Abstract
Magnetic ZnFe2O4 nanotubes (ZFONTs) with numerous pores on their walls were synthesized and characterized. They are shown to be a viable sorbent for dispersive micro-solid phase extraction of the trivalent ions of rare earth elements (REEs), specifically of lanthanum, praseodymium, europium, gadolinium, holmium and ytterbium. The specific surface area of ZFONTs is large (57 m2⋅g−1) and much bigger than that of ZnFeO4 nanoparticles (16 m2⋅g−1). It is shown that REEs are quantitatively retained on ZFONTs in the pH range of 7.0–9.0. The separation of the sorbent from the aqueous phase was achieved by an external magnetic field. Following elution with 0.5 mol⋅L−1 HNO3, REEs were quantified by inductively coupled plasma mass spectrometry. The main parameters influencing preconcentration and determination of the REEs were studied. Under optimum conditions, detection limits for REEs range from 0.01 (Ho) to 0.75 (La) pg⋅mL−1. Relative standard deviations are less than 6.5% (for n = 9; at 1.0 ng⋅mL−1). The method was applied to the determination of trace REEs in spiked biological and environmental samples and gave satisfactory results.

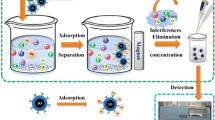
Schematic presentation of a new adsorbent for dispersive micro-solid phase extraction (DMSPE) combined with ICP-MS. Magnetic ZnFe2O4 nanotubes with many pores on their walls were used for preconcentration and determination of rare earth elements (REEs) in environmental and biological samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Owing to their unique characteristics, rare earth elements (REEs) have attracted considerable interest in high-technology fields, including electronics, superconductors, super-magnets, ceramics, catalysts and laser materials [1,2,3]. Moreover, because REEs can promote the growth of plants and animals, they are also widely used as feed additives and microelement fertilizer in agricultural production [4]. As a result, more and more REEs are spread in the environment, and may enter human bodies via food chain. It was reported that long-term intake of low dose REEs may result in the aberration of bone structure and tissue, and even bring about the generation of genetic toxicity in bone marrow cells [5]. REEs can invade the central nervous system because they are susceptible to cerebral cortex and cause subclinical damage [6]. Therefore, it is of great importance to develop new methods for the determination of trace/ultra-trace REEs in environmental and biological samples.
Various analytical techniques have been used for the determination of REEs in real samples, such as neutron activation analysis, X-ray fluorescence, isotopic dilution mass spectrometry, inductively coupled plasma atomic emission spectrometry and inductively coupled plasma mass spectrometry (ICP-MS). Relatively, ICP-MS is the most favorable choice because of its advantages, including high sensitivity, wide linear range and multi-elemental detection capability. However, direct determination of REEs in environmental and biological samples by ICP-MS is sometimes restricted due to very low concentration of REEs and high contents of matrices. This is the reason why sample pretreatment techniques are often necessary prior to analysis [7, 8]. Among the preconcentration method, solid phase extraction (SPE) is a preferred procedure because of its advantages, such as simple operation, low cost, high enrichment factor and the ability to combine with other detection techniques [9,10,11]. However, this technique suffers from certain shortcomings, including solvent loss, large secondary wastes and a long procedure [12]. In addition, a liquid sample is passed through a column containing an adsorbent in conventional SPE. Small-size adsorbent such as nanoparticles can escape from SPE column, and can cause high pressure in SPE system.
In order to overcome the above-mentioned drawback of SPE, many researches have focused on developing simplified and miniaturized sample pretreatment methods. Among them, dispersive micro-solid phase extraction (DMSPE) has received an increasing attention. Compared with traditional SPE, DMSPE has many merits of reduced solvent consumption, less adsorbent usage, short extraction time and high extraction efficiency [13,14,15]. In DMSPE, physicochemical properties of sorbents are very important to achieve an accurate, sensitive and selective determination of analytes. Therefore, development of new adsorbent materials has become a focus of interests to analysts.
Due to their unique properties, nanomaterials are attracting more and more attention in analytical sciences [16,17,18,19,20,21]. Among them, magnetic nanoparticles have shown great potential as adsorbent because of their smaller particle size, large surface area, high adsorption activity and magnetic property. Some magnetic nanomaterials have been employed for the preconcentration and separation of inorganic and organic substances in DMSPE [22,23,24,25,26,27]. Magnetic ZnFe2O4 nanotubes (ZFONTs) not only possess tubular structure, but also exhibit many pores on their wall, which result in their larger specific surface area. These features reveal that ZFONTs maybe have a great analytical application as an adsorbent in DMSPE. To the best of our knowledge, however, study on this topic has received little attention so far.
In this work, ZFONTs were investigated as adsorbent for the first time in DMSPE for the preconcentration of REEs before their determination by ICP-MS. The experimental results showed that ZFONTs have a strong adsorption capacity for REEs due to their large specific surface area. DMSPE using ZFONTs coupled to ICP-MS provides the merits of high enrichment factor, large dynamic capacity, low detection limits. In addition, the separation of the sorbent containing target analytes from aqueous phase was achieved by an external magnetic field to avoid time consuming column passing or filtration/centrifugation process. The accuracy and applicability of this were validated by analyzing REEs in environmental water samples and two certified reference materials.
Experimental
Instrumentation
An X Series 7 ICP-MS system (Thermo Fisher Scientific, USA, www.thermofisher.com) with a Babington nebulizer was applied in this work. 1.0 ng⋅mL−1 115In was used as internal standard to compensate for signal error. The optimal conditions for ICP-MS are listed in Table S1. A pH meter with a combined electrode was used to control the pH values of solutions (Thermo Fisher Scientific, USA, www.thermofisher.com). The mixture was sonicated using a KQ-50E ultrasonic bath (Kunshan Ultrasonic Instrument Co., Ltd., Suzhou, China, www.kscsyq.com). A strong neodymium-iron-boron (Nd2Fe12B) magnet was used for phase separation. Sample digestion was performed by an Ethos T microwave digestion device (Milestone, Italy, www.milestonesci.com).
Reagents and solutions
Stock standard solutions of REEs (1.0 mg⋅mL−1) were purchased from the National Analysis Center of Iron & Steel (Beijing, China, www.nacis cn.com). Working solutions were prepared by stepwise dilution of the above stock solutions just before use. All reagents used in this experiment were of analytical grade unless otherwise noted and bought from Shanghai Reagent Factory (Shanghai, China, www.zardzfp.cn.gtobal.com). High purity deionized water was obtained from Milli-Q® A10 service (Millipore Corporation, USA, www.millipore.com) and used during this work. The synthesis of ZFONTs was carried out in our laboratory. All plastic and glass containers in this work were stored in 20% (v/v) nitric acid over 24 h and rinsed with high purity water prior to their use.
Dispersive micro-solid phase extraction procedure
The model solution of 30 mL containing REEs was placed in a 50 mL test tube and adjusted to pH 8.0 with diluted NH3⋅H2O. Afterwards, 10 mg of ZFONTs was immediately added to the test tube. The mixture was dispersed by ultra-sonication for 1.5 min at room temperature, followed by a strong magnet placed at the bottom of the test tube to separate ZFONTs from the aqueous solution. The aqueous phase was discarded, and ZFONTs remained in the test tube. To desorb the analytes, 1.0 mL of 0.5 mol⋅L−1 HNO3 solution was added to the test tube containing ZFONTs, and the test tube was ultra-sonicated for 1 min. Finally, the eluate was isolated by the magnet again. The analytes in the eluent was determined by ICP-MS. A blank solution was also run by the same procedure.
Sample preparation
The water samples, including lake water and wastewater water (collected from Wuhan, China), were filtered through a 0.45 μm membrane filter, and then acidified to a pH of 5.0 for their storage. Prior to use, the sample solution was adjusted to a pH 8.0 with 0.1 mol⋅L−1 NH3⋅ H2O.
Two certified reference materials of tea leaf (GBW 07605, Institute of Geophysical and Geochemical Exploration, Langfang, China) and human hair (GBW 09101a, NRCSM, Beijing, China) were used to validate this method. An accurately weighed sample portion of 0.1000 g was mixed with 4.0 mL of HNO3 (65–68%, w/w) and 2.0 mL of H2O2 (30%, w/w) in a PTFE pressure vessel. The PTFE vessel was closed and left to stand overnight. Then, the vessels were placed into a microwave digestion device. After that, the samples were digested at 180 °C (ramp, 10 °C⋅min−1; hold, 15 min) with a power of 1.0 kW. After cooling, the vessels were opened and heated to near dryness on a hot plate at 200 °C. The residues were dissolved with 0.1 mol⋅L−1 HNO3, diluted to a required volume with ultrapure water, and kept in a refrigerator at 4 °C for future analysis. The blanks were prepared exactly as the samples without addition of target ions.
Results and discussion
Preparation of magnetic magnetic ZnFe2O4 nanotubes (ZFONTs)
Magnetic ZFONTs were fabricated by a facile electrospinning and direct annealing method. Firstly, a certain amount of Zn(Ac)2⋅2H2O and Fe(acac)3 with molar ratio of 1:2 were completely dissolved in 6 mL N, N-dimethylformamide with vigorous stirring to form homogeneous solution. Then, 1 g poly(vinyl pyrrolidone) (PVP, Mw~1,300,000) powder was added into the above solution, followed by stirring for 6 h at ambient temperature to obtain uniform brown-red precursor solution. Finally, the mixture precursor was loaded into a syringe equipped steel needle, a high voltage of 11 kV was supplied by a direct-current power supply, and the collector was placed 15 cm away from the tip of steel needle. Magnetic ZFONTs were fabricated by direct annealing of as-spun ZnFeO4 precursor nanofibers mat at 550 °C for 3 h with a heating rate of 1 °C ⋅ min−1. For comparison, ZnFeO4 nanoparticles were prepared by a hydrothermal method [28].
Choice of materials
Among various materials such as RGO, γ-Fe2O3-SiO2, MIPs, LDH, Ni-doped CoFe2O4, magnetite @MOFs and ZnO, ZFONTs can stand above due to its remarkable advantages. Preparation of ZFONTs is facile, and used precursors possess no drastic toxicity. However, the materials like MIPs are too complex, and in some cases use toxic precursors such as vinyl compounds. For magnetite @MOFs, MOFs were activated at a required temperature under vacuum condition. Thus, their synthesis is also an extremely tedious work. The separation of the non-magnetic adsorbents such as RGO, LDH and ZnO from aqueous phase was achieved using time-consuming filtration or centrifugation process.
Compared with the magnetic γ-Fe2O3-SiO2 and Ni-doped CoFe2O4 nanoparticle, ZFONTs exhibit hollow tubular structure and many pores on their wall. This unique structure results in the great increase of specific surface area and active sites of ZFONTs, which is beneficial for adsorbing more ions or molecules. Besides, ZFONTs is polar, unlike some polymers and mesoporous carbon materials, and can highly disperse in water, which leads to more contact between the adsorbent and analytes.
Characterization of ZFONTs
The formation of ZFONTs was evaluated by an X-ray diffractometer with Cu Kα radiation (XRD 7000, Shimazu, Japan, https://www.shimadzu.com). It can be seen from Fig. 1a that the sharp and intense diffraction peaks located at 2 Theta of 29.9o, 35.3o, 42.9o, 53.2o, 56.7o, 62.2o and 73.5° corresponded to the (220), (311), (400), (420), (511), (440) and (533) planes of ZFONTs, respectively. It is demonstrated that ZFONTs possessed standard spinel structure after calcination treatment.
The surface morphology and composition of ZFONTs was characterized by scanning electron microscopy (SEM) and energy dispersive X-ray spectrometry (EDS, attached to SEM) (MAIA 3 LMH, TESCAN, Czech Republic, www.tescan.com). In Fig. 1b, it is vividly indicated that ZFONTs possess a mean outer diameter of about 258 nm and many nanopores on their walls which consist of ZnFeO4 nanoparticles. Moreover, the hollow structure of ZFONTs with mean inner diameter of approximate 150 nm can be detected from the transection image (Fig. 1c). Additionally, ZFONTs maintain the 1D nanotube morphology. The formation of the nanopores can be attributed to the void space between the stacking ZnFeO4 nanoparticles during annealing process. The results of EDS spectrum indicate that ZFONTs were composed of Zn, Fe and O except for Au from the sample treatment, which suggests that the pure ZFONTs were obtained in this work.
Sorption isotherm and pore size distribution of ZFONTs were measured by a Micromeritics ASAP 2010 analyzer (Norcross, GA, USA, www.micromeritics.com). Fig. 1d indicates that the sorption isotherm belongs to the type IV with a distinct hysteresis loop, illustrating the presence of mesoporous structures on ZFONTs. In addition, the pore size distribution of ZFONTs was examined by Barrett-Joyner-Halenda method (Fig. 1e). The specific surface area and pore volume of ZFONTs were measured to be 57.42 m2⋅g−1 and 0.16 cm3⋅g−1 by Barrett-Emmett-Teller method, respectively. It is noting that the specific surface area of ZFONTs is much bigger than that of ZnFeO4 nanoparticles (16.09 m2⋅g−1) reported in the literature [28].
The experimental facts mentioned above suggest that ZFONTs not only possess tubular structure, but also have many nanopores on their wall, which result in their large specific surface area. ZFONTs may be likely to become an excellent adsorbent.
Optimization of experimental conditions
To achieve good analytical results, the following parameters were optimized: a sample pH of 8.0 (Fig. S1), 10 mg of ZFONTs as an adsorbent, 1.5 min as an extraction time, 1.0 mL of 0.5 mol⋅L−1 HNO3 as an eluent, and 1.0 min as an elution time. Relevant experimental steps, respective data, Tables and Figures were given in the Electronic Supplementary Material.
Study of memory effect
Memory effect as a significant parameter was investigated in DMSPE. The results revealed that all analytes retained on ZFONTs can be completely desorbed under the selected conditions, and no carryover is observed in the next analysis. The reason for this fact is that DMSPE allow a close contact between the sorbent and the eluent in the elution step, which favors the kinetics of elution.
Effect of sample volume
To explore its capability of extracting analytes at very low content levels from large volume of real sample, the effect of sample volume on the extraction of the analytes was investigated by different sample volumes ranging from 10 to 150 mL containing 6.0 ng of REEs. It is found from the results that 120 mL is the largest sample volume at which quantitative extraction of analytes was achieved. However, the recovery of analytes slightly decreased with further increase of sample volume to 150 mL. Thus, an enrichment factor of 120 was obtained by 1.0 mL of 0.5 mol⋅L−1 HNO3 as the elution solution.
Influence of diverse ions
In order to investigate the selectivity of ZFONTs for DMSPE of REEs, the effect of various interfering anions and cations, most probably present in biological and environmental samples, on the recoveries of analytes was examined. In this experiment, solutions containing 3.0 ng⋅mL−1 analytes and various amounts of the interfering ions were treated according to this procedure. The tolerance limit of coexisting ions is defined as the largest amount making the recovery of the analyte maintained in the range of 90–110%. It was found that 10,000 mg⋅L−1 Cl− and NO3−, 8000 mg⋅L−1 SO42−, SiO32− and PO43−, 10,000 mg⋅L−1 K+ and Na+, 5000 mg⋅L−1 Ca2+ and Mg2+, 10 mg⋅L−1 Al3+ and Fe3+ did not influence on recoveries of the analytes. Based on the experimental results, it can be concluded that this method has an excellent selectivity for the adsorption of REEs, and is suitable for the analysis of samples with complicated matrix.
Adsorption capacity
Adsorption capacity is an important parameter to evaluate the performance of sorbent. In this work, the adsorption capacity of REEs on ZFONTs was measured by the procedure reported in the literature [29]. 30 mL aliquots of sample solutions containing the analytes in the concentration range of 10–40 μg⋅mL−1 were preconcentrated and eluted under the selected conditions. The amount of the analytes adsorbed on ZFONTs (mg⋅g−1) at each concentration level was determined by this method. Breakthrough curves were plotted by concentration of analytes versus their amount adsorbed on per gram of adsorbent. The adsorption capacities for La, Pr, Eu, Gd, Ho and Yb calculated from the breakthrough curves were found to be 31.2, 27.6, 28.9, 26.1, 25.9 and 32.7 mg⋅g−1, respectively.
Analytical performance of method
The analytical performances of DMSPE using ZFONTs coupled with ICP-MS for the determination of trace REEs were evaluated under the optimum conditions, including precision, detection limits, and linear range of calibration. The detection limits (DLs), defined as the concentration of the analytes equal to 3 times the standard deviation for nine replicate detections of the blanks, were in the range of 0.01(Ho) - 0.75 (La). The relative standard deviations (RSDs) ranged from 2.5% (La) to 6.3% (Yb) (n = 9, c = 1.0 ng⋅mL−1). The linear range (LR) of calibration covered over four orders of magnitude with correlation coefficient (R2) higher than 0.9986. The relative data were listed in Table 1.
Table 2 gives the data of the analytical performances of this method and other methods reported in the literatures [30,31,32,33,34,35]. The results in Table 2 indicate that DLs of this method are lower than those reported in the literatures. The RSDs, LR and R2 are superior or similar to other methods. In addition, this method has a higher enrichment factor.
Validation and application of method
The accuracy and reliability of this method were examined by the determination of REEs in environmental water samples, including lake water and wastewater. The results were listed in Table S2. The recoveries for the spiked samples were in the range of 93–107%. To further validate this method, two certified reference materials of tea leaf (GBW 07605) and human hair (GBW 09101a) were also analyzed by this method (Table 3). The analytical results in Table 3 showed that the determined values agreed well with the certified values.
Conclusions
Magnetic ZnFe2O4 nanotubes (ZFONTs) were used as a new adsorbent for dispersive micro-solid phase extraction (DMSPE) of rare earth elements followed by ICP-MS detection. Compared with traditional SPE, DMSPE allow a close contact between the sorbent and the analytes in the preconcentration step, which greatly improves the extraction efficiency. Moreover, ZFONTs exhibit hollow tubular structure and many pores on their wall. This unique structure results in the great increase of specific surface area and adsorptive sites of ZFONTs. The mechanism for extraction of target ions mainly attributes to the different surface charges of ZFONTs at different pH values. Thus, ZFONTs have a great application potential in preconcentration, separation and analysis of metal ions, speciation of elements, non-metal ions and polar organic compounds. At the same time, it should be pointed out here that the structure of ZFONTs may be destroyed in harsh acidic or basic media. Thus, this material suffers from limited tolerance for an eluent with a high concentration level of strong acid or base.
References
Zhang S, Shan X (2001) Speciation of rare earth elements in soil and accumulation by wheat with rare earth fertilizer application. Environ Pollut 112:395
Chen S, Cheng X, He Y, Zhu S, Lu D (2013) Determination of the rare earth elements La, Eu, and Yb using solidified floating organic drop microextraction and electrothermal vaporization ICP-MS. Microchim Acta 180:1479
Premadas A (2012) Determination of trace level lanthanides and thorium by inductively coupled plasma atomic emission spectrometry in different types of geological, red mud, and coal fly ash samples after separation as oxalates using calcium as carrier. At Spectrosc 33(1):14
Buckingham S, Maheswaran J, Meehan B, Peverill K (1999) The role of applications of rare earth elements in enhancement of crop and pasture production. Mater Sci Forum 315:339
Aniwashi J, Kaleri HA, Sulaiman Y, Qifa L, Zhuang X (2011) Interactions between rare-earth ions and DNA of Bashibai sheep. Genet Mol Res 10:1075
Zhu W, Xu S, Shao P, Zhang H, Wu D, Yang W, Feng J (1997) Bioelectrical activity of the central nervous system among populations in a rare earth element area. Biol Trace Elem Res 57:71–77
Liang P, Fa W (2005) Determination of La, Eu and Yb in water samples by inductively coupled plasma atomic emission spectrometry after solid phase extraction of their 1-phenyl-3-methyl-4-benzoylpyrazol-5 one complexes on silica gel column. Microchim Acta 150(1):15
Zhu X, Lin J, Gao A, Wang D, Zhu M (2017) Simultaneous determination of rare earth elements in aqueous samples by online preconcentration system and inductively coupled plasma mass spectrometry. At Spectrosc 38(4):77
Hemmati M, Rajabi M, Asghari A (2018) Magnetic nanoparticle based solid-phase extraction of heavy metal ions: a review on recent advances. Microchim Acta 185: 160
Chen S, Yan J, Li J, Lu D (2017) Study on titanium dioxide: nanofibers as new solid phase extraction adsorbent for column preconcentration and separation of trace rare earth elements prior to their determination by ICP-MS. At Spectrosc 38(4):86
Dai H, Xiao D, He H, Li H, Yuan D, Zhang C (2015) Synthesis and analytical applications of molecularly imprinted polymers on the surface of carbon nanotubes: a review. Microchim Acta 182(5–6):893
Shirkhanloo H, Khaligh A, Mousavi HZ, Rashid A (2016) Ultrasound assisted-dispersive-micro-solid phase extraction based on bulky amino bimodal mesoporous silica nanoparticles for speciation of trace manganese (II)/(VII) ions in water samples. Microchem J 124:637
Pytlakowska K (2016) Dispersive micro solid-phase extraction of heavy metals as their complexes with 2-(5-bromo-2-pyridylazo)-5-diethylaminophenol using graphene oxide nanoparticles. Microchim Acta 183(1):91
Chen S, Zhu S, Lu D (2018) Dispersive micro-solid phase extraction coupled with dispersive liquid-liquid microextraction for speciation of antimony in environmental water samples by electrothermal vaporization ICP-MS. At Spectrosc 39(2):55
Krawczyk-Coda M (2017) Halloysite nanotubes as a solid sorbent in ultrasound-assisted dispersive micro solid-phase extraction for the determination of bismuth in water samples using high-resolution continuum source graphite-furnace atomic absorption spectrometry. Spectrochim Acta Part B 129:21
Soylak M, Erbas Z (2017) Magnetic solid phase extraction of trace lead and copper on chromotrope FB impregnated magnetic multiwalled carbon nanotubes from cigarette and hair samples for measurement by flame AAS. At Spectrosc 38(3):57
Martinez A, Vazquez S, Lara R, Martinez L, Pacheco P (2018) Selenium analysis by an integrated microwave digestion-needle trap device with hydride sorption on carbon nanotubes and electrothermal atomic absorption spectrometry determination. Spectrochim Acta Part B 140:22
Rahman M, Khan S, Marwani H, Asiri AA (2015) SnO2-Sb2O3 nanocomposite for selective adsorption of lead ions from water samples prior to their determination by ICP-OES. Microchim Acta 182(3–4):579
Yan J, Chen S, Li J, He Y, Lu D (2017) Study on the determination of trace lead and cadmium by ICP-MS after preconcentration and separation on carbon nanofibers loaded with 8-hydroxyquinoline. At Spectrosc 38(2):42
Mazaher A, Hatem E, Tayyebeh M, Mohamed AR (2017) Nanomaterials as sorbents for sample preparation in bioanalysis: A review. Anal Chim Acta 958:1
De la Calle I, Menta M, Seby F (2016) Current trends and challenges in sample preparation for metallic nanoparticles analysis in daily products and environmental samples: A review. Spectrochim Acta Part B 125:66
Erkan Y, Mustafa S (2013) Ionic liquid-linked dual magnetic microextraction of lead(II) from environmental samples prior to its micro-sampling flame atomic absorption spectrometric determination. Talanta 116:882
Fahimirad B, Asghari A, Rajabi M (2017) Magnetic graphitic carbon nitride nanoparticles covalently modified with an ethylenediamine for dispersive solid-phase extraction of lead(II) and cadmium(II) prior to their quantitation by FAAS. Microchim Acta 184(8):3027
Eshaghi Z, Bardajee GR, Azimi S (2016) Magnetic dispersive micro solid-phase extraction for trace mercury pre-concentration and determination in water, hemodialysis solution and fish samples. Microchem J 127:170
Jalilian N, Ebrahimzadeh H, Asgharinezhad AA (2018) Determination of acidic, basic and amphoteric drugs in biological fluids and wastewater after their simultaneous dispersive micro-solid phase extraction using multiwalled carbon nanotubes/magnetite nanoparticles@poly(2-aminopyrimidine) composite. Microchem J 143:337
Magoda C, Nomngongo PN, Mabuba N (2016) Magnetic iron-cobalt/silica nanocomposite as adsorbent in micro solid-phase extraction for preconcentration of arsenic in environmental samples. Microchem J 128:242
Rajabi M, Moghadam AG, Bari B, Asghari A (2016) Air-assisted dispersive micro-solid phase extraction of polycyclic aromatic hydrocarbons using a magnetic graphitic carbon nitride nanocomposite. Microchim Acta 183(4):1449
Yao L, Hou X, Hu S, Wang J, Li M, Su C, Tade M, Shao Z, Liu X (2014) Green synthesis of mesoporous ZnFe2O4/C composite microspheres as superior anode materials for lithium-ion batteries. J Power Sources 258:305
Maqieira A, Elmahadi HAM, Puchades R (1994) Immobilized cyanobacteria for on-line trace metal enrichment by flow injection atomic absorption spectrometry. Anal Chem 66:3632
Shaowei W, Man H, Bin H, Zucheng J (2007) Determination of trace rare earth elements in natural water by electrothermal vaporization ICP-MS with pivaloyltrifluoroacetone as chemical modifier. Microchim Acta 159:269
Evelina KV, Plamen AA, Violeta MS (2016) Study of 3-Ethylamino-but-2-enoic acid phenylamide as a new ligand for preconcentration of lanthanides from aqueous media by liquid-liquid extraction prior to ICP-MS analysis. Talanta 160:389
Yabing C, Beibei C, Man H, Xiaolan L, Hu B (2017) Gold nanoparticles as intermediate ligands for polymer monolithic capillary microextraction of trace rare earth elements followed by inductively coupled plasma mass spectrometry detection. Spectrochim Acta Part B 127:56
Zhang J, Wang X, Dong Y, Xu Z, Li G (2016) Solid phase extraction of rare earth elements in deep groundwater with multi-wall carbon nanotubes as adsorbent for the determination by inductively coupled plasma mass spectrometry. At Spectrosc 37(1):1
Celik I, Kara D, Karadas C, Fisher A, Hill S (2015) A novel ligandless-dispersive liquid-liquid microextraction method for matrix elimination and the preconcentration of rare earth elements from natural waters. Talanta 134:476
Shaowei S, Beibei C, Man H, Bin H, Zuowei X (2014) Determination of trace/ultratrace rare earth elements in environmental samples by ICP-MS after magnetic solid phase extraction with Fe3O4@SiO2@polyaniline–graphene oxide composite. Talanta 119:458
Acknowledgments
This work was supported by the Research and Innovation Initiatives of Wuhan Polytechnic University (No. 2018 J04) and Special Fund for Agro-scientific Research in the Public Interest (Project No. 201503135-22).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 75 kb)
Rights and permissions
About this article
Cite this article
Chen, S., Yan, J., Li, J. et al. Magnetic ZnFe2O4 nanotubes for dispersive micro solid-phase extraction of trace rare earth elements prior to their determination by ICP-MS. Microchim Acta 186, 228 (2019). https://doi.org/10.1007/s00604-019-3342-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-019-3342-8