Abstract
Background
The purpose of this study was to evaluate the surgical results of the single-stage surgery only from posterior approach for the management of thoracic dumbbell tumor and to discuss its usefulness and limitations.
Methods
Sixteen cases of large thoracic dumbbell tumor (11 men and 5 woman, mean age, 44 years) were analyzed retrospectively. Pathologic findings included schwannoma in 10 patients, neurofibroma in 2 patients (Recklinghausen in 1 patient), meningioma in 2 patients, myxolipoma in 1 and ganglioneuroma in 1. They underwent single-stage removal of dumbbell tumor using the posterior approach followed by laminectomy and often costotransversectomy combined with instrumentation. Clinical and radiologic outcomes are reviewed.
Results
The mean follow-up period for clinical and radiographic outcome variables was 66 months (range, 24–120 months). Operative time ranged from 185 to 420 min (mean, 320 min), with estimated blood loss ranging from 71 to 1830 ml (mean, 540 ml). Postoperative complications were pleural injury during the enucleation of paravertebral tumors, which could be repaired, and the chest tube was detained to prevent postoperative pneumothorax. Postoperative complications included atelectasis in one case. All patients had tumors successfully removed with no neurological deterioration. One patient underwent both posterior and anterior surgery because of attachment to and compression of an artery. We were not able to diagnose this case preoperatively, although a biopsy had been performed.
Conclusions
Single-stage surgery may be a useful method for removing thoracic dumbbell tumors without the combined anterior approach, unless they are attached to and compressing the artery and the diagnosis cannot be made preoperatively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
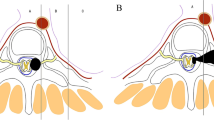
Dumbbell tumors are relatively rare neoplasms that can arise from neurogenic elements within the posterior mediastinum. They often become very large and involve surrounding structures before any neurological symptoms appear. Therefore, surgical removal is the treatment of choice, and this may require an extensive surgical approach. There are various approaches for managing thoracic dumbbell tumors [1–18]. Surgical strategies for managing these tumors depend on the type of tumor classification as developed by Eden [7, 19]. Laminectomy to remove the intraspinal component of a dumbbell tumor should be performed first to prevent a spinal cord injury caused by traction and compression when manipulating the tumor [5, 6]. This is followed by removal of the paravertebral tumor component.
Although a combined posteroanterior approach has been reported for removing a large paravertebral tumor component, this technique requires thoracotomy, two stages, and technical accuracy of the neurosurgeon [7]. Furthermore, a sternum cleavage may be required for the anterior approach with a large, upper thoracic tumor. It may be more rational to perform a single-stage removal of a thoracic dumbbell tumor without thoracotomy.
This report presents 15 cases of thoracic dumbbell tumors removed using only a posterior approach and costotransversectomy for large paravertebral tumors. The patients were observed for a minimum of 5 years postoperatively. We also report on 1 tumor removed through a combined laminectomy and thoracotomy in response to the limitation of only approaching posteriorly. We describe the surgical techniques and clinical findings of single-stage removal of these tumors using 3-dimensional computed tomography (3D-CT) to evaluate the surrounding components. We discuss the usefulness and limitations of this posterior approach.
Patients and methods
Sixteen patients were admitted for thoracic dumbbell tumor removal at the Department of Orthopedic Surgery of Nagoya University School of Medicine and 15 were observed for a minimum of 5 years postoperatively. The tumor in one patient was removed through a combined laminectomy and thoracotomy in response to the limitation of only approaching posteriorly (Table 1).We evaluated the following items in these patients: preoperative symptoms and their duration; plain radiography of the chest, thoracic-spine, and total-spine; magnetic resonance imaging (MRI); CT, including reconstructed 3D, which can show arterial involvement; the specific surgical procedure performed, any complications, and postoperative recovery. We evaluated the severity of a patient’s myelopathy before and after surgery using the JOA scoring system. We evaluated postoperative improvement of symptoms using the recovery ratio of the JOA score and the Hirabayashi method:
with a recovery ratio of 100 % indicating the best postoperative improvement [20].
Postoperatively, we performed annual MRIs for a minimum of 5 years to document that there was no tumor re-growth. The study protocol was approved by the Committee on Ethics in Human Research of Nagoya University, and informed consent was obtained from each patient.
Surgical technique
Preoperatively, we acquired all relevant information on the involvement of any artery, bone, or other peritumoral structures by 3D-CT. During surgery we monitored evoked potentials (SSEPs) and motor-evoked potentials (MEPs) with the patient in the prone position under general anesthesia. We made a vertical midline incision to expose the laminae bilaterally at the selected levels and the transverse process with the costotransverse joint and rib on the affected side. We performed a costotransversectomy (CTV) on the affected side for a large paravertebral tumor followed by bilateral laminectomy and facetectomy (FCT) at the affected level using Kerrison’s punch, and drilled as required to expose the intraforaminal tumor. The dura was opened for an intradural tumor excision (Eden type II) using an operating microscope extended laterally over the nerve root sleeve. After removing this component of the tumor and sacrificing the entire affected spinal nerve root to prevent traction on the spinal cord during excision of the paravertebral tumor, we primarily closed the dura in a watertight fashion and placed fat harvested from subcutaneous tissue using fibrin glue. Then, the paravertebral tumor with its distal stump and an encapsulated smooth surface tumor at the back side were exposed and carefully enucleated to prevent the need for a thoracotomy. Finally, we inserted the pedicle screws under image guidance.
The fusion area encompassed 1 vertebrae above and below the affected levels if we were able to insert the pedicle screws (Patient no. 5, 10, 15), or 2 vertebrae above or below the affected levels if we could not insert pedicle screws to the affected side because of pedicle scalloping, plus one level CTV (no. 1, 6, 8, 11, 12, 13, 14, 16), or 2 vertebrae above and below the affected level if multilevel CTV was performed because of a large paravertebral tumor. We applied contoured rods to prevent deformities that might occur because of instability, followed by a bone graft using a spinous process and lamina resected to the decorticated lamina and facet joints of the non-affected side. A Valsalva maneuver to check for a pleural breach was performed, and, where necessary, a temporary chest tube was inserted. Finally, we performed a meticulous closure of the wound in layers over a drain. Postoperatively, patients were put on bed rest for 3–4 days and then mobilized by the physiotherapist after removal of the drain.
Results
Clinical data (Table 1)
There were 11 men and 5 women ranging in age from 17 to 73 years (mean age, 44 years). Eight (50.0 %) patients had long tract signs with gait disturbance, 7 (43.7 %) patients had radicular symptoms, and 3 patients had bowel and bladder dysfunction. The duration of preoperative symptoms ranged from 3 to 72 months (mean duration, 16.1 months). According to the Eden classification [19] (Table 2), 11 patients were classified as type II and 5 patients as type III. Six patients underwent CT-guided preoperative biopsy to rule out malignancy, and 1 patient received preoperative embolization (no. 4).
Fifteen patients underwent single-stage removal with instrumentation. One patient underwent both posterior and anterior surgery because of the attachment to and compression of an artery. We also could not diagnose this case preoperatively, although a biopsy had been performed (no. 16). Histopathology revealed schwannoma in 10 patients, meningioma in 2 patients, neurofibroma in 2 patients (neurofibromatosis type 1 in 1 patient), and ganglioneuroma and myxolipoma in 1 patient each. Extraforaminal tumor extension ranged from 3 to 8.4 cm (mean, 5.3 cm). The involved nerve had to be sacrificed in all patients for complete intraspinal tumor removal.
Outcome and follow-up
Outcomes are documented in Table 1. The mean follow-up period for clinical and radiographic outcome variables was 85 months (range, 60–135 months) except for one patient. Operative time ranged from 185 to 420 min (mean, 320 min), with estimated blood loss ranging from 71 to 1830 ml (mean, 540 ml). There was a large amount of bleeding during surgery on the patient with neurofibromatosis type I (no. 9). Postoperative complications included pleural injury during the enucleation of the paravertebral tumor, which was repaired (nos. 4, 9), and a chest tube was needed to prevent postoperative pneumothorax (no. 4). Because the atelectasis that occurred was on the opposite side of the tumor, we concluded that it was not related to the surgical technique but to the prone position during the long surgery (no. 3). Tumor resection was subtotal using this procedure with a remnant capsule because enucleation was performed on the paravertebral tumor. The mean preoperative JOA score was 6.9 (range 2–10). The mean recovery rate was 68.4 % (range 33.3–100) at our most recent follow-up. The patients experienced no postoperative neurological deterioration. Radicular symptoms resolved in all patients. Gait disturbance due to myelopathy improved in all patients to some extent, although bowel and bladder dysfunction did not improve. The 5-year follow-up MRI revealed no re-growth in any of the patients. There was only 1 deformity or instability at the proximal adjacent areas as determined by plain X-ray and CT. This was case no. 16, who had proximal adjacent disease and has been wearing a cervical collar and followed carefully.
Illustrative case 1: patient no. 4
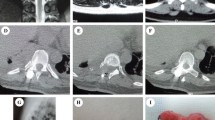
An 18-year-old man presented to our department with a history of left chest radicular symptoms and numbness (12 months), which gradually deteriorated. Plain radiography of the chest showed a circular-shaped shadow at the left, middle portion of the lung (Fig. 1a). CT and MRI findings were highly suggestive of a dumbbell thoracic cord tumor, and an axial view MRI demonstrated an intraspinal and extraspinal soft tissue mass at T8–T9 (Fig. 1b, c) with the extraforaminal portion extending 8.4 cm at a maximum diameter on the left side. The 3D-CTs showed the relationships between the tumor and peritumoral structures, that is, the aorta, ribs, and vertebrae (Fig. 1d).
a Plain radiography of the chest showing a circular-shaped shadow at the left, middle portion of the lung. b, c MRI findings highly suggestive of a dumbbell thoracic cord tumor. An extraspinal soft tissue mass at T8–T9 with the extraforaminal portion extending 8.4 cm (maximum diameter) on the left side. d 3D-CT showing the relationships between the tumor and peritumoral structures, that is, the aorta, ribs, and vertebrae. e Plain radiography showed no implant failure at the last follow-up. f, g MRI showed no re-growth of the remnant tumor
We performed surgery on the patient using the procedure described above (T6–T10 instrumentation and local bone graft) to directly observe the relationship between the dura mater and tumor and avoid spinal cord injury, after which the patient's redicular pain disappeared postoperatively. No relapse was seen in the MRIs, and the plain radiography showed no implant failure at the last follow-up (Fig. 1e). The MRI showed no re-growth of the remnant tumor (Fig. 1f, g).
Case 2: patient no. 16: limitation of the single, posterior surgical approach [10]
A 17-year-old woman presented to our department with a history of backache (6 months). Plain radiography of the chest showed a circular-shaped shadow at the right, upper portion of the lung (Fig. 2a). CT and MRI findings were highly suggestive of a dumbbell thoracic cord tumor. There was an intraspinal and extraspinal soft tissue mass at T1–T2 (Fig. 2b, c) with the extraforaminal portion extending 7.0 cm on the right side with attachment to and compression of the artery. Moreover, we were unable to diagnose the tumor, although a biopsy was carried out.
a Plain radiography of the chest showing a circular-shaped shadow at the right, upper portion of the lung. b–d CT and MRI demonstrating the intraspinal and extraspinal soft tissue mass at T1–T2 with the extraforaminal portion extending 7.0 cm on the right side with attachment to and compression of the artery. e, f Plain radiography showed no implant failure at the last follow-up. g, h MRI showed no relapse
We performed surgery on the patient using both a posterior and anterior approach with open thoracotomy (T1–T2 one sided-instrumentation and local bone graft) and total resection because we believed that extirpation was impossible approaching only posteriorly (Fig. 2d, e). No relapse was seen in the MRIs, however, at the last follow-up. However, this patient had a proximal adjacent kyphotic change and has been wearing a cervical collar and followed carefully. Fortunately, the patients had no symptoms until the fusion of the adjacent area.
Discussion
Thoracic dumbbell tumors are relatively rare [21]. In a series of 674 spinal cord tumors, Ozawa et al. reported 4.7 % thoracic dumbbell tumors. Approximately 10 % of all neurogenic tumors located in the posterior mediastinum are dumbbell tumors having intraspinal extensions via the intervertebral foramen [9]. Most tumors are benign, neurogenic tumors, including schwannoma, neurofibroma, ganglioneuroma, and neuroblastoma, with schwannomas accounting for 90 % of all dumbbell tumors [14]. Sometimes, the tumors are large by the time they are detected because they grow slowly and are entirely asymptomatic. The paravertebral tumor component is usually larger than the intraspinal component [9].
There are various approaches for managing thoracic dumbbell tumors [1–18]. An important aim of the surgical method is to remove both the intraspinal and the extraspinal tumor mass. Combined or single posterior approaches are mainly reported, although removing the tumor from only an anterior approach may be possible when there is only a paravertebral tumor, such as an Eden type IV. Akwari reported on a combination of 2 approaches [9]. First, a posterior laminectomy is performed by the neurosurgeon, followed by a postero-lateral thoracotomy performed by a thoracic team. This method avoids the risk of bleeding from remnant tumor tissue, compression of the spinal cord, leakage of cerebrospinal fluid, and damage to the spinal cord, which can be encountered with the two-stage procedure. Recently, the focus of interest has been on a combined approach involving laminectomy by a neurosurgeon followed by videothoracoscopic removal of the intrathoracic component [2–8]. However, it is difficult to use thoracoscopic instruments precisely when there is unexpected bleeding or other emergencies [3].
The procedure described in our case report is reasonable for a single surgeon employing a single surgical approach to perform. A single posterior approach has several advantages: it leaves the patient with only 1 wound and it avoids use of a postoperative chest tube, which is required after transthoracic transpleural procedures. In these cases, there was only one patient with pleural injury who required a chest tube to prevent postoperative pneumothorax.
For resection of the extraspinal component, we performed a simple enucleation without thoracotomy. The most serious complication that can develop during this surgery is spinal cord ischemia due to injury of the Adamkiewicz artery. But we do not touch the aorta and segmental artery during surgery. Although the extraspinal components, including the aorta, cannot be fully seen, enucleation is thought to be the safest method since ablation from the aorta is unnecessary. The goal of surgery is to entirely remove the tumor at one time to relieve symptoms and eliminate recurrence and involvement with surrounding structures. Malignancy has been rare aside from an occasional case report in the literature on malignant transformation [22, 23]. Moreover, as schwannoma, neurofibroma, and ganglioneuroma tend to encapsulate well [24, 25], enucleation from the posterior approach is not difficult for a surgeon to perform, and there is less chance for injury to peritumoral structures such as an artery with enucleation, which is an intracapsular manipulation. We have detected no re-growth in our patients; however, the follow-up period was relatively short at only 5–10 years, and continued evaluation is needed.
Advocates of the combined approach claim that the posterior approach can be restricted to a vertical midline incision centered over the tumor and that less removal of facet joints, transverse processes, and ribs is required if the extraspinal portion of the tumor is removed through a transthoracic transpleural approach [3, 5]. A biomechanical study revealed that sequential costo-vertebral joint releases resulted in a decrease in the force required for axial rotation and lateral bending, coupled with an increase in the displacement of vertebral bodies [26]. Moreover, postoperative instability and subluxation or deformity were reported [27–29], although Agrawal et al. [30] reported on single-stage excision by the posterior approach without instrumentation. Vecil et al. reported that multi-level rib resections and laminectomy necessitated posterior spinal stabilization.
We believe that for safer surgery for the spinal cord, intra- and large extraspinal lesions connected through the foramen should not be resected without facetectomy and often costotransversectomy. Moreover, partial facetectomy with scalloping lesions has the risk for postoperative instability. Therefore, spinal instrumentation with pedicle screws and bone grafting was added in our reported cases to prevent deformity. There were no complications such as instrumentation failure or adjacent segmental disorder at the last follow-up. Takamura et al. [15] reported that it is essential to individualize each patient to plan a suitable preoperative surgical strategy. In all our cases, surgical strategies were discussed preoperatively with 3D-CT images showing relationships between tumors and peritumoral structures, such as arteries, ribs, and vertebrae.
Of course, there are several disadvantage to this procedure. First, extensive reconstruction with instrumentation is needed to remove a tumor that is in the thoracic cavity through a posterior approach. Using instrumentation on seven patients who received only one-level facetectomy is considered controversial. The stabilization may be unnecessary for them. Moreover, we should especially take care of postoperative infection. Second, as a limitation of this procedure, we presented case no. 16, who underwent both posterior and anterior surgery because making a preoperative diagnosis of malignancy was impossible in spite of having a biopsy performed, and preoperative CT and MRI revealed attachment to and compression of an artery, which was meant a risk for separation of the tumor.
Nevertheless, we believe that this procedure is one of the important surgical methods to treat thoracic dumbbell tumors in which most dumbbell tumors can be effectively resected by posterior surgery only regardless of the tumor size.
Conclusion
A single posterior approach has several advantages: it provides the surgeon with a familiar orientation of all intraspinal structures throughout the entire surgical procedure, it leaves the patient with only 1 wound, and it avoids use of a postoperative chest tube, which is required after transthoracic transpleural procedures. Single-stage surgery by laminectomy and costotransversectomy for large paravertebral tumors may be a useful method for removing thoracic dumbbell tumors except when the tumor is attached to and compressing an artery and making an accurate preoperative diagnosis is impossible, especially the inability to exclude the malignancy.
References
Grillo HC, Ojemann RG, Scannell JG, Zervas NT. Combined approach to “dumbbell” intrathoracic and intraspinal neurogenic tumors. Ann Thorac Surg. 1983;36:402–7.
Heltzer JM, Krasna MJ, Aldrich F, McLaughlin JS. Thoracoscopic excision of a posterior mediastinal “dumbbell” tumor using a combined approach. Ann Thorac Surg. 1995;60:431–3.
Vallieres E, Findlay JM, Fraser RE. Combined microneurosurgical and thoracoscopic removal of neurogenic dumbbell tumors. Ann Thorac Surg. 1995;59:469–72.
Fiumara E, D’Angelo V, Florio FP, Nardella M, Bisceglia M. Preoperative embolization in surgical treatment of spinal thoracic dumbbell schwannoma. A case report. J Neurosurg Sci. 1996;40:153–6.
Citow JS, Macdonald RL, Ferguson MK. Combined laminectomy and thoracoscopic resection of a dumbbell neurofibroma: technical case report. Neurosurgery. 1999;45:1263–5. (discussion 5–6).
Dickman CA. Combined laminectomy and thoracoscopic resection of a dumbbell neurofibroma: technical case report—Comment. Neurosurgery. 1999;45:1266.
Konno S, Yabuki S, Kinoshita T, Kikuchi S. Combined laminectomy and thoracoscopic resection of dumbbell-type thoracic cord tumor. Spine (Phila Pa 1976). 2001;26:E130–4.
Jules JA, Guarnieri JM, Alkofer B, Le Rochais JP, Icard P. Posterior intrathoracic neurinoma cure: a transforaminal resection after a thoracotomy. Ann Thorac Surg. 2005;79:1411–2.
Akwari OE, Payne WS, Onofrio BM, Dines DE, Muhm JR. Dumbbell neurogenic tumors of the mediastinum. Mayo Clin Proc. 1978;53:353–8.
Joseph SG, Tellis CJ. Posterior mediastinal mass with intraspinous extension. Chest. 1988;93:1101–3.
Shamji FM, Todd TR, Vallieres E, Sachs HJ, Benoit BG. Central neurogenic tumours of the thoracic region. Can J Surg. 1992;35:497–501.
Lucas S, Cendan E, Auque J, Civit T, Caremelle S, Braun D. [Asymptomatic giant thoracic dumbbell neurinoma. Apropos of a case]. J Chir (Paris). 1992;129:81–7.
Yuksel M, Pamir N, Ozer F, Batirel HF, Ercan S. The principles of surgical management in dumbbell tumors. Eur J Cardiothorac Surg. 1996;10:569–73.
McCormick PC. Surgical management of dumbbell and paraspinal tumors of the thoracic and lumbar spine. Neurosurgery. 1996;38:67–74. (discussion–5).
Takamura Y, Uede T, Igarashi K, Tatewaki K, Morimoto S. Thoracic dumbbell-shaped neurinoma treated by unilateral hemilaminectomy with partial costotransversectomy–case report. Neurol Med Chir (Tokyo). 1997;37:354–7.
Onesti ST, Ashkenazi E, Michelsen WJ. Transparaspinal exposure of dumbbell tumors of the spine. Report of two cases. J Neurosurg. 1998;88:106–10.
Miura J, Doita M, Miyata K, Yoshiya S, Kurosaka M, Yamamoto H. Horner’s syndrome caused by a thoracic dumbbell-shaped schwannoma: sympathetic chain reconstruction after a one-stage removal of the tumor. Spine (Phila Pa 1976). 2003;28:E33–6.
Payer M, Radovanovic I, Jost G. Resection of thoracic dumbbell neurinomas: single postero-lateral approach or combined posterior and transthoracic approach? J Clin Neurosci. 2006;13:690–3.
Eden K. The dumb-bell tumours of the spine. Br J Surg. 1941;28:549–70.
Hirabayashi K, Miyakawa J, Satomi K, Maruyama T, Wakano K. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine (Phila Pa 1976). 1981;6:354–64.
Ozawa H, Kokubun S, Aizawa T, Hoshikawa T, Kawahara C. Spinal dumbbell tumors: an analysis of a series of 118 cases. J Neurosurg Spine. 2007;7:587–93.
Martins MD, Anunciato de Jesus L, Fernandes KP, Bussadori SK, Taghloubi SA, Martins MA. Intra-oral schwannoma: case report and literature review. Indian J Dent Res. 2009;20:121–5.
Singer RL. Thoracoscopic excision of a malignant schwannoma of the intrathoracic vagus nerve. Ann Thorac Surg. 1995;59:1586–7.
Radulovi DV, Branislav D, Skender-Gazibara MK, Igor NM. Cervical dumbbell ganglioneuroma producing spinal cord compression. Neurol India. 2005;53:370–1.
Sasaki K, Kohno T, Mun M, Yoshiya T. Thoracoscopic removal of middle mediastinal schwannoma originating from recurrent nerve. Thorac Cardiovasc Surg. 2008;56:375–7.
Yao X, Blount TJ, Suzuki N, Brown LK, van der Walt CJ, Baldini T, Lindley EM, Patel VV, Burger EL. A biomechanical study on the effects of rib head release on thoracic spinal motion. Eur Spine J. 2012;21:606–12.
Amhaz HH, Fox BD, Johnson KK, Whitehead WE, Curry DJ, Luerssen TG, Jea A. Postlaminoplasty kyphotic deformity in the thoracic spine: case report and review of the literature. Pediatr Neurosurg. 2009;45:151–4.
Hida S, Naito M, Arimizu J, Morishita Y, Nakamura A. The transverse placement laminoplasty using titanium miniplates for the reconstruction of the laminae in thoracic and lumbar lesion. Eur Spine J. 2006;15:1292–7.
Thorat JD, Rajendra T, Thirugnanam A, Ng IH. Single-stage posterior midline approach for dumbbell tumors of the thoracic spine, with intraoperative CT guidance. Surg Neurol Int. 2011;2:31.
Agrawal A, Srivastava S, Joharapurkar SR, Gharde P, Ubeja G. Single stage complete excision of large thoracic dumbbell schwannoma by modified posterior approach. Surg Neurol. 2008;70:432–6.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Ando, K., Imagama, S., Ito, Z. et al. Removal of thoracic dumbbell tumors through a single-stage posterior approach: its usefulness and limitations. J Orthop Sci 18, 380–387 (2013). https://doi.org/10.1007/s00776-013-0370-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00776-013-0370-9