Abstract
Virtually every patient affected by Parkinson’s disease (PD) eventually requires treatment with l-3,4-dihydroxyphenylalanine (l-DOPA), which leads to complications such as dyskinesia and psychosis. Whereas blockade of serotonin 2A (5-HT2A) receptors appears to be an effective way to reduce both dyskinesia and psychosis, whether it has the potential to eliminate the two phenomena remains to be determined. In a previous study, we showed that highly selective 5-HT2A receptor blockade with EMD-281,014, at plasma levels comparable to those achieved in the clinic, reduced dyskinesia and psychosis-like behaviours (PLBs), in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned marmoset. Here, we sought to determine whether further increasing the dose would result in greater therapeutic benefit or if maximal effectiveness was achieved at lower doses. Six MPTP-lesioned marmosets with stable dyskinesia and PLBs were administered EMD-281,014 (0.1, 1 and 10 mg/kg) or vehicle in combination with l-DOPA and the effect on dyskinesia, PLBs and parkinsonism was assessed. Administration of EMD-281,014 (0.1, 1 and 10 mg/kg) in combination with l-DOPA resulted in a significant reduction in the severity of dyskinesia, by up to 63%, 64% and 61% (each P < 0.001), when compared to l-DOPA/vehicle. Similarly, the addition of EMD-281,014 (0.1, 1 and 10 mg/kg) to l-DOPA also significantly decreased the severity of PLBs, by up to 54%, 55% and 53% (each P < 0.001), when compared to l-DOPA/vehicle. Our results suggest that there might be a ceiling to the reduction of dyskinesia and psychosis that can be achieved through antagonism of 5-HT2A receptors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The dopamine precursor l-3,4-dihydroxyphenylalanine (l-DOPA) remains the most effective treatment for relief of motor symptoms of Parkinson’s disease (PD) (Fox et al. 2011; Connolly and Lang 2014). However, with long-term administration of l-DOPA, patients experience complications such as dyskinesia and psychosis (Hely et al. 1999, 2005). Given that treatments for these complications are few and their efficacy is partial, there is a major unmet need to develop therapies for patients with PD that are afflicted with these conditions.
There is post-mortem evidence of altered serotonin (5-HT) 2A (5-HT2A) receptor levels in l-DOPA-induced dyskinesia (Riahi et al. 2011; Huot et al. 2012) and visual hallucinations (Huot et al. 2010) in PD. Pharmacologically, 5-HT2A receptor blockade (Hamadjida et al. 2018a) or partial agonism (Huot et al. 2011) reduced each of dyskinesia and psychosis-like behaviours (PLBs) in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-lesioned marmoset, while in the clinic, the 5-HT2A inverse agonist pimavanserin reduced psychosis (Cummings et al. 2014). Other molecules such as clozapine and ritanserin, both of which harbour affinity for 5-HT2A receptors, also alleviated dyskinesia (Maertens de Noordhout and Delwaide 1986; Meco et al. 1988; Durif et al. 2004) and psychosis (French Clozapine Parkinson Study Group 1999; Parkinson Study Group 1999) in the clinic, but their lack of selectivity makes it difficult to determine the extent to which 5-HT2A receptor antagonism contributed to their benefit.
EMD-281,014 (also known as pruvanserin or LY-2,422,347) is a highly selective 5-HT2A neutral antagonist, with approximately 2000-fold selectivity (Bartoszyk et al. 2003). EMD-281,014 entered clinical testing and target plasma levels and in vivo brain receptor occupancy have been determined (Mamo et al. 2004). Recently, we have shown that doses of EMD-281,014 leading to plasma levels in the range of those that were achieved in the clinic were effective at reducing both dyskinesia and PLBs in the parkinsonian marmoset (Hamadjida et al. 2018a). In this study, EMD-281,014 (0.03 and 0.1 mg/kg) reduced dyskinesia by ≈ 54%, while PLBs were diminished by ≈ 45%. Although these were important reductions, whether higher doses of EMD-281,014 could further alleviate dyskinesia and PLBs has not been demonstrated. Whereas such doses would not be clinically relevant, they may nevertheless be informative as to whether there is a ceiling to the potential anti-dyskinetic and anti-psychotic benefit conferred by 5-HT2A receptor blockade. Here, we sought to find an answer to this question, by administering high doses of EMD-281,014 in combination with l-DOPA to the MPTP-lesioned marmoset, a non-human primate model of PD with high predictive value and low false positive rate of the translational success of drugs (Veyres et al. 2018).
Materials and methods
Animals
Six common marmosets (Callithrix jacchus; WorldWide Primates, USA and McGill University breeding colony; 3 males and 3 females), weighing 300–450 g, were housed in groups of 2–3 under controlled conditions of temperature (23 ± 1 °C), humidity (50%) and light (12 h light/dark cycle, lights on at 07:15). Animals were cared for in accordance with a protocol approved by McGill University and the Montreal Neurological Institute Animal Care Committees, in accordance with the regulations defined by the Canadian Council on Animal Care. Food, fresh fruits and water were available ad libitum and home cages were enriched with primate toys and perches. Prior to the beginning of studies, animals were acclimatised to handling, administration of sub-cutaneous (s.c.) treatments, and transfer to observation cages for behavioural assessment.
Induction of parkinsonism, dyskinesia and PLBs in the marmoset
Animals were rendered parkinsonian by daily injection of MPTP hydrochloride (MilliporeSigma, Canada) dissolved in 0.9% sterile saline (2 mg/kg s.c.) for up to 5 days. Following a 6-week recovery period, a stable parkinsonian phenotype was observed. Dyskinesia and PLBs were then induced by chronic administration of l-DOPA/benserazide (henceforth termed l-DOPA, 15/3.75 mg/kg, MilliporeSigma, Canada) per os, once daily for at least 1 month. This treatment regimen has been shown to produce a stable phenotype of dyskinesia and PLBs (Huot et al. 2011; Hamadjida et al. 2017, 2018c).
Administration of EMD-281,014 with l-DOPA to MPTP-lesioned marmosets
On experimental days, at 08:00, marmosets received therapeutic doses of l-DOPA, 15/3.75 mg/kg s.c. (MilliporeSigma, Canada), in combination with either vehicle (0.9% NaCl) or EMD-281,014 (0.1, 1 or 10 mg/kg s.c.; Cedarlane Laboratories, Canada). Drugs were administered according to a randomised within-subject design, in which all animals received all treatments, in a random order. After administration of a given treatment, each marmoset was placed individually into an observation cage (36 × 33 × 22 in.) containing food, water and a wooden perch, and left undisturbed for 6 h. At least 72 h were left between each treatment in any animal and behaviour was recorded via webcam and analysed post hoc by a movement disorder neurologist blinded to the treatment given.
Behavioural analysis
Behavioural analysis was performed according to scales previously described (Fox et al. 2010; Huot et al. 2014; Hamadjida et al. 2017, 2018b, 2018c).
Parkinsonian disability
Parkinsonian disability was scored for 5 min every 10 min using a scale that combined measures of range of movement, bradykinesia, posture, and attention/alertness. Range of movement was rated on a 0–9 scale: 0 = running, jumping between roof, walls, perch, using limbs through a wide range of activity; 1: climbing up and down the cage walls or along perch; 2: climbing onto cage wall or perch; 3: hopping on floor of cage; 4: walking around floor; 5: on cage wall or perch, movement of limbs, but no locomotion; 6: on cage wall or perch, movement of head or trunk; 7: on the floor of the cage, movement of limb, but no locomotion; 8: on the floor of the cage, movement of head; 9 = no movement. Bradykinesia was rated from 0 to 3: 0 = normal initiation and speed of movement; 1: slight slowing of movement; 2: moderate slowing of movement, marked freezing, difficulty initiating and maintaining movement; 3 = prolonged freezing, akinesia, inability to move. Postural abnormalities were rated 0 or 1: 0 = normal balance, upright posture, head held up; 1 = impaired balance, crouched posture, head down. Attention/alertness was rated 0 or 1; 0 = normal head checking movements, movement of neck in variable directions, smooth, small movements; 1 = reduced or absent head checking, head in one position for more than 50% of observation period. The score attributed to each of the behaviours assessed was the most representative of the 5 min observation period. The higher the score, the more severe the disability. The global parkinsonian disability was calculated as a combination of the above behaviours according to the following formula: (range of movement × 1) + (bradykinesia × 3) + (posture × 9) + (attention/alertness × 9). With this method, the maximal parkinsonian disability score per 5 min observation period was 36.
l-DOPA induced dyskinesia and PLBs
Dyskinesia and PLBs were simultaneously assessed and scored using a scale from 0 to 4. For dyskinesia, choreiform and dystonic dyskinesia were assessed separately for 5 min every 10 min as follow: 0 = absent; 1 = mild, present less than 30% of the observation period; 2 = moderate, not interfering with normal activity, present more than 30% of the observation period; 3 = marked, at times interfering with normal activity, present less than 70% of the observation period; 4 = severe, continuous, replacing normal activity, present more than 70% of the observation period. The most disabling dyskinesia score, either chorea or dystonia, reflected the severity of the dyskinesia disability for each observation period.
For PLBs, hyperkinesia, response to non-apparent stimuli (hallucinatory-like behaviour), repetitive grooming and stereotypies were assessed in 5 min observation periods every 10 min for 6 h. Each of these was rated according to a validated scale (Fox et al. 2010), where 0 = absent; 1 = mild, present less than 30% of the observation period; 2 = moderate, not interfering with normal activity, present more than 30% of the observation period; 3 = marked, at times interfering with normal activity, present less than 30% of the observation period; 4 = severe, at times interfering with normal activity, present more than 30% of the observation period. The PLBs score attributed reflected the most disabling of any of the four sub-scores during the observation period.
The scores for parkinsonism, dyskinesia and PLBs were cumulated for each hour across the entire 6 h observation period and during the peak-effect period (60–150 min following l-DOPA administration). Duration of anti-parkinsonian action, referred to as on-time, was defined as the number of minutes for which bradykinesia was absent (score 0).
Data analysis
Time course data for parkinsonian disability, dyskinesia and PLBs scores were sorted by ranking in ascending order (Howell 2011) and analysed by two-way repeated measures analysis of variance (RM ANOVA) followed by Tukey’s post hoc test. On-time parameters are presented as the mean ± standard error of the mean (SEM) and were analysed by one-way RM ANOVA followed by Tukey’s post hoc test. Statistical significance was assigned when P < 0.05. Statistical analyses were computed using GraphPad Prism 7.03 (GraphPad Software Inc, La Jolla, USA).
Results
EMD-281,014 attenuates the severity of l-DOPA-induced dyskinesia
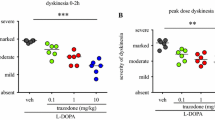
As illustrated in Fig. 1a, a significant effect of treatment and interaction between treatment and time on the severity of dyskinesia were observed [Ftime(5, 120) = 0, P > 0.05; Ftreatment(3, 120) = 24.92, P < 0.001; and Finteraction(15, 120) = 4.41, P < 0.001; two-way RM ANOVA following ranking of data]. Thus, EMD-281,014 (0.1, 1 and 10 mg/kg) significantly reduced the severity of dyskinesia when administered in combination with l-DOPA, especially during the first 3 h. During the first hour, EMD-281,014 (0.1, 1 and 10 mg/kg) combined with l-DOPA reduced the severity of dyskinesia by ≈ 41% (P < 0.001), ≈ 44% (P < 0.001) and ≈ 31% (P < 0.05), respectively, when compared to l-DOPA/vehicle (Tukey’s post hoc test). By the second hour, EMD-281,014 (0.1, 1 and 10 mg/kg), when added to l-DOPA, diminished the severity of dyskinesia by ≈ 36%, ≈ 34% and ≈ 33%, when compared to l-DOPA/vehicle (each P < 0.001, Tukey’s post hoc test). At the third hour, dyskinesia was significantly decreased, by ≈ 63%, ≈ 64% and ≈ 61%, respectively, when l-DOPA/EMD-281,014 (0.1, 1 and 10 mg/kg) was compared to l-DOPA/vehicle (each P < 0.001, Tukey’s post hoc test).
Effect of EMD-281,014 on dyskinesia in the MPTP-lesioned marmoset. a Time course of l-DOPA-induced dyskinesia in marmosets administered EMD-281,014 (0.1, 1 and 10 mg/kg) or vehicle in combination with l-DOPA. The maximal possible dyskinesia score (most severe disability) was 24. On the Y-axis, mild = 6, moderate = 12, marked = 18 and severe = 24. b On-time with disabling dyskinesia in MPTP-lesioned marmosets treated with l-DOPA in combination with EMD-281,014 (0.1, 1 and 10 mg/kg) or vehicle. Data are expressed as the median (a) and the mean ± SEM (b). *P < 0.05; ***P < 0.001
Duration of on-time with disabling dyskinesia significantly decreased when EMD-281,014 was administered in combination with l-DOPA, as shown in Fig. 1b [Ftreatment(3, 15) = 25.15, P < 0.001; one-way RM ANOVA]. Thus, after administration of l-DOPA/vehicle, duration of on-time with disabling dyskinesia was ≈ 123 ± 9 min, whereas it was ≈ 30 ± 6 min following administration of l-DOPA/EMD-281,014 0.1 mg/kg, (76% reduction, P < 0.001, Tukey’s post hoc test), while it was ≈ 40 ± 13 min and ≈ 40 ± 14 min after administration of l-DOPA/EMD-281,014 1 and 10 mg/kg, respectively (both 68% reduction, both P < 0.001, Tukey’s post hoc test).
EMD-281,014 diminishes l-DOPA-induced PLBs
As shown in Fig. 2a, the addition of EMD-281,014 to l-DOPA resulted in a significant reduction of the severity of PLBs [Ftime(5, 120) = 0, P > 0.05; Ftreatment(3, 120) = 21.6, P < 0.001; and Finteraction(15, 120) = 3.919, P < 0.001; two-way RM ANOVA following ranking of data]. Thus, during the first hour, the addition of EMD-281,014 (0.1, 1 and 10 mg/kg) led to a significant decrease in the severity of PLBs, by ≈ 31% (P < 0.001), ≈ 38% (P < 0.001) and ≈ 27% (P < 0.01), respectively, when compared to l-DOPA/vehicle (Tukey’s post hoc test). Subsequently, at the second hour, the severity of PLBs was reduced by ≈ 27%, ≈ 33% and ≈ 23%, when compared to l-DOPA/vehicle (each P < 0.001, Tukey’s post hoc test). By the third hour, EMD-281,014 (0.1, 1 and 10 mg/kg) diminished PLBs by ≈ 54%, ≈ 55% and ≈ 53% when compared to l-DOPA/vehicle (each P < 0.001, Tukey’s post hoc test).
Effect of EMD-281,014 on PLBs in the MPTP-lesioned marmoset. a Time course of l-DOPA-induced PLBs in marmosets administered EMD-281,014 (0.1, 1 and 10 mg/kg) or vehicle in combination with l-DOPA. The maximal possible PLBs score (most severe disability) was 24. On the Y-axis, mild = 6, moderate = 12, marked = 18 and severe = 24. b Duration of on-time with disabling PLBs in MPTP-lesioned marmosets administered l-DOPA in combination with EMD-281,014 (0.1, 1 and 10 mg/kg) or vehicle. Data are expressed as the median (a) and the mean ± SEM (b). **P < 0.01; ***P < 0.001
As shown in Fig. 2b, administration of l-DOPA/EMD-281,014 resulted in a significant reduction of duration of on-time with disabling PLBs [Ftreatment(3, 15) = 20.33, P < 0.001; one-way RM ANOVA]. Following administration of l-DOPA/vehicle, duration of on-time with disabling PLBs was ≈ 152 ± 7 min, whereas treatment with l-DOPA/EMD-281,014 0.1 mg/kg shortened the duration to 70 ± 10 min (54% of reduction, P < 0.001, Tukey’s post hoc test), while it diminished to 53 ± 12 min and 72 ± 17 min, respectively, following treatment with l-DOPA/EMD-281,014 1 and 10 mg/kg (65% and 53% of reduction, both P < 0.001, Tukey’s post hoc test).
High dose EMD-281,014 may alter l-DOPA anti-parkinsonian action
As illustrated in Fig. 3a, the addition of EMD-281,014 to l-DOPA mildly but significantly increased parkinsonian disability [Ftime(5, 120) = 0, P > 0.05; Ftreatment(3, 120) = 3.817, P < 0.05; and Finteraction(15, 120) = 1.368, P > 0.05; two-way RM ANOVA following ranking of data]. Thus, 5 h after treatment administration, the severity of parkinsonism was significantly higher with EMD-281,014 10 mg/kg (by 7%, P < 0.05, Tukey’s post hoc test), whereas neither EMD-281,014 0.1 nor 1 mg/kg worsened parkinsonian disability (both P > 0.05, Tukey’s post hoc test), when compared to l-DOPA/vehicle. In contrast, duration of on-time was similar across all treatments and averaged approximately 186 min (Fig. 3b).
Effect of EMD-281,014 on l-DOPA anti-parkinsonian action in the MPTP-lesioned marmoset. a Time course of parkinsonian disability in MPTP-lesioned marmosets following administration of l-DOPA in combination with EMD-281,014 (0.1, 1 and 10 mg/kg) or vehicle. The maximal possible parkinsonian score (most severe disability) was 216. On the Y-axis, mild = 54, moderate = 108, marked = 162 and severe = 216. b Duration of on-time in MPTP-lesioned marmosets treated with l-DOPA in combination with EMD-281,014 (0.1, 1 and 10 mg/kg) or vehicle. Data are expressed as the median (a) and the mean ± SEM (b). *P < 0.05
Discussion
In this study, we expanded on previous work performed by our group with EMD-281,014 (Hamadjida et al. 2018a), and demonstrated that higher doses of EMD-281,014 reduced the severity of dyskinesia and duration of on-time with disabling dyskinesia. Moreover, treatment with EMD-281,014, also resulted in a significant decrease in the severity of PLBs and duration of on-time with disabling PLBs in parkinsonian marmosets. Our results also suggest that, at high dose, 5-HT2A receptor blockade might compromise, albeit slightly, l-DOPA therapeutic efficacy.
In that previous study, we determined the pharmacokinetic profile of EMD-281,014 in the marmoset and found that administration of doses of 0.1 mg/kg s.c. led to plasma levels comparable to those that were achieved and reportedly well-tolerated in the clinic, ≈ 11 ng/ml (Mamo et al. 2004). At these levels, ≈ 95% 5-HT2A receptor occupancy was reached in the frontal cortex of human subjects (Mamo et al. 2004), but the 5-HT2A receptor occupancy of other brain areas, notably the basal ganglia, was not mentioned, although there is little reason to believe that it would markedly differ to the neocortex. Nevertheless, because dopamine acts as a partial agonist at 5-HT2A receptors (Bhattacharyya et al. 2006), we hypothesised that it could displace a certain amount of EMD-281,014 from its target, which is why we have administered doses that were, respectively, 10- and 100-fold higher than 0.1 mg/kg s.c. Although we did not determine plasma levels associated with EMD-281,014 1 and 10 mg/kg s.c., it is plausible to assume that they were significantly higher than these attained with 0.1 mg/kg in our first study.
Unlike what we expected, we did not achieve greater anti-dyskinetic or anti-psychotic effects with these higher doses. This suggests that selective 5-HT2A receptor blockade may not reduce l-DOPA-induced dyskinesia and psychosis over a certain limit, around 50% for each. Unlike activation of 5-HT1A receptors (Iravani et al. 2006; Goetz et al. 2007), however, antagonising 5-HT2A receptors does not seem to hinder l-DOPA anti-parkinsonian action at clinically relevant doses, although high dose may slightly reduce the effect of l-DOPA. Our results are in agreement with previous experiments with 5-HT2A antagonists performed in the MPTP-lesioned primate (Table 1), where ceiling effects were apparently reached, without impairing l-DOPA anti-parkinsonian effect (Vanover et al. 2008; Huot et al. 2011). However, caution is advised when interpreting these last two studies, as the drugs under investigation, R-3,4-methylenedioxymethamphetamine (R-MDMA) (Huot et al. 2011) and pimavanserin (Vanover et al. 2006), are not as selective as EMD-281,014 for 5-HT2A receptors. Moreover, R-MDMA is a partial agonist (Nash et al. 1994), while pimavanserin is an inverse agonist (Vanover et al. 2006) at 5-HT2A receptors; how these different actions at 5-HT2A receptors compare to the neutral antagonism of EMD-281,014 (Bartoszyk et al. 2003) in experimental parkinsonism is uncertain.
To the best of our knowledge, EMD-281,014, pimavanserin and R-MDMA are the most selective 5-HT2A “antagonists” that were assessed in the MPTP-lesioned primate. Of these, only pimavanserin was tested in clinical settings for PD-related endpoints. Pimavanserin apparently had a modest anti-dyskinetic effect in an early report (Roberts 2006), but randomised controlled trials on dyskinesia have not been published, and this early report awaits confirmation. In contrast, pimavanserin reduced PD psychosis in a Phase III trial, but here again the effect was relatively modest, ≈ 13% (Cummings et al. 2014). Whereas these modest benefits achieved clinically with pimavanserin are not necessarily indicative of a ceiling effect of the drug or of antagonising 5-HT2A receptors, they nevertheless suggest that the benefit conferred by 5-HT2A blockade for both dyskinesia and psychosis may be limited.
Perhaps more surprisingly, given the benefits encountered in the parkinsonian primate and in the clinic, 5-HT2A blockade does not appear to be an effective anti-dyskinetic approach in the 6-hydroxydopamine (6-OHDA)-lesioned rat (Table 2). Thus, the selective 5-HT2A inverse agonist volinanserin [MDL-100,907, M-100,907] (Vanover et al. 2006) was ineffective at reducing l-DOPA-induced abnormal involuntary movements (AIMs) in the rat (Taylor et al. 2006). In addition, we recently showed that EMD-281,014, at doses leading to clinically relevant plasma levels, was also ineffective at reducing l-DOPA-induced AIMs in the parkinsonian rat (Frouni et al. 2018). Taken together, these two studies in the rodent suggest that 5-HT2A blockade, whether with an inverse agonist or a neutral antagonist, does not stand out as an effective anti-dyskinetic approach in the 6-OHDA-lesioned rat.
In summary, this study provides further support that highly selective 5-HT2A receptor blockade is effective at alleviating both l-DOPA-induced dyskinesia and psychosis in PD. However, our results suggest that the magnitude of the benefits conferred by 5-HT2A blockade may be limited and that, at very high dose, an interference with the anti-parkinsonian effect of l-DOPA may emerge. This study, coupled with the reviewed literature, also suggest that, while antagonising 5-HT2A receptors reduces both dyskinesia and psychosis, it may be impossible to eradicate these two treatment-related complications by relying solely on selective modulation of this target.
References
Bartoszyk GD, van Amsterdam C, Bottcher H, Seyfried CA (2003) EMD 281014, a new selective serotonin 5-HT2A receptor antagonist. Eur J Pharmacol 473:229–230
Bhattacharyya S, Raote I, Bhattacharya A, Miledi R, Panicker MM (2006) Activation, internalization, and recycling of the serotonin 2A receptor by dopamine. Proc Natl Acad Sci USA 103:15248–15253. https://doi.org/10.1073/pnas.0606578103
Connolly BS, Lang AE (2014) Pharmacological treatment of Parkinson disease: a review. JAMA 311:1670–1683
Cummings J, Isaacson S, Mills R et al (2014) Pimavanserin for patients with Parkinson’s disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet 383:533–540. https://doi.org/10.1016/S0140-6736(13)62106-6
Durif F, Debilly B, Galitzky M et al (2004) Clozapine improves dyskinesias in Parkinson disease: a double-blind, placebo-controlled study. Neurology 62:381–388
Fox SH, Visanji N, Reyes G, Huot P, Gomez-Ramirez J, Johnston T, Brotchie JM (2010) Neuropsychiatric behaviors in the MPTP marmoset model of Parkinson’s disease. Can J Neurol Sci 37:86–95
Fox SH, Katzenschlager R, Lim SY et al (2011) The Movement Disorder Society evidence-based medicine review update: treatments for the motor symptoms of Parkinson’s disease. Mov Disord 26(Suppl 3):S2–S41
French Clozapine Parkinson Study Group (1999) Clozapine in drug-induced psychosis in Parkinson’s disease. The French Clozapine Parkinson Study Group. Lancet 353:2041–2042
Frouni I, Kwan C, Bedard D et al (2018) Effect of the selective 5-HT2A receptor antagonist EMD-281,014 on l-DOPA-induced abnormal involuntary movements in the 6-OHDA-lesioned rat. Exp Brain Res. https://doi.org/10.1007/s00221-018-5390-4
Goetz CG, Damier P, Hicking C et al (2007) Sarizotan as a treatment for dyskinesias in Parkinson’s disease: a double-blind placebo-controlled trial. Mov Disord 22:179–186. https://doi.org/10.1002/mds.21226
Hamadjida A, Nuara SG, Veyres N et al (2017) The effect of mirtazapine on dopaminergic psychosis and dyskinesia in the parkinsonian marmoset. Psychopharmacology 234:905–911
Hamadjida A, Nuara SG, Bedard D, Gaudette F, Beaudry F, Gourdon JC, Huot P (2018a) The highly selective 5-HT2A antagonist EMD-281,014 reduces dyskinesia and psychosis in the l-DOPA-treated parkinsonian marmoset. Neuropharmacology 139:61–67. https://doi.org/10.1016/j.neuropharm.2018.06.038
Hamadjida A, Nuara SG, Gourdon JC, Huot P (2018b) The effect of mianserin on the severity of psychosis and dyskinesia in the parkinsonian marmoset. Prog Neuropsychopharmacol Biol Psychiatry 81:367–371
Hamadjida A, Nuara SG, Gourdon JC, Huot P (2018c) Trazodone alleviates both dyskinesia and psychosis in the parkinsonian marmoset model of Parkinson’s disease. J Neural Transm (Vienna)125(9):1355–1360
Hely MA, Morris JG, Traficante R, Reid WG, O’Sullivan DJ, Williamson PM (1999) The sydney multicentre study of Parkinson’s disease: progression and mortality at 10 years. J Neurol Neurosurg Psychiatry 67:300–307
Hely MA, Morris JG, Reid WG, Trafficante R (2005) Sydney Multicenter Study of Parkinson’s disease: non-l-dopa-responsive problems dominate at 15 years. Mov Disord 20:190–199. https://doi.org/10.1002/mds.20324
Howell DC (2011) Fundamental statistics for the behavioral sciences. Wadsworth Cengage Learning, Belmont
Huot P, Johnston TH, Darr T et al (2010) Increased 5-HT2A receptors in the temporal cortex of parkinsonian patients with visual hallucinations. Mov Disord 25:1399–1408
Huot P, Johnston TH, Lewis KD et al (2011) Characterization of 3,4-methylenedioxymethamphetamine (MDMA) enantiomers in vitro and in the MPTP-lesioned primate: R-MDMA reduces severity of dyskinesia, whereas S-MDMA extends duration of ON-time. J Neurosci 31:7190–7198. https://doi.org/10.1523/JNEUROSCI.1171-11.2011
Huot P, Johnston TH, Winkelmolen L, Fox SH, Brotchie JM (2012) 5-HT2A receptor levels increase in MPTP-lesioned macaques treated chronically with l-DOPA. Neurobiol Aging 33:194 e195-115. https://doi.org/10.1016/j.neurobiolaging.2010.04.035
Huot P, Johnston TH, Lewis KD et al (2014) UWA-121, a mixed dopamine and serotonin re-uptake inhibitor, enhances l-DOPA anti-parkinsonian action without worsening dyskinesia or psychosis-like behaviours in the MPTP-lesioned common marmoset. Neuropharmacology 82:76–87. https://doi.org/10.1016/j.neuropharm.2014.01.012
Iravani MM, Tayarani-Binazir K, Chu WB, Jackson MJ, Jenner P (2006) In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates, the selective 5-hydroxytryptamine 1a agonist (R)-(+)-8-OHDPAT inhibits levodopa-induced dyskinesia but only with\ increased motor disability. J Pharmacol Exp Ther 319:1225–1234. https://doi.org/10.1124/jpet.106.110429
Maertens de Noordhout A, Delwaide PJ (1986) Open pilot trial of ritanserin in parkinsonism. Clin Neuropharmacol 9:480–484
Mamo D, Sedman E, Tillner J, Sellers EM, Romach MK, Kapur S (2004) EMD 281014, a specific and potent 5HT2 antagonist in humans: a dose-finding PET study. Psychopharmacology 175:382–388. https://doi.org/10.1007/s00213-004-1817-7
Meco G, Marini S, Linfante I, Modarelli F, Agnoli A (1988) Controlled single-blind crossover study of ritanserin and placebo in l-dopa-induced dyskinesias in Parkinson’s disease. Curr Ther Res 43:262–270
Nash JF, Roth BL, Brodkin JD, Nichols DE, Gudelsky GA (1994) Effect of the R(−) and S(+) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors. Neurosci Lett 177:111–115
Parkinson Study Group (1999) Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. The Parkinson Study Group. N Engl J Med 340:757–763
Riahi G, Morissette M, Parent M, Di Paolo T (2011) Brain 5-HT(2A) receptors in MPTP monkeys and levodopa-induced dyskinesias. Eur J Neurosci 33:1823–1831. https://doi.org/10.1111/j.1460-9568.2011.07675.x
Roberts C (2006) ACP-103, a 5-HT2A receptor inverse agonist. Curr Opin Investig Drugs 7:653–660
Taylor JL, Bishop C, Ullrich T, Rice KC, Walker PD (2006) Serotonin 2A receptor antagonist treatment reduces dopamine D1 receptor-mediated rotational behavior but not l-DOPA-induced abnormal involuntary movements in the unilateral dopamine-depleted rat. Neuropharmacology 50:761–768. https://doi.org/10.1016/j.neuropharm.2005.12.004
Vanover KE, Weiner DM, Makhay M et al (2006) Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropylo xy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther 317:910–918. https://doi.org/10.1124/jpet.105.097006
Vanover KE, Betz AJ, Weber SM et al (2008) A 5-HT2A receptor inverse agonist, ACP-103, reduces tremor in a rat model and levodopa-induced dyskinesias in a monkey model. Pharmacol Biochem Behav 90:540–544. https://doi.org/10.1016/j.pbb.2008.04.010
Veyres N, Hamadjida A, Huot P (2018) Predictive value of parkinsonian primates in pharmacologic studies: a comparison between the macaque, marmoset, and squirrel monkey. J Pharmacol Exp Ther 365:379–397. https://doi.org/10.1124/jpet.117.247171
Acknowledgements
PH has research support from Parkinson Canada, Fonds de Recherche Québec—Santé, the Natural Sciences and Engineering Research Council of Canada, the Michael J Fox Foundation for Parkinson's Research and the Weston Brain Institute.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest. PH has received payments from UCB.
Rights and permissions
About this article
Cite this article
Kwan, C., Frouni, I., Bédard, D. et al. 5-HT2A blockade for dyskinesia and psychosis in Parkinson’s disease: is there a limit to the efficacy of this approach? A study in the MPTP-lesioned marmoset and a literature mini-review. Exp Brain Res 237, 435–442 (2019). https://doi.org/10.1007/s00221-018-5434-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-018-5434-9