Abstract
Background
Paraspinal neurogenic tumors usually expand into the mediastinum and retroperitoneum and can reach a considerable size before they become symptomatic. Such large tumors are rare. We describe 14 cases of large schwannomas (>2.5 cm ø) with mild and late onset of symptoms, which were treated with total surgical resection through a single-approach surgery.
Methods
In 2013 14 patients with paraspinal large schwannomas were treated in our institutions. Data were analyzed retrospectively. Magnetic resonance imaging (MRI) showed lesions suspicious for a paraspinal schwannoma with partial intraforaminal growth. In case of ambiguity regarding tumor dignity, a needle biopsy was performed before final treatment. Three different approaches and their indications were discussed.
Results
Fourteen patients (7 female and 7 male, ages 18–58 years, mean: 39.8 years) requiring surgical exploration because of a thoracic (6) or lumbar/lumbosacral (8) lesion were treated in our institutions. Two patients received CT-guided needle biopsy preoperatively. Complete resection of the schwannoma was possible through a mini-thoracotomy in 1 case (7 %), a retroperitoneal approach in 2 cases (14 %), and dorsal interlaminar and intercostal fenestration in 11 cases (79 %). Histological examination revealed the diagnosis of schwannoma (WHO grade I) in all cases except one with neurofibroma (WHO grade I). There were no major complications in any case.
Conclusion
Large benign schwannomas are rare. They need a tailored treatment, which in most cases works through one surgical approach. Usually it is possible to perform a complete resection with a good postoperative prognosis.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Schwannomas are neurogenic tumors that arise from the Schwann cells of the neural sheath. Benign paraspinal schwannomas usually remain asymptomatic until they reach a considerable size and expand into the mediastinal or retroperitoneal spaces. Diagnosis is often an incidental finding on a routine radiograph or ultrasound or the tumor presents with mild symptoms such as mild pain or intermittent neurological symptoms.
To confirm the diagnosis, CT, MRI and CT-guided needle biopsy are the methods of choice. The surgical procedure should be tailored according to the size, expansion, location and the anatomy of surrounding structures. To treat real dumbbell-type schwannomas, combined intraspinal and extraspinal approaches are often described. However, only 10 % of these thoracic or retroperitoneal masses extend through an intervertebral foramen into the spinal canal [2]. The reported 14 patients also present large paraspinal tumor masses with their origin from the intervertebral foramen, recessus or paraspinal costal nerve but without intraspinal involvement. In those cases the two or even more operations and approaches that are often described can be avoided, and very often the patients can be treated with one surgical approach [13, 24].
The existing classifications for spinal schwannomas do not support the decision-making process for choosing the surgical approach [6, 24]. The aim of this study is to demonstrate that often only one approach is needed as a result of precise tailored surgical planning in order to achieve complete tumor removal, which we illuminate on the basis of 3 cases out of our 14 patients. Additionally, we discuss additive stabilization procedures, which are not needed in most of the cases in our opinion.
Clinical material and methods
Between January 2013 and December 2013, 14 patients with large paraspinal schwannomas were treated in our institutions. Data were analyzed retrospectively.
In all cases, magnetic resonance imaging, with or without an additional CT scan, showed lesions suspicious for a paraspinal schwannoma with partial intraforaminal growth. In case of ambiguity regarding tumor dignity, a needle biopsy was performed before final treatment.
Results and illustrative cases
Fourteen patients (7 female and 7 male, ages 18–58 years, mean: 39.8 years) requiring surgical exploration because of a thoracic (6) or lumbar/lumbosacral (8) lesion were treated in our institutions. All patients except one had a history of 1 to 2 years of mild symptoms such as back pain, radicular pain and intermittent dysesthesias. One patient had a 7-year history of dyspnea and mild chest pain and was found to have a tumor measuring nearly 9.5 cm in diameter (case no. 8). Two patients received CT-guided needle biopsy preoperatively. Patients with lumbar or lumbosacral tumors underwent electrophysiological testing.
Complete resection of the tumor was possible through a mini-thoracotomy in 1 case (7 %), a retroperitoneal approach in 2 cases (14 %) and dorsal interlaminar and intercostal fenestration in 11 cases (79 %). The complete nerve root had to be sacrificed only in one thoracic case. In three of the lumbar cases the sensory root was sacrificed; in all other cases, the tumor just originated from a small part of the parent nerve and could be preserved by microdissection from the nerve and resection at the origin. There were no CSF leaks during the operations due to the fact that the tumors did not originate from the intraspinal area intradurally but from the extraspinal nerve roots. The intraforaminal tumor part could be excized out of the foramen without injuring the spinal dura because this part generally appears to have grown in a retrograde fashion into the foramen. Nevertheless, in three cases the dura was covered with a medical sponge (TachoSil, Takeda, Osaka, Japan). In cases of complete surgical sectioning of the nerve root, ligation had been performed previously. No case with new sensory or other neurological deficits occurred in the presented series.
Histological examination revealed the diagnosis of schwannoma (WHO grade I) in all cases except one with neurofibroma (WHO grade I). There were no major complications except for a mild hypesthesia of the forearm of unknown origin in one patient and prolonged treatment with a chest tube postoperatively because of mild hematothorax in another.
Patient characteristics are summarized in Table 1.
Case I
A 33-year-old female (case no. 10) presented with intermittent lumbar pain and episodes of hypesthesias in the left lower leg in the supine position for 2 years, which was aggravated during a pregnancy. Further, there was a palpable resistance in the upper abdomen. The physical examination showed a healthy young female without any neurological deficits. Ultrasound revealed a large tumor in the left retroperitoneal space. An MRI scan of the abdomen and lumbar spine showed a mass originating from the left L4 nerve root, eroding the third lumbar vertebra, infiltrating the left iliopsoas muscle and displacing the aorta and vena cava (Fig. 3). The tumor measured 9.0 × 6.9 × 4.0 cm with an intraforaminal component. A needle biopsy confirmed the diagnosis of a benign schwannoma WHO grade I. The patient underwent surgery via a left retroperitoneal approach, and the whole tumor was excized in one sitting by microdissection from the parent nerve. The sensory root had to be sacrificed in this case, but there were no new neurological deficits postoperatively. The part of the tumor infiltrating the vertebral body could easily be curetted out of the erosive canals. The walls of the cavities were cortical and bone-like in terms of the “neocorticalis” with no sign of instability. There was no need for drainage insertion, and the patient could be discharged a week later. Final histopathology confirmed the diagnosis of the biopsy. Follow-up MRI after 1 year showed complete removal of the tumor.
Case II
Another 37-year-old female patient (case no. 9) suffered from progressive pain in the left neck and arm for 1 year without any neurological deficits. An MRI scan showed a paravertebral, sharply defined cystic tumor arising from the second thoracic nerve root in the left upper chest with intraforaminal and extraspinal components, measuring 3.6 × 2.8 × 5.0 cm and compressing the pleura (Fig. 4). The tumor could be removed completely in one sitting by a left intercostal approach with an external foraminotomy. Although we saw no cerebrospinal fluid and the dura was not directly injured, we covered the intraspinal dura with a medical sponge (TachoSil, Takeda, Osaka, Japan). Five days after surgery, the patient could be discharged without any new neurological deficits.
Case III
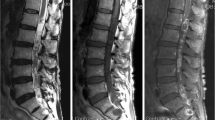
The third case was a 49-year-old female (case no. 8) with the finding of a paravertebral thoracic mass (Th 8–11) diagnosed 7 years prior to surgery measuring 3.5 cm in diameter (Fig. 1a/b). Because of the only moderate symptoms with little local pain and numbness of the chest wall, the patient received regular MRI scans, which revealed continuous tumor growth. When symptoms became progressive and included dyspnea and fatigue, the tumor measured almost 9.5 cm (Fig. 1 e–f). A CT-guided needle biopsy showed a benign schwannoma (WHO grade I). The resection of the tumor arising from an intercostal nerve without intraspinal involvement could be performed in a single-stage mini-thoracotomy from the left side (Fig. 2). Here, the nerve had to be sacrificed and ligated to perform a complete resection. There were no new neurological deficits postoperatively, and the patient could be discharged 7 days after the operation in good condition. Histopathology confirmed WHO grade I schwannoma (Fig. 3).
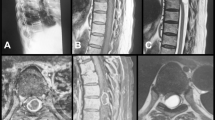
Schematic drawing of the surgical approach to the tumor illustrated in Fig. 1a : View in the direction of the neurinoma (asterisk) (the lung is not illustrated). b Intraoperative photo after opening the pleura and collapsing of the left lung with a good view of the neurinoma (broken lines visualizing the removed rib). c After removing the neurinoma, the pleura was reconstructed (#: left lung). d Completely removed neurinoma
Extensive growth through the expanded neuroforamen widely to the ventral aspect of the vertebral column and erosion of the vertebral body. a–c Displacement of the psoas muscle due to tumor growth. A clearly ventrally located tumor mass, which indicates the ventral retroperitoneal approach. b–c Retroperitoneal approach. d On the way the psoas muscle must be partially transected to reach the tumor mass. Displaced psoas muscle (asterisk). Oblique and transverse muscles (white square), tumor mass 
Two months after the operation there was good improvement of the dyspnea and the patient’s overall strength. Follow-up MRI showed no tumor recurrence.
Discussion
Neurogenic tumors arise from two cell types: nerve sheaths, which include schwannomas and neurofibromas, and autonomic ganglia, which include ganglioneuromas, neuroblastomas, ganglioneuroblastomas and paragangliomas. Schwannomas often occur in the extremities, head and neck [11]. Paraspinal neurogenic tumors are rather uncommon. In adults only 20 % of all mediastinal soft tissue tumors are neurogenic compared to 35 % in children [3], but they account for almost 75 % of posterior mediastinal masses [23]. The tumors most frequently grow in the posterior and posterolateral region of the spinal column, in the thoracic region and laterally and anterolaterally of the spinal column in the retroperitoneum.
At the time of diagnosis, symptoms are based on the size and location, and as the extraspinal component is usually much larger than the intraforaminal or potential intraspinal component, symptoms occur late and patients often suffer from only mild pain, intermittent paresthesias or symptoms related to compression of other structures [7, 10]. Because of these unspecific symptoms, other possible diagnoses are often ruled out first. In case no. 8, increasing dyspnea was diagnosed with reduced pulmonary function in clinical testing. This could be explained retrospectively by pulmonary compression from the growing tumor and pulmonary testing normalized after surgical tumor removal in this patient.
Malignancy in nerve sheath tumors is rare (2–5 %) unless they are related to neurofibromatosis (von Recklinghausen’s disease) or radiation exposure (10–20 %) [16, 17]. These malignant peripheral nerve sheath tumors (MPNSTs) normally have a shorter history with a more sudden onset of symptoms because of their infiltrative nature and rapid growth [19].
In the literature, benign schwannomas mostly vary between 3 and 7 cm in diameter; however, tumors up to 19 cm in the largest dimension have been described [14, 25]. The bigger ones are usually MPNSTs.
For diagnosis and differential diagnoses, in all newer reports an MRI scan is considered the best method. A CT, or even better an MRI scan, or both are essential to evaluate the exact location, shape, extent and border to other tissues. Often it is even possible to find the parent nerve. The use of contrast agents allows for making the distinction between normal and abnormal blood vessels [12, 22].
Schwannomas often present as a solid, sharply defined mass with an ovoid or round shape. They can be homo- or heterogeneous on contrast-enhanced imaging because of differences in cellularity (Antoni type A and Antoni type B with cellular and myxoid components). Large schwannomas can also undergo spontaneous degeneration, e.g., cystic degeneration or calcification. Bony erosion can be seen in benign tumors but is more likely in malignant tumors [5, 8, 9, 23]. Other extraspinal tumors of the mediastinum or retroperitoneum such as sarcoma, lymphoma, pulmonary or renal tumors have to be taken into consideration when planning therapy [15].
In order to classify spinal schwannomas, Eden published a classification in 1941 based on radiological findings [6]. Type I described an intra- and extradural type. Type II is an additional paravertebral mass with intra- and extradural growth. Purely extradural growth with a paravertebral tumor mass characterizes type III, and a tumor limited to the paravertebral and intraforaminal space is type IV.
Sridhar et al. presented a new classification in 2001 including giant and invasive schwannomas. Type I characterizes an intraspinal tumor <2 vertebral segments in length: Ia, intradural; Ib, extradural. Type II is an intraspinal tumor >2 vertebral segments in length (defined as a giant tumor). Type III describes an intraspinal tumor with extension into the nerve root foramen. Type IV is an intraspinal tumor with extraspinal extension (defined as a “dumbbell” tumor); IVa, extraspinal component <2.5 cm; IVb, extraspinal component >2.5 cm (defined as giant tumor). Type V tumors erode into vertebral bodies (giant invasive tumors) with lateral and posterior extensions into the myofascial planes [24].
Park et al. added two additional types in 2009: type VI tumors with an entirely intravertebral location without intraspinal involvement and type VII as an intraspinal tumor with erosion into vertebral bodies (invasive tumor) and extension into the nerve root foramen [18].
The tumors presented in this series correspond best with Eden’s type IV tumors because of the missing intraspinal component in most cases. However, in cases with erosion into the vertebral body, type VII tumors according to the modified Sridhar classification may be the right category even if the authors rather mean a primary growth in the vertebral body with additional extension to the nerve root foramen.
Basically, we are describing an additional type with extraforaminal, sometimes intraforaminal genesis and an extraforaminal large tumor mass (all >2.5 cm) with partial erosive growth into the vertebral body.
Surgical treatment
The preoperative tissue diagnosis in terms of biopsy is controversially discussed. Malignancy or ambiguous tumor dignity is a contraindication to resection in parts, e.g., endoscopically or in two sittings. The aim should be an en bloc resection to minimize seeding and in order to obtain clean surgical margins, although seeding is also a risk in the preoperative biopsy. Some authors consider biopsy unreliable for the diagnosis of large or giant schwannomas because of their secondary degenerative changes. Few cases of malignant transformation of benign schwannoma are described in the literature. Complete surgical resection is the therapy of choice, with an excellent prognosis [1, 21]. When total resection is not achieved, consideration of the Ki-67 index and percentage of p53-positive cells is recommended as an indicator of growth and malignant transformation potential [20, 26].
Nevertheless, regular follow-up is necessary in all cases of schwannoma, especially in patients who have undergone subtotal resection and for patients with a higher Ki-67 index (>2 %) [26].
The approach to the tumor depends primarily on the accessibility and need for potential additional fusion of the spine. Paraspinal schwannomas sometimes have specific characteristics that lead to the possibility of minimally invasive treatment, even in grade V types according to Sridhar. Intraperitoneal and especially intrapleural pressure leads to a dilatation of the pleura or peritoneum during tumor excision, pressing the remaining tumor in the direction of the approach (Fig. 4). Therefore, many paraspinal tumors can be resected through a dorsal transcostal or extraforaminal lumbar approach. In case of intraforaminal tumor involvement, the approach can be extended to a foraminal decompression and tumor excision in the neuroforamen.
A large tumor mass on the thoracic level with intraforaminal involvement. Striped arrows show the dorsal transcostal approach. a-b Empty arrows indicate dilatation of the pleura during the tumor excision, pressing the remaining tumor in the direction of the approach. The broken line shows optional bone removal to reach the whole tumor mass and decompress the neuroforamen
Some large schwannomas, especially in the paraspinal thoracic region without intraforaminal involvement, can be better reached surgically through a ventral approach (Figs. 1 and 2). The advantage is the direct and quick approach and the possibility to remove the tumor in one piece (Fig. 2d). A disadvantage can be chest pain after surgery and the potential transient reduction of pulmonary function test parameters [4].
In case of the tumor crossing the ventral midline of the vertebral body (Fig. 3) without complete erosion of the spinal column on the approach side, a ventral/ventro-retroperitoneal approach is recommended. In these cases the approach-related morbidity using a solely dorsal approach would be disproportionally high.
Tumors according to type V of Sridhar's classification or the later added types VI and VII show erosion into the vertebral body. In fast-growing cancer metastases, this can lead to instability, requiring additional spinal fusion. In schwannomas the slow-growing and therefore slow erosion of the vertebral body induces stabilization of the vertebral body in terms of “neocortical bone,” which is located between the tumor and vertebral spongiosa. Schwannoma tissues can easily be curetted out of the tumor cavities in most cases, and stability is not affected in terms of the remaining half of the vertebral body. Therefore, most schwannoma cases do not require additional spinal fusion.
Conclusion
Large thoracic or lumbar benign schwannomas are rare. Most are asymptomatic or present with only minor symptoms, but they can reach a considerable size. Biopsy may be helpful if dignity is unclear. The aim should be a total resection of the tumor, preferably through a minimally invasive approach in one sitting. Stabilization is rarely required.
References
Ando K, Imagama S, Ito Z, Tauchi R, Muramoto A, Matsui H, Matsumoto T, Ishiguro N (2013) Removal of thoracic dumbbell tumors through a single-stage posterior approach: its usefulness and limitations. J Orthop Sci 18:380–387
Akwari OE, Payne WS, Onofrio BM, Dines DE, Muhm JR (1978) Dumbbell neurogenic tumors of the mediastinum. Diagnosis and management. Mayo Clin Proc 53(6):353–8
Azarow KS, Pearl RH, Zurcher R, Edwards FH, Cohen AJ (1993) Primary mediastinal masses a comparison of adult and pediatric populations. J Thorac Cardiovasc Surg 106:67–72
Bullmann V, Schulte TL, Schmidt C, Gosheger G, Osada N, Liljenqvist UR (2013) Pulmonary function after anterior double thoracotomy approach versus posterior surgery with costectomies in idiopathic thoracic scoliosis. Eur Spine J 22(Suppl 2):164–71
Cohen LM, Schwartz AM, Rockoff SD (1986) Benign schwannomas: pathologic basis for CT inhomogeneities. AJR Am J Roentgenol 147:141–143
Eden K (1941) The dumb-bell tumors of the spine. Br J Surg 28:549–70
Frahm S, Mautner VF, Brems H, Legius E, Debiec-Rychter M, Friedrich RE, Knöfel WT, Peiper M, Kluwe L (2004) Genetic and phenotypic characterization of tumor cells derived from malignant peripheral nerve sheath tumors of neurofibromatosis type 1 patients. Neurobiol Dis 16:85–91
Hu S, Chen Y, Wang Y, Chen KM, Song Q (2012) Clinical and CT manifestation of pleural schwannoma. Acta Radiol 53:1137–1141
Hughes MJ, Thomas JM, Fisher C, Moskovic EC (2005) Imaging features of retroperitoneal and pelvic schwannomas. Clin Radiol 60:886–893
Ishikawa E, Matsumura A, Ishikawa S, Nakamura K, Nose T (2002) Combined minimally invasive approach using microsurgery and thoracoscopic surgery for resecting a dumbbell-type thoracic schwannoma. Minim Invase Neurosurg 45:251–25
Kara M, Ozkan M, Sak SD, Aksu O, Kavukçu S (2002) Giant ancient schwannoma of the posterior mediastinum cytologically misdiagnosed as a malignant tumour. A case report. Acta Chir Belg 102:464–466
Khanlou H, Khanlou N, Eiger G (1998) Schwannoma of posterior mediastinum: a case report and concise review. Heart Lung 27:344–347
Konno S, Yabuki S, Kinoshita T, Kikuchi S (2001) Combined laminectomy and thoracoscopic resection of dumbbell-type thoracic cord tumor. Spine (Phila Pa 1976) 26:E130–4
Kumar S, Rafiq MU, Ahamed I, Ansari J, Cowen ME (2006) Asymptomatic giant thoracic schwannoma. Ann Thorac Surg 82:e26
McCormick PC (1996) Surgical management of dumbbell and paraspinal tumors of the thoracic and lumbar spine. Neurosurgery 38:67–74
Morbidini-Gaffney S, Alpert TE, Hatoum GF, Sagerman RH (2005) Benign pleural schwannoma secondary to radiotherapy for Hodgkin disease. Am J Clin Oncol 28:640–641
Pandiyan MS, Kavunkal AM, Cherian VK, Christopher DJ (2006) Chest wall mass with double pathology. Eur J Cardiothorac Surg 29:625–626
Park SC, Chung SK, Choe G, Kim HJ (2009) Spinal intraosseous schwannoma: a case report and review. J Korean Neurosurg Soc 46:403–8
Patnaik A, Mishra SS, Senapati SB, Patra SK, Tripathy K, Burma S (2012) Primary intraosseous malignant peripheral nerve sheath tumor of spine with a giant paraspinal and retrospinal subcutaneous extension. Surg Neurol Int 3:157
Peng X, Chen L, Du H, Lai Y, Li F, Zou X (2011) Malignant transformation of benign intraosseous schwannoma in the cervical spine: a case report with an immunohistochemical study. Int Surg 96:337–344
Reed JC, Hallet KK, Feigin DS (1978) Neural tumors of the thorax: subject review from the AFIP. Radiology 126:9–17
Sakai F, Sone S, Kiyono K, Maruyama Y, Oguchi K, Imai N, Li F, Matsubara M, Ueda H, Haniuda M, Kubo K, Honda T, Ishii K (1996) Magnetic resonance imaging of neurogenic tumors of the thoracic inlet: determination of the parent nerve. J Thorac Imaging 11:272–278
Shields TW, Reynolds M (1988) Neurogenic tumors of the thorax. Surg Clin North Am 68:645–668
Sridhar K, Ramamurthi R, Vasudevan MC, Ramamurthi B (2001) Giant invasive spinal schwannomas: definition and surgical management. J Neurosurg 94(2 Suppl):210–5
Vecil GG, McCutcheon IE, Mendel E (2008) Extended lateral parascapular approach for resection of a giant multi-compartment thoracic schwannoma. Acta Neurochir (Wien) 150:1295–300
Yu NH, Lee SE, Jahng TA, Chung CK (2012) Giant invasive spinal schwannoma: its clinical features and surgical management. Neurosurgery 71:58–67
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Theresa Krätzig and Marc Dreimann contributed equally
Rights and permissions
About this article
Cite this article
Krätzig, T., Dreimann, M., Klingenhöfer, M. et al. Treatment of large thoracic and lumbar paraspinal schwannoma. Acta Neurochir 157, 531–538 (2015). https://doi.org/10.1007/s00701-014-2320-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-014-2320-5