.001]. Thus, when trazodone 10 mg/kg was added to l-DOPA, dyskinesia was reduced by ≈ 48% (P < 0.001, Dunn’s post hoc test), when compared to l-DOPA/vehicle. The addition of trazodone 0.1 or 1 mg/kg to l-DOPA did not diminish dyskinesia severity, when compared to l-DOPA/vehicle.
a Dyskinesia severity in the first 2 h following administration of l-DOPA in combination with trazodone (0.1, 1 and 10 mg/kg) or vehicle. b Peak dose dyskinesia severity in MPTP-lesioned marmosets treated with l-DOPA in combination with trazodone (0.1, 1 and 10 mg/kg) or vehicle. Data are presented as the median with individual values. **P < 0.01; ***P < 0.001
As shown in Fig. 1b, trazodone significantly decreased the severity of peak dose dyskinesia (FS = 12.56, P < 0.01). Thus, when trazodone 10 mg/kg was added to l-DOPA, peak dose dyskinesia was reduced by ≈ 39% (P < 0.01, Dunn’s post hoc test), when compared to l-DOPA/vehicle. The addition of trazodone 0.1 or 1 mg/kg to l-DOPA did not diminish peak dose dyskinesia severity, when compared to l-DOPA alone.
Trazodone reduces the severity of l-DOPA-induced psychosis-like behaviours
As illustrated in Fig. 2a, adding trazodone to l-DOPA resulted in a significant reduction of PLBs severity in the first 2 h following treatment administration (FS = 14.53, P < 0.001). Thus, when trazodone 10 mg/kg was added to l-DOPA, PLBs were reduced by ≈ 30% (P < 0.01, Dunn’s post hoc test), when compared to l-DOPA/vehicle. The addition of trazodone 0.1 or 1 mg/kg to l-DOPA did not reduce PLBs, when compared to l-DOPA/vehicle.
a PLBs severity in the first 2 h following administration of l-DOPA in combination with trazodone (0.1, 1 and 10 mg/kg) or vehicle. b Peak dose PLBs severity in MPTP-lesioned marmosets treated with l-DOPA in combination with trazodone (0.1, 1 and 10 mg/kg) or vehicle. Data are presented as the median with individual values. *P < 0.05; **P < 0.01
As shown in Fig. 2b, trazodone significantly decreased the severity of peak dose PLBs (FS = 8.38, P < 0.05). Thus, when trazodone 10 mg/kg was added to l-DOPA, peak dose PLBs were reduced by ≈ 17% (P < 0.05, Dunn’s post hoc test), when compared to l-DOPA/vehicle. The addition of trazodone 0.1 or 1 mg/kg to l-DOPA had no effect on peak dose PLBs, when compared to l-DOPA/vehicle.
Trazodone hinders l-DOPA anti-parkinsonian action
As illustrated in Fig. 3a, adding trazodone to l-DOPA resulted in a significant increase of parkinsonian disability in the first 2 h after treatment administration (FS = 11.58, P < 0.01). Thus, when trazodone 10 mg/kg was added to l-DOPA, parkinsonism severity was increased by ≈ fivefold (P < 0.05, Dunn’s post hoc test), when compared to l-DOPA/vehicle, while it was increased by ≈ fourfold (P < 0.05, Dunn’s post hoc test), when l-DOPA/trazodone 10 mg/kg was compared to l-DOPA/trazodone 0.1 mg/kg. The addition of trazodone 0.1 or 1 mg/kg to l-DOPA did not worsen parkinsonian disability, when compared to l-DOPA/vehicle.
a Parkinsonism severity in the first 2 h following administration of l-DOPA in combination with trazodone (0.1, 1 and 10 mg/kg) or vehicle. b On-time, in MPTP-lesioned marmosets treated with l-DOPA in combination with trazodone (0.1, 1 and 10 mg/kg) or vehicle. In a data are presented as the median with the individual values, while in b they are displayed as the mean with standard error. *P < 0.05
As shown in Fig. 3b, trazodone significantly decreased the duration of on-time (F (3,15) = 4.09, P < 0.05, one-way RM ANOVA). Thus, after administration of l-DOPA/vehicle, duration of on-time was ≈ 195 min, while it was ≈ 170 min after l-DOPA/trazodone 0.1 mg/kg (P > 0.05, Tukey’s post hoc test), ≈ 188 min after l-DOPA/trazodone 1 mg/kg (P > 0.05, Tukey’s post hoc test), and ≈ 155 min after l-DOPA/trazodone 10 mg/kg (≈ 21% reduction, P < 0.05, Tukey’s post hoc test).
Discussion
In this study, we have demonstrated that trazodone effectively reduces both dyskinesia and PLBs, in the MPTP-lesioned marmoset. However, at effective dose, trazodone appeared to sedate the animals and to hamper l-DOPA anti-parkinsonian action, which may limit its use in the PD population. The extent to which this sedative effect of trazodone contributed to the reduction of dyskinesia and PLBs encountered here remains to be determined.
To the best of our knowledge, this is the first study that assesses the potential effect of trazodone on both l-DOPA-induced dyskinesia and dopaminergic psychosis, although a case-report had previously suggested that trazodone could have a beneficial effect on dyskinesia (El-Awar et al. 1987). Case-series, open-label and uncontrolled studies suggested that trazodone might alleviate parkinsonian disability, mostly tremor, in PD subjects, but many patients experienced sedation in these trials (Cerone et al. 1977a, b; Mastrosimone et al. 1980; Piccinin et al. 1981; Ruggieri et al. 1977; Werneck et al. 2009). In contrast, trazodone did not alleviate parkinsonian tremor in a double-blind trial (Sanson et al. 1986), somewhat shedding doubt on its potential effect against parkinsonian tremor.
Because trazodone is a non-selective drug, as mentioned in the “Introduction”, its anti-dyskinetic and anti-psychotic actions likely stem from an interaction with several targets, notably 5-HT1A and 5-HT2A receptors, as well as α-adrenoceptors. Its affinity for 5-HT1A receptors could explain its deleterious effect on l-DOPA anti-parkinsonian action, as a reduction of l-DOPA therapeutic benefit has often been encountered when 5-HT1A receptors are activated, in both primate studies (Iravani et al. 2006) and clinical trials (Goetz et al. 2007). Trazodone also exhibits high affinity for histamine 1 receptors (Owens et al. 1997), which could underlie the sedative effect encountered here (Yanai et al. 2017). Trazodone has little affinity for dopamine receptors (Richelson and Nelson 1984); for that reason, dopamine receptor blockade is unlikely to be a major contributor to the reduction of anti-parkinsonian action encountered here.
In summary, trazodone was effective in alleviating both l-DOPA-induced dyskinesia and PLBs in our study. However, it was impossible to separate these therapeutic effects of trazodone from sedation and a reduction of the benefit conferred by l-DOPA on parkinsonian disability. This suggests that, at beneficial dose, trazodone may be poorly tolerated by PD subjects, which may limit its potential usefulness in PD.
References
Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA (1993) Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med 329:162–167
Ashby CR Jr, Wang RY (1996) Pharmacological actions of the atypical antipsychotic drug clozapine: a review. Synapse 24:349–394
Cerone G, Ruggieri S, Agnoli A (1977a) Parkinsonian tremor: a neuropharmacological study. Acta Neurol Belg 77:213–229
Cerone G, Ruggieri S, Aloisi P, Cappenberg L, Agnoli A (1977b) Pharmacological study of parkinsonian tremor. III. Role of the serotonin-histamine system. Boll Soc Ital Biol Sper 53:354–360
Crissman AM, O’Donnell JM (2002) Effects of antidepressants in rats trained to discriminate centrally administered isoproterenol. J Pharmacol Exp Ther 302:606–611
Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, Dhall R, Ballard C (2014) Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 383:533–540
Durif F, Debilly B, Galitzky M, Morand D, Viallet F, Borg M, Thobois S, Broussolle E, Rascol O (2004) Clozapine improves dyskinesias in Parkinson disease: a double-blind, placebo-controlled study. Neurology 62:381–388
El-Awar M, Freedman M, Seeman P, Goldenberg L, Little J, Solomon P (1987) Response of tardive and l-DOPA-induced dyskinesias to antidepressants. Can J Neurol Sci 14:629–631
Fox SH, Visanji NP, Johnston TH, Gomez-Ramirez J, Voon V, Brotchie JM (2006) Dopamine receptor agonists and levodopa and inducing psychosis-like behavior in the MPTP primate model of Parkinson disease. Arch Neurol 63:1343–1344
Fox SH, Visanji N, Reyes G, Huot P, Gomez-Ramirez J, Johnston T, Brotchie JM (2010) Neuropsychiatric behaviors in the MPTP marmoset model of Parkinson’s disease. Can J Neurol Sci 37:86–95
French Clozapine Parkinson Study Group (1999) Clozapine in drug-induced psychosis in Parkinson’s disease. The French Clozapine Parkinson Study Group. Lancet 353:2041–2042
Goetz CG, Damier P, Hicking C, Laska E, Muller T, Olanow CW, Rascol O, Russ H (2007) Sarizotan as a treatment for dyskinesias in Parkinson’s disease: a double-blind placebo-controlled trial. Mov Disord 22:179–186
Hamadjida A, Nuara SG, Veyres N, Frouni I, Kwan C, Sid-Otmane L, Harraka MJ, Gourdon JC, Huot P (2017) The effect of mirtazapine on dopaminergic psychosis and dyskinesia in the parkinsonian marmoset. Psychopharmacology 234:905–911
Hamadjida A, Nuara SG, Gourdon JC, Huot P (2018) The effect of mianserin on the severity of psychosis and dyskinesia in the parkinsonian marmoset. Prog Neuropsychopharmacol Biol Psychiatry 81:367–371
Hely MA, Morris JG, Reid WG, Trafficante R (2005) Sydney Multicenter Study of Parkinson’s disease: non-l-DOPA-responsive problems dominate at 15 years. Mov Disord 20:190–199
Huot P, Johnston TH, Lewis KD, Koprich JB, Reyes MG, Fox SH, Piggott MJ, Brotchie JM (2011) Characterization of 3,4-methylenedioxymethamphetamine (MDMA) enantiomers in vitro and in the MPTP-lesioned primate: R-MDMA reduces severity of dyskinesia, whereas S-MDMA extends duration of on-time. J Neurosci 31:7190–7198
Huot P, Johnston TH, Gandy MN, Reyes MG, Fox SH, Piggott MJ, Brotchie JM (2012a) The monoamine re-uptake inhibitor UWA-101 improves motor fluctuations in the MPTP-lesioned common marmoset. PLoS One 7:e45587
Huot P, Johnston TH, Koprich JB, Aman A, Fox SH, Brotchie JM (2012b) l-745,870 reduces l-DOPA-induced dyskinesia in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J Pharmacol Exp Ther 342:576–585
Huot P, Johnston TH, Lewis KD, Koprich JB, Reyes MG, Fox SH, Piggott MJ, Brotchie JM (2014) UWA-121, a mixed dopamine and serotonin re-uptake inhibitor, enhances l-DOPA anti-parkinsonian action without worsening dyskinesia or psychosis-like behaviours in the MPTP-lesioned common marmoset. Neuropharmacology 82:76–87
Iravani MM, Tayarani-Binazir K, Chu WB, Jackson MJ, Jenner P (2006) In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates, the selective 5-hydroxytryptamine 1a agonist (R)-(+)-8-OHDPAT inhibits levodopa-induced dyskinesia but only with increased motor disability. J Pharmacol Exp Ther 319:1225–1234
Johnston TH, Millar Z, Huot P, Wagg K, Thiele S, Salomonczyk D, Yong-Kee CJ, Gandy MN, McIldowie M, Lewis KD, Gomez-Ramirez J, Lee J, Fox SH, Martin-Iverson M, Nash JE, Piggott MJ, Brotchie JM (2012) A novel MDMA analogue, UWA-101, that lacks psychoactivity and cytotoxicity, enhances l-DOPA benefit in parkinsonian primates. FASEB J 26:2154–2163
Johnston TH, Huot P, Damude S, Fox SH, Jones SW, Rusche JR, Brotchie JM (2013) RGFP109, a histone deacetylase inhibitor attenuates l-DOPA-induced dyskinesia in the MPTP-lesioned marmoset: a proof-of-concept study. Parkinsonism Relat Disord 19:260–264
Lewitt PA, Hauser RA, Lu M, Nicholas AP, Weiner W, Coppard N, Leinonen M, Savola JM (2012) Randomized clinical trial of fipamezole for dyskinesia in Parkinson disease (FJORD study). Neurology 79:163–169
Mason P, Skinner J, Luttinger D (1987) Two tests in rats for antianxiety effect of clinically anxiety attenuating antidepressants. Psychopharmacology 92:30–34
Mastrosimone F, Colucci d’Amato C, De Angelis G, Iaccarino C, Giordano L, Marmo E (1980) Personal experience with a combination of drugs in subjects with dopa resistant Parkinson’s disease. J Med 11:377–383
Owens MJ, Morgan WN, Plott SJ, Nemeroff CB (1997) Neurotransmitter receptor and transporter binding profile of antidepressants and their metabolites. J Pharmacol Exp Ther 283:1305–1322
Parkinson Study Group (1999) Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. The Parkinson Study Group. N Engl J Med 340:757–763
Piccinin GL, Piccirilli M, Agostini L (1981) Treatment of parkinson disease with the combination of l-DOPA (plus carbidopa) and trazodone. Clin Ter 96:621–626
Richelson E, Nelson A (1984) Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro. J Pharmacol Exp Ther 230:94–102
Rudissaar R, Pruus K, Vaarmann A, Pannel P, Skrebuhhova-Malmros T, Allikmets L, Matto V (2001) Acute trazodone and quipazine treatment attenuates apomorphine-induced aggressive behaviour in male rats without major impact on emotional behaviour or monoamine content post mortem. Pharmacol Res 43:349–358
Ruggieri S, Cerone G, Aloisi P, Cappenberg L, Agnoli A (1977) Pharmacological study of parkinsonian tremor. I. Methods. Boll Soc Ital Biol Sper 53:344–348
Sanson F, Schergna E, Semenzato D, Trevisan CP, Bizzarini M, Violante F, Santagostino I, Ravenna C, Maccarone G (1986) Therapeutic effects of trazodone in the treatment of tremor. Multicentric double-blind study. Riv Neurol 56:358–364
Svenningsson P, Rosenblad C, Af Edholm Arvidsson K, Wictorin K, Keywood C, Shankar B, Lowe DA, Bjorklund A, Widner H (2015) Eltoprazine counteracts l-DOPA-induced dyskinesias in Parkinson’s disease: a dose-finding study. Brain 138:963–973
Vanover KE, Betz AJ, Weber SM, Bibbiani F, Kielaite A, Weiner DM, Davis RE, Chase TN, Salamone JD (2008) A 5-HT2A receptor inverse agonist, ACP-103, reduces tremor in a rat model and levodopa-induced dyskinesias in a monkey model. Pharmacol Biochem Behav 90:540–544
Visanji NP, Gomez-Ramirez J, Johnston TH, Pires D, Voon V, Brotchie JM, Fox SH (2006) Pharmacological characterization of psychosis-like behavior in the MPTP-lesioned nonhuman primate model of Parkinson’s disease. Mov Disord 21:1879–1891
Werneck AL, Rosso AL, Vincent MB (2009) The use of an antagonist 5-HT2a/c for depression and motor function in Parkinson’ disease. Arq Neuropsiquiatr 67:407–412
Yanai K, Yoshikawa T, Yanai A, Nakamura T, Iida T, Leurs R, Tashiro M (2017) The clinical pharmacology of non-sedating antihistamines. Pharmacol Ther 178:148–156
Acknowledgements
PH has research support from Parkinson Canada, Fonds de Recherche Québec—Santé, the Weston Brain Institute, the Natural Sciences and Engineering Research Council of Canada and the Montreal Neurological Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest.
Rights and permissions
About this article
Cite this article
Hamadjida, A., Nuara, S.G., Gourdon, J.C. et al. Trazodone alleviates both dyskinesia and psychosis in the parkinsonian marmoset model of Parkinson’s disease. J Neural Transm 125, 1355–1360 (2018). https://doi.org/10.1007/s00702-017-1830-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-017-1830-8