Abstract
Background
Resection of giant thoracic schwannomas is challenging and usually requires a staged approach. The resection of the intraspinal component, usually via laminectomy, is done in one sitting and the intrathoracic component, via thoracotomy, follows at another. We describe the complete resection of a massive multi-compartmental thoracic schwannoma by an extended lateral parascapular approach.
Method and findings
The tumor, which presented with local pain and scapular displacement, had intrathoracic paraspinal (10 × 5 × 4 cm), posterolateral upper thoracic paramuscular (19 × 7 × 4 cm), foraminal, and epidural components. It was removed at a single sitting, via a posterior extended lateral parascapular approach that did not require staged procedures, multiple incisions, or repositioning of the patient. This operation included resection of the thoracic, foraminal, and intraspinal components and posterior stabilization with pedicle screws and rods. There were no postoperative neurological complications.
Conclusions
The extended lateral parascapular approach allows complete resection of giant multi-compartment schwannomas of the thoracic spine that extend from the canal into the thoracic cavity. It also permits posterior stabilization through the same incision used for tumor removal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
When benign neurogenic tumors extend transforaminally from the paraspinal space into the spinal canal, their removal becomes difficult. Until recently, this often required separate approaches to deal with the intrathoracic and intraspinal components, but the two-stage approach, using laminectomy followed by thoracotomy, risks neurological complications regardless of the initial approach [10]. In recent years the value of a single-stage procedure has become more apparent [1, 15, 27, 31]. In particular, Fessler and colleagues have described a lateral parascapular approach which they used to approach small upper thoracic lesions without violating the pleura [9]. Recently, the trend towards minimally invasive approaches has fostered transthoracic microsurgical endoscopy (thoracoscopy) for resection of intrathoracic neurogenic tumors [3, 7, 12, 30]. However, this approach is not recommended when intraspinal extension is present [13]. This report describes the successful resection of a large dumbbell thoracic schwannoma, during which the sheer size of the tumor and multi-level extension precluded staying extrapleural. We describe the use of a previously unreported intrapleural extension of the lateral parascapular approach that facilitated complete resection of a massive schwannoma via a single-stage procedure.
Case report
History and Clinical Findings
This 34-year-old man presented with a 14-year history of an enlarging posterior thoracic mass. At the time of presentation the lesion produced an obvious physical deformity with a palpable mass beneath and displacement of the right scapula. He had no external stigmata of neurofibromatosis. Radiographs demonstrate the resulting scoliosis (Fig. 1). The mass caused local discomfort and pain that radiated along the anterior chest wall on the right. The pain was aggravated by deep inspiration and any activity that required use of his right arm or shoulder. The patient described occasional episodes of “buckling” of his legs, but complained of no specific weakness. He denied incontinence of bowel or bladder. Neurological examination revealed no focal motor deficit and intact sensation to all modalities.
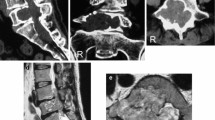
(a) Preoperative chest X-ray demonstrating the extent of the intrathoracic component of the mass and the associated scoliosis. (b) Corresponding coronal MRI demonstrating the multilobulated and dumbbell nature of the lesion. (c) Sagittal MRI depicting both the intrathoracic and extrathoracic components of the lesion
Neuroimaging
Magnetic resonance imaging (MRI) study of the thoracic spine demonstrated a 19 × 7 × 4 cm schwannoma in the right posterior paramuscular region (Figs. 1 and 2). The mass was isointense on T1 and heterogeneously hyperintense on T2 with marked contrast enhancement. It had a multilobulated 10 × 5 × 4 cm intrathoracic component that extended from T2 to T7. At the right T4/5 foramen, the tumor extended into the spinal canal and the majority of the intraspinal component was at T5. The epidural component produced a leftward displacement of the thecal sac at this level. The ipsilateral neural foramina at T4/5 and T5/6 were widened.
Surgical Technique
To allow for single-lung ventilation a double-lumen endotracheal tube was placed. The patient was then placed in the prone position. Intraoperative somatosensory evoked potentials (SSEPs) were monitored. The posterior paramuscular deformity was obvious. Ultrasonography was used to delineate its subsurface extent, but computer-assisted neuronavigation was not applied. A linear incision was made from the posterior midline at C7 down to T12 where it was curved around the base of the lesion onto the right flank. In this manner the tumor was outlined and a musculocutaneous flap was elevated. During the creation of the flap the tumor became visible and we found a dissection plane between the flap and the tumor on the chest wall (Fig. 2). Chest wall resection with disarticulation and partial resection of the right third through seventh ribs was then performed by a thoracic surgeon, providing a wide transpleural view into the thoracic cavity.
This allowed us to visualize the intrathoracic/paravertebral component of the mass which was multilobulated and intimately associated with the vertebral bodies from T3 to T6. Tumor extended transforaminally at T3/4, T4/5, and T5/6 into the intraspinal space. The disarticulation of the ribs from T3 to T7 allowed meticulous dissection both above and below the mass. Laminectomy from T2 to T7 was then performed to expose the intraspinal component of the lesion as well as the normal dura above and below the mass. A significant amount of epidural tumor was present causing cord compression. Through careful dissection the nerve roots from which the tumor arose were isolated. The right T3, T4, T5, and T6 nerve roots were ligated and divided. We then used blunt dissection to free the tumor (and its overlying pleura) within the thoracic cavity from the anterolateral vertebral bodies. Having already taken the nerve roots, we were then able to separate, and remove in one piece, the intrathoracic and intracanalicular components from the spine (Fig. 3).
With the tumor resection completed, we performed posterior stabilization. This consisted of thoracic pedicle screw placement bilaterally performed under fluoroscopic control from T2 to T10. The pedicle screws (6 mm diameter, polyaxial top-loading type; Universal Spine Set, Synthes Corp., Paoli, PA, USA) were connected with rods and cross connectors were placed and secured (Fig. 4). Some correction of the deformity was achieved using a combination of compression and distraction across the screws. Chest wall reconstruction was performed using a polypropylene (Marlex) mesh graft, necessitated by the en bloc resection of a portion of five contiguous ribs [6, 19]. Bony fusion was initiated by extensive decortication of laminae and transverse processes of instrumented segments, followed by placement of allograft bone croutons mixed with demineralized bone matrix. No changes in the SSEPs occurred during the case.
Pathology
Routine hematoxylin and eosin staining of slides taken from the specimen confirmed the diagnosis of a benign neural sheath tumor. Positive S-100 staining was consistent with the diagnosis of schwannoma. No sarcomatous degeneration was found.
Postoperative Course
Except for minor chest wall numbness secondary to the nerve root sacrifice on the right, the patient had no neurological deficit after the procedure. He developed a postoperative leukocytosis and thrombocytosis. Initially, these were thought to be secondary to infection (elevated white blood cell count and low grade temperature) but despite all efforts to detect a source of infection, none was found. Hematological workup suggested the thrombocytosis was likely a reactive process secondary to the stress of the procedure and the large size of the mass removed; the hematological abnormalities corrected within two weeks of surgery. The patient was discharged home in stable condition. He has had no recurrence of the tumor in the four years since surgery.
Discussion
The surgical resection of posterior mediastinal masses is best performed by a team with thoracic and neurosurgical expertise. Clearly, the optimal treatment is complete removal in a single-stage operation. This reduces the risk of bleeding, time under anesthesia, and may decrease traction or compression of the spinal cord. Factors to consider when planning the surgical approach to these lesions include the overall size of the lesion and the extent of intraspinal involvement [20]. Traditionally, resection of “dumbbell” lesions has required two procedures, a laminectomy and thoracotomy. The laminectomy is usually done first to release the intraspinal component and free the nerve root(s). In patients with intradural extension, this gives the neurosurgeon direct control of the dural closure minimizing the risk of CSF leakage and subarachnoid-pleural fistulae. Subsequently, an open thoracotomy is performed to resect the remaining intrathoracic component.
Grillo et al. proposed a posterolateral approach in which the thoracotomy incision is extended to allow the laminectomy to be completed [10]. Transthoracic, costotransversectomy, and lateral extracavitary approaches each have their advantages and limitations, which have been well described [2, 20, 26, 28]. Because lesions in the upper thoracic spine are difficult to reach via a transthoracic approach, Fessler and colleagues described a lateral parascapular extrapleural approach to the upper thoracic vertebrae in which they overcame the anatomical limitations of other techniques [9]. Their approach is designed to reach vertebral body lesions [7]. This extrapleural approach has been used successfully by others to remove relatively small dumb-bell neurofibromas at T1 and at T6 [23, 25].
In recent years, thoracoscopic resection of posterior mediastinal masses has been advocated by some groups [22, 32]. The advantages of thoracoscopy over open thoracotomy include less postoperative pain, a shortened hospital stay, and an earlier return to work [4, 17, 18]. However, the procedure has a steep learning curve, and dural closure through an endoscope is tricky regardless of operator experience and is the limiting factor in thoracoscopic resection of dumbbell lesions [11, 22]. Thus, an endoscopic approach is not recommended for tumors like the one described here, in which a significant intraspinal component is present [13]. Combining a laminectomy to release the intraspinal component with thoracoscopy for complete resection of these lesions under the same anesthetic was initially described by Vallieres [29] and subsequently by others [5, 14, 16, 21, 24].
Staged procedures require two separate incisions, allowing the surgeon to do the thoracotomy or the laminectomy first, and then to reposition the patient for the second part. The various ways to organize and perform the two operations have been reviewed elsewhere [29], but the consenus is that the intraspinal component should be freed prior to manipulation of the thoracic component [5, 29]. The imaging in our patient suggested, however, that the lesion could be removed via a single posterior approach with the patient in a prone position.
We extended the lateral parascapular approach (originally described by Fessler et al. for the vertebral body and pedicle) into the paraspinal, intrathoracic, and mediastinal compartments [9]. These modifications were necessitated by the extensive intrathoracic/mediastinal component in our case that precluded simple exposure via an extrapleural approach. In addition, the technique of Fessler et al. was devised for use in the upper thoracic spine (T1 through T4) whereas our exposure included elements from T2 through T7.
As in the lateral parascapular technique, we curved the incision off midline at the caudal end to facilitate removal of the posterior paramuscular mass as a single unit. Unlike the vertebral fractures and small tumors for which the lateral parascapular approach is typically used, the very large schwannoma in our patient had itself performed most of the muscle dissection, which made the musculocutaneous flap easy to create. Furthermore, the patient’s scapula was already displaced by the tumor, which provided internal retraction for us. By strategically placing the chest rolls during patient positioning, we kept additional scapular retraction to a minimum. By disarticulating the ribs from T3 to T7 the intrathoracic component became easily accessible. This component was adherent to the lateral vertebral bodies, but a safe dissection plane between the mass and the vertebral bodies was present and easy to follow. The chest wall defect left by the rib resection was easily filled using polypropylene (Marlex) mesh. This technique has been shown to be safe and effective for major chest wall reconstructions [19].
Finally, the prone position facilitated the laminectomy and foraminotomies to decompress the epidural component. Thus, all components of the schwannoma were removed in a single procedure without requiring repositioning of the patient. Because the patient in this case had an underlying scoliosis, the multi-level rib resections and laminectomy necessitated posterior spinal stabilization. Posterior spinal fixation was also facilitated with the patient in a prone position.
Conclusions
Massive schwannomas with multiforaminal involvement and intraspinal extension can be successfully resected via a single open procedure. In the past, if an intraspinal component was present a laminectomy followed by a thoracotomy was the usual process. Although staged procedures are still occasionally necessary, with careful planning a single operation may be possible even for large tumors. The complete resection of this massive schwannoma was completed using an extended lateral parascapular approach under a single session of anesthesia. The lateral parascapular approach can easily be modified, a reflection of its versatility, allowing for management of both anterior as well as posterolateral disease involving the upper and middle thoracic spine. Chest wall reconstruction and posterior stabilization are also facilitated by this approach.
References
Akwari OE, Payne WS, Onofrio BM, Dines DE, Muhm JR (1978) Dumbbell neurogenic tumors of the mediastinum. Diagnosis and management. Mayo Clin Proc 53:353–358
Amadei F, Carnevali G, Facchetti S, Griner A, Pozzi G, Tonnarelli G, Wizemann G (1996) Our experience in the treatment of intrathoracic and intraspinal hourglass neurogenic neoplasms. Chir Ital 48:13–19
Assaker R, Fromont G, Reyns N, Louis E, Chastanet P, Lejeune JP (2001) Video-assisted thoracoscopic surgery. Neurochirurgie 47:93–104
Bousamra M 2nd, Haasler GB, Patterson GA, Roper CL (1996) A comparative study of thoracoscopic vs open removal of benign neurogenic mediastinal tumors. Chest 109:1461–1465
Citow JS, Macdonald RL, Ferguson MK (1999) Combined laminectomy and thoracoscopic resection of a dumbbell neurofibroma: technical case report. Neurosurgery 45:1263–1265 discussion 1265-1266
Deschamps C, Tirnaksiz BM, Darbandi R, Trastek VF, Allen MS, Miller DL, Arnold PG, Pairolero PC (1999) Early and long-term results of prosthetic chest wall reconstruction. J Thorac Carciovasc Surg 117:588–591
Dickman CA, Apfelbaum RI (1998) Thoracoscopic microsurgical excision of a thoracic schwannoma. Case report. J Neurosurg 88:898–902
Dietze DD Jr, Fessler RG (1992) Lateral parascapular extrapleural approach to spinal surgery. Compr Ther 18:34–37
Fessler RG, Dietze DD Jr, Millan MM, Peace D (1991) Lateral parascapular extrapleural approach to the upper thoracic spine. J Neurosurg 75:349–355
Grillo HC, Ojemann RG, Scannell JG, Zervas NT (1983) Combined approach to “dumbbell” intrathoracic and intraspinal neurogenic tumors. Ann Thorac Surg 36:402–407
Han PP, Dickman CA (2002) Thoracoscopic resection of thoracic neurogenic tumors. J Neurosurg 96:304–308
Hazelrigg SR, Boley TM, Krasna MJ, Landreneau RJ, Yim AP (1999) Thoracoscopic resection of posterior neurogenic tumors. Am Surg 65:1129–1133
Ishida T, Maruyama R, Saitoh G, Mitsudomi T, Sugimachi K (1996) Thoracoscopy in the management of intrathoracic neurogenic tumors. Int Surg 81:347–349
Ishikawa E, Matsumura A, Ishikawa S, Nakamura K, Nose T (2002) Combined minimally invasive approach using microsurgery and thoracoscopic surgery for resecting a dumbbell-type thoracic schwannoma. Minim Invasive Neurosurg 45:251–253
Joseph SG, Tellis CJ (1988) Posterior mediastinal mass with intraspinous extension. Chest 93:1101–1103
Konno S, Yabuki S, Kinoshita T, Kikuchi S (2001) Combined laminectomy and thoracoscopic resection of dumbbell-type thoracic cord tumor. Spine 26:E130–E134
Landreneau RJ, Hazelrigg SR, Mack MJ, Dowling RD, Burke D, Gavlick J, Perrino MK, Ritter PS, Bowers CM, DeFino J et al (1993) Postoperative pain-related morbidity: video-assisted thoracic surgery versus thoracotomy. Ann Thorac Surg 56:1285–1289
Landreneau RJ, Wiechmann RJ, Hazelrigg SR, Mack MJ, Keenan RJ, Ferson PF (1998) Effect of minimally invasive thoracic surgical approaches on acute and chronic postoperative pain. Chest Surg Clin N Am 8:891–906
Mansour KA, Thourani VH, Losken A, Reeves JG, Miller JI Jr, Carlson GW, Jones GE (2002) Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg 73:1720–1725 discussion 1725-1726
McCormick PC (1996) Surgical management of dumbbell and paraspinal tumors of the thoracic and lumbar spine. Neurosurgery 38:67–74 discussion 74-65
McKenna RJ Jr, Maline D, Pratt G (1995) VATS resection of a mediastinal neurogenic dumbbell tumor. Surg Laparosc Endosc 5:480–482
Ohtsuka T, Kohno T, Nakajima J, Yagyu K, Furuse A (1994) Thoracoscopic resection of schwannoma: a report of two cases. Kyobu Geka 47:719–722
O’Reilly G, Jackowski A, Weiner G, Thomas D (1994) Lateral parascapular extrapleural approach for single-stage excision of dumb-bell neurofibroma. Br J Neurosurg 8:347–351
Osada H, Aoki H, Yokote K, Taira Y, Yamate N (1991) D umbell neurogenic tumor of the mediastinum: a report of three cases undergoing single-stage complete removal without thoracotomy. Jpn J Surg 21:224–228
Payer M, Radovanovic I, Jost G (2006) Resection of thoracic dumbbell neurinomas: single postero-lateral approach or combined posterior and transthoracic approach. J Clin Neurosci 13:690–693
Ricci C, Rendina EA, Venuta F, Pescarmona EO, Gagliardi F (1990) Diagnostic imaging and surgical treatment of dumbbell tumors of the mediastinum. Ann Thorac Surg 50:586–589
Shamji FM, Todd TR, Vallieres E, Sachs HJ, Benoit BG (1992) Central neurogenic tumours of the thoracic region. Can J Surg 35:497–501
Takamura Y, Uede T, Igarashi K, Tatewaki K, Morimoto S (1997) Thoracic dumbbell-shaped neurinoma treated by unilateral hemilaminectomy with partial costotransversectomy–case report. Neurol Med Chir (Tokyo) 37:354–357
Vallieres E, Findlay JM, Fraser RE (1995) Combined microneurosurgical and thoracoscopic removal of neurogenic dumbbell tumors. Ann Thorac Surg 59:469–472
Visocchi M, Masferrer R, Sonntag VK, Dickman CA (1998) Thoracoscopic approaches to the thoracic spine. Acta Neurochir (Wien) 140:737–743 discussion 743-734
Yuksel M, Pamir N, Ozer F, Batirel HF, Ercan S (1996) The principles of surgical management in dumbbell tumors. Eur J Cardiothorac Surg 10:569–573
Zierold D, Halow KD (2000) Thoracoscopic resection as the preferred approach to posterior mediastinal neurogenic tumors. Surg Laparosc Endosc Percutan Tech 10:222–225
Acknowledgment
The authors would like to thank Wei-Ming Shi, M.D., for his assistance with the figures.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
The paper is well done and deserves publication in Acta Neurochirurgica. The problem of the combined approach of these challenging and quite rare diseases is well stressed in the current literature. Spinal surgery develops from the neurosurgical and orthopedic spine experiences but in this case also thoracic surgery strongly contributes to implement the neurosurgical armamentarium for this very special disease. From the surgical point of view the Authors give a convincing list of advantages related to the approach descibed. Data provided by the literature lacks of unusual surgical details. The text is clear and “charming” as the surgical experiences the Authors show us.
References
1. Tahir MZ, Fatimi SH, Enam SA: Ancient schwannoma presenting as a thoracic mass. Surg Neurol. 2007 Nov;68(5):534-6. Epub 2007 Sep 4.
2.Kumar S, Rafiq MU, Ahamed I, Ansari J, Cowen ME: Asymptomatic giant thoracic schwannoma. Ann Thorac Surg. 1: 2006 Sep;82(3):e26
3. Endo S, Murayama F, Yamaguchi T, Hasegawa T, Sohara Y, Fuse K, Kuriki K, Saito K.: Subcarinal neurogenic tumor: report of a case. Surg Today. 1998;28(12):1307-9.
Massimiliano Visocchi
Catholic University Rome, Italy
Rights and permissions
About this article
Cite this article
Vecil, G.G., McCutcheon, I.E. & Mendel, E. Extended lateral parascapular approach for resection of a giant multi-compartment thoracic schwannoma. Acta Neurochir (Wien) 150, 1295–1300 (2008). https://doi.org/10.1007/s00701-008-0154-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-008-0154-8