Abstract
A nano-carbon paste electrode was modified with nitrogen doped graphene and applied to square wave anodic stripping voltammetric determination of lead(II) and cadmium(II). The modified electrode was investigated by cyclic voltammetry and electrochemical impedance spectroscopy. Key parameters affecting the performance of the sensor such as pH value, content of modifier, deposition potential and time were optimized. Under optimized conditions, the electrode exhibits linear response to lead (at about −0.56 V) and cadmium (at about −0.77 V) in the range between 10 pM to 1 nM, and the detection limits are 8.0 pM for cadmium(II) and 5.0 pM for lead(II), respectively. The sensitivity, selectivity and simplicity of the method are comparable to, or even better than those reported earlier.

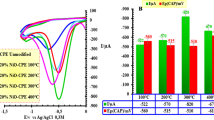
Schematic presentation showing that the modified CPE possesses much faster electron transfer ability. Charge transfer resistance values are shown for (a) a traditional carbon paste electrode (CPE); (b) a nano-carbon paste electrode; and (c) a nano-carbon paste electrode modified with nitrogen-doped graphene
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The heavy metals widely exist in the industrial processes of electroplating, batteries, papermaking and paint [1]. Lead (Pb2+) and cadmium (Cd2+) are toxic heavy metals, and their pollution is one of the serious environmental problems. Pb2+ and Cd2+ are non-biodegradability, and they can be accumulated in the human body during the long-term process, resulting in serious disorders to human organs [2, 3]. Thus, the trace quantification determination of Pb2+ and Cd2+ can provide important information for human health and environmental monitoring.
Some spectroscopic techniques have been utilized for the determination of lead and cadmium [4–6], those methods require specialized operator, sophisticated and expensive devices, and they are not appropriate for field measurement [7]. In contrast, electrochemical methods are proved to be easily operated, low-cost, rapid and portable [3, 8]. Initially, mercury based electrodes combined with anodic stripping voltammetry (ASV) are used for trace heavy metals analysis. The environmental friendly electrodes have gradually replaced the mercury-modified electrodes due to the high toxicity of mercury. They exhibit low toxicity, simple preparation, good sensitivity and selectivity. We used the chemically modified nano-carbon paste electrodes for the determination of Pb2+ and Cd2+.
Various green electrode modification materials are utilized for improving electrochemical performance. Graphene is a two-dimensional single atom thick of sp2-hybridized carbon material, and it exhibits excellent electrical conductivity, high mechanical strength, large surface area, and rapid heterogeneous electron transfer [9, 10]. Therefore, graphene plays an important role in the field of electrochemistry. Comparing with graphene, the properties of nitrogen-doped graphene (N-GE) have been further improved, including better electric conductivity and enhanced electrocatalytic activity [11, 12]. Nitrogen-doped nanomaterials have great potential for preparing the electrochemical sensors. For example, Shi et al. [13] prepared an enzymeless H2O2 sensor based on nitrogen-doped graphene nanoribbons modified electrode. The nano-modified electrode exhibited enhanced electron transfer ability, and possessed a large active surface and a large number of catalytically active sites that originated from the presence of nitrogen atoms. Giribabu et al. [14] utilized a nitrogen doped reduced graphene oxide modified glassy carbon electrode (GCE) for electrochemical sensing of 4-nitrophenol. Due to the electrocatalytic property of nitrogen doped reduced graphene oxide, the oxidation peak current of 4-nitrophenol improved significantly on the modified GCE compared to bare GCE. Tsierkezos et al. [15] fabricated pristine multi-walled carbon nanotubes (MWCNTs) and nitrogen-doped MWCNTs (N-MWCNTs) decorated with gold nanoparticles (AuNPs). N-MWCNTs/AuNPs exhibited better electrochemical response towards simultaneous oxidation of ascorbic acid (AA), uric acid (UA), and dopamine (DA) compared to that of MWCNTs/AuNPs. This was due to the presence of nitrogen that enhanced the electrocatalytic activity of MWCNTs.
We fabricated nano-carbon paste electrode using nano-graphite powder instead of common graphite powder. Compared with traditional carbon paste electrode (CPE), nano-carbon paste electrode (nano-CPE) revealed lower impedance and faster electron transfer. Then nitrogen-doped graphene was used to modify nano-carbon paste electrode for trace analysis of Pb2+ and Cd2+. The electrochemical properties of the modified electrodes were evaluated by cyclic voltammetry and electrochemical impedance spectroscopy. Due to large surface area and good electrocatalytic activity of nitrogen-doped graphene, the modified nano-carbon paste electrode exhibited a remarkably improved sensitivity for Pb2+ and Cd2+ using square wave anodic stripping voltammetry. The method was applied for the determination of Pb2+ and Cd2+ in real samples.
Experimental
Reagents and materials
Graphite powder (spectrographic grade), paraffin oil, lead nitrate and cadmium nitrate were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China, http://www.sinoreagent.com/). Nano-graphite powder (thickness: <40 nm, sheet size: about 400 nm), plain graphene and nitrogen-doped graphene (nitrogen content: 3.0 wt% ~ 5.0 wt%; surface area: >500 m2/g) were purchased from Nanjing XFNANO Materials Tech Co., Ltd. (Nanjing, China, http://www.xfnano.com/). Stock standard solutions of 2.5 mM Cd2+ and 2.5 mM Pb2+ were prepared by dissolution of corresponding nitrate salts and working standard solutions were obtained by appropriate dilution. 0.2 M acetate buffer with various pH values were used as the supporting electrolytes. All other reagents were of analytical grade, and were used without further purification.
Apparatus
All electrochemical measurements (except electrochemical impedance spectroscopy measurements) were performed using a CHI660E electrochemical workstation (Shanghai Chenhua Instruments Corporation, Shanghai, China, http://www.chinstr.com/). The electrochemical impedance spectroscopy (EIS) measurements were carried by a PARSTAT 4000 (Princeton Applied Research, America, http://www.par-solartron.com.cn/). A three electrode system was used containing a nitrogen doped graphene modified nano-carbon paste electrode (3.0 mm in diameter) as working electrode, a platinum wire as auxiliary electrode, and a saturated calomel electrode (SCE) as reference electrode. All potentials were reported with respect to the reference electrode. A KMO2 basic magnetic stirrer (IKA, Germany, http://www.ika.cn/) was used to stir the testing solution before measurements. All the experiments were carried out at room temperature.
Electrode preparation
The nitrogen doped graphene modified nano-carbon paste electrode was prepared by the following procedures: A certain proportion of nano-graphite powder, nitrogen doped graphene, and paraffin oil were mixed together in a mortar, then the mixture was packed firmly into the cavity of the teflon holder (3.0 mm in diameter) and the electrical contact was established via a copper wire. A new surface was obtained by smoothing the electrode onto a piece of weighing paper. The prepared electrode was denoted as N-GE/nano-CPE.
For comparison, traditional carbon paste electrode (CPE) was made up of common graphite powder and paraffin oils. Nano-carbon paste electrode (nano-CPE) was prepared using nano-graphite powder instead of common graphite powder. Plain graphene modified nano-carbon paste electrode (GE/nano-CPE) was prepared by mixing nano-graphite powder, plain graphene, and paraffin oil. When not in use, the modified electrodes were kept in room temperature with a cover to protect them from dust.
Analytical procedure
Electrochemical measurements were performed in 0.2 M acetate buffer containing various concentrations of Pb2+ and Cd2+ using square wave anodic stripping voltammetry (SWASV). Firstly, the pre-concentration step was carried out in a stirred solution at the potential of −1.0 V for 210 s. After a quiet time of 2 s, and the voltammograms were recorded from −1.0 V to −0.2 V with step potential 4 mV, pulse amplitude 30 mV and frequency 15 Hz. After each measurement the modified electrode was regenerated at potential of 0.0 V for 60 s in order to remove the residual deposits.
Results and discussion
Choice of materials
Carbon-based nanomaterials have widely applied in the construction of electrochemical sensors, such as multi-walled carbon nanotubes (MWCNTs), graphene (GE) and their derivatives. Compared to MWCNTs, graphene exhibits increased electron transfer ability and enlarged surface area. Nitrogen-doped graphene (N-GE) is derived from graphene. In comparison with graphene, enhancement of the electrocatalytic activity and the conductivity is found in N-GE due to the doping of nitrogen. This is because the doped nitrogen atoms can increase active sites for electrocatalysis. Nitrogen atom contains five valence electrons, and it can form strong valence bonds with carbon atoms. The distribution of electrons changes and free electrons are introduced when nitrogen is doped into the graphene structure [13]. Therefore, we chose the N-GE as modifier due to its superior performance. The results indicated that the current responses of Cd2+ and Pb2+ increased obviously on the N-GE/nano-CPE compared to those on the GE/nano-CPE.
Electrochemical characterization of of the modified electrode
Figure 1a shows cyclic voltammograms of different electrodes in 1 mM [Fe(CN)6]3−/4− solution containing 0.1 M KCl. As seen in the figure, the peak potential separations (ΔEp) were: CPE>nano-CPE>N-GE/nano-CPE, and the peak currents (Ip) were: CPE<nano-CPE<N-GE/nano-CPE. A pair of weak redox peaks with the largest ΔEp was observed on CPE (curve a) due to the poor conductivity of paraffin oil, indicating the slow electron transfer rate at the interface. By using the nano-CPE (curve b), well-defined and enhanced redox peaks with smaller ΔEp were obtained. This is ascribed to the nano-structure and excellent electrocatalytic activity of nano-graphite powder. Compared with CPE and nano-CPE, the highest peak currents and the smallest ΔEp were observed on N-GE/nano-CPE (curve c) due to the large surface area and good conductivity of N-GE.
Electrochemical impedance spectroscopy (EIS) was further utilized to investigate the properties of different modified electrodes. The typical impedance spectrum is made up of a semicircle portion at higher frequencies indicating electron transfer process, and a linear portion at lower frequencies corresponding to the diffusion process. The charge transfer resistance (Rct) value is calculated by measuring the diameter of the high-frequency semicircle in the Nyquist plots. In addition, an equivalent circuit consists of the ohmic resistance of the electrolyte (Rs), the charge transfer resistance (Rct), the Warburg impedance (Zw), and interfacial capacitance (Cdl). Figure 1b depicts the Nyquist diagrams of the different modified electrodes. By fitting the equivalent circuit (the inset of Fig. 1b), the Rct value at the CPE (curve a) was obtained as 39.28 ± 1.83 kΩ, indicating a very high electron transfer resistance. This is attributed to the poor conductivity of paraffin oil. The Rct value (19.66 ± 0.74 kΩ) of the nano-CPE (curve b) remarkably decreased. This indicates that the use of nano-CPE accelerates the electron transfer between redox probe and electrode. The Rct value (16.27 ± 0.67 kΩ) of N-GE/nano-CPE (curve c) further decreased due to the good conductivity and large surface area of N-GE. This exhibits that the N-GE modified electrode possesses excellent electron transport ability. The impedance changes of the modified electrode also indicated that N-GE was successfully modified on the surface of nano-CPE.
Electrode surface area
The effective surface areas of nano-CPE and N-GE/nano-CPE were investigated by cyclic voltammetry at different scan rates in 5 mM [Fe(CN)6]3−/4- solution containing 0.1 M KCl, and the results were shown in Fig. 2. When gradually increasing the scan rate from 25 to 200 mV s−1, the peak currents linearly increased with the square root of scan rate. For a reversible process, the function of anodic peak current (Ip, A) versus the square root of scan rate (ν, V s−1) can be expressed by Randles-Sevčik equation [16]:
where n is the electron transfer number, A is effective surface area of the electrode (cm2), C0 is the concentration for K3[Fe(CN)6] (mol L−1) and DR is the diffusion coefficient of K3[Fe(CN)6] (cm2 s−1). For K3[Fe(CN)6], n = 1, DR = 7.60 × 10−6 cm2 s−1. From the slope of the Ip-ν1/2 relation, the electrochemical active area of N-GE/nano-CPE and nano-CPE were calculated to be 0.073 ± 0.003 cm2 and 0.051 ± 0.002 cm2, respectively. This indicated that the active area of the N-GE/nano-CPE increased significantly and was about 1.4 times larger than that of the nano-CPE.
Electrochemical response of lead(II) and cadmium(II)
Figure 3 shows the square wave anodic stripping voltammograms of 1 μM Pb2+ and 1 μM Cd2+ on different electrodes. Two weak stripping peaks for Cd2+ (0.34 μA) and Pb2+ (3.27 μA) were observed on the CPE (Curve a). The response signals on the nano-CPE (Curve b) were nearly 35 times and 13 times larger for Cd2+ and Pb2+ than those obtained on the CPE, respectively. This exhibits that nano-CPE promotes the accumulation of Cd2+ and Pb2+ on the electrode surface and shows good electrocatalytic activities. Compared to nano-CPE, an enhancement of current responses of about 130 % and 42 % for Cd2+ and Pb2+ was obtained on the GE/nano-CPE (Curve c), respectively. The current responses of Cd2+ and Pb2+ further increased for about 26 % and 107 % on the N-GE/nano-CPE compared to those on GE/nano-CPE, respectively. This phenomenon can be explained by the following reasons: On one hand, the modifier N-GE with good conductivity can promote electron transfer of lead and cadmium, and more ions can be deposited on the surface of the electrode in shorter time; On the other hand, N-GE possesses large surface area, and the doped nitrogen atoms can provide more active sites for electrocatalysis of heavy metals, which improves the sensitivity of the modified electrode.
Optimization of method
The following parameters were optimized: (a) Sample pH value; (b) Amount of modifier; (c); Deposition time (d) Deposition potential. Respective data and Figures are given in the Electronic Supporting Material. The following experimental conditions were found to give best results: (a) A sample pH value of 5.0; (b) 2.5 % of N-GE in carbon paste; (c) Deposition time of 210 s; (d) Deposition potential of −1.0 V.
Determination of lead(II) and cadmium(II)
Figure 4 presents the square wave anodic stripping voltammograms of different concentrations of Cd2+ and Pb2+ in 0.2 M acetate buffer (pH 5.0) under the optimal conditions. Two sharp and well-defined stripping peaks occured at potentials of about −0.77 V for Cd2+ and about −0.56 V for Pb2+, respectively. The peak currents of Cd2+ and Pb2+ exhibited excellent linear dependence on their corresponding concentrations in the range from 1 × 10−11 M to 1 × 10−9 M, and the linear regression equations were: I(μA) = 41.8463 c (nM) + 7.1534 (r2 = 0.9920) for Pb2+ and I(μA) = 24.3859 c (nM) + 1.5889 (r2 = 0.9904) for Cd2+, respectively. The detection limits at signal to noise ratio of 3 were 5 × 10−12 M for Pb2+ and 8 × 10−12 M for Cd2+, respectively.
A comparison of the N-GE/nano-CPE with other reported modified electrodes [17–21] for the determination of Cd2+ and Pb2+ is listed in Table 1. The method exhibits comparative or even better linear range and detection limit. Moreover, the modified electrode has the advantages of simple preparation, ease of renewable surface, and convenient operation.
Reproducibility and stability
The reproducibility of the modified electrode was evaluated by repetitive measurement of 0.5 μM Pb2+ and 0.5 μM Cd2+. For one electrode, the relative standard deviation (R.S.D.) was 3.4 % for Cd2+ and 3.7 % for Pb2+ in six times measurements. For six different electrodes prepared in the same procedure, the R.S.D. was 4.1 % for Cd2+ and 4.5 % for Pb2+, respectively. The storage stability of the sensor was also studied. The electrode remained about 94.5 % and 95.3 % of the original responses for Cd2+ and Pb2+ after its storage at ambient conditions for three weeks. These results indicate that the sensor can be successfully applied to the simultaneous determination of heavy metal ions with excellent repeatability and stability.
Potentially interfering ions
The effect of various cations and anions on the simultaneous determination of Pb2+ and Cd2+ was investigated. The results exhibited that 1000-fold Na+, K+, Ca2+, Ba2+, Mg2+, Mn2+, Zn2+, Al3+, Fe3+, Cr3+, Cl−, NO3 −, SCN−, CO3 2−, SO4 2−, PO4 3− had no significant effect on the signals of Pb2+ and Cd2+ (tolerated ratios: ≤ ± 5.0 %). 100-fold Cu2+ was found to have a large influence on the stripping responses of target ions, peak currents of Pb2+ and Cd2+ decreased remarkably. This suppression effect was probably abscribed to the competition between copper and target ions on the electrode surface. The interference of copper can be eliminated by ferrocyanide in the form of a stable and insoluble copper-ferrocyanide complex [22]. In addition, 100-fold Hg2+ increased the stripping responses significantly. This is attributed to the formation of mercury film at the modified electrode surface, which promotes the reduction of Cd2+ and Pb2+ by formatting amalgam [23]. Therefore, most common ions did not interfere with the determination and the sensor exhibited good selectivity.
Analysis applications
To evaluate the feasibility of the modified electrode for practical analysis, the N-GE/nano-CPE was applied to detect Cd2+ and Pb2+ in real water samples. A certain volume of lake water samples were added in 0.2 M acetate buffer and no further sample treatment was done. Table 2 illustrates the results obtained for the water samples using standard addition method. The results are satisfying and indicate that this is an effective method for the simultaneously determination of Pb2+ and Cd2+ in practical samples.
Conclusions
Simultaneous determination of cadmium and lead at trace levels by SWASV was firstly carried out on the N-GE/nano-CPE with high sensitivity. The N-GE/nano-CPE exhibited better enrichment ability and excellent electron transfer for lead and cadmium due to large surface area and good conductivity of N-GE. The fabricated electrode had the advantages of simple preparation, ease of renewable surface, as well as low cost, which was expected to be applied in environmental monitoring.
References
Viyannalage LT, Bliznakov S, Dimitrov N (2008) Electrochemical method for quantitative determination of trace amounts of lead. Anal Chem 80:2042–2049
Nguyen PKQ, Lunsford SK (2013) Square wave anodic stripping voltammetric analysis of lead and cadmium utilizing titanium dioxide/zirconium dioxide carbon paste composite electrode. J Electroanal Chem 711:45–52
Wang ZQ, Wang H, Zhang ZH, Liu G (2014) Electrochemical determination of lead and cadmium in rice by adisposable bismuth/electrochemically reduced graphene/ionic liquidcomposite modified screen-printed electrode. Sensors Actuators B 199:7–14
Gasparik J, Vladarova D, Capcarova M, Smehyl P, Slamecka J, Garaj P, Stawarz R, Massanyi P (2010) Concentration of lead, cadmium, mercury and arsenic in leg skeletal muscles of three species of wild birds. J Environ Sci Health A 45:818–823
Pohl P (2009) Determination of metal content in honey by atomic absorption and emission spectrometries. TrAC Trends Anal Chem 28:117–128
Sen I, Shandil A, Shrivastava VS (2011) Study for determination of heavy metals in fish species of the river Yamuna (Delhi) by inductively coupled plasma-optical emission spectroscopy (ICP-OES). Adv Appl Sci Res 2:161–166
Kempegowda RG, Malingappa P (2012) A binderless, covalently bulk modified electrochemical sensor: application to simultaneous determination of lead and cadmium at trace level. Anal Chim Acta 728:9–17
Promphet N, Rattanarat P, Rangkupan R, Chailapakul O, Rodthongkum N (2015) An electrochemical sensor based ongraphene/polyaniline/polystyrene nanoporous fibers modifiedelectrode for simultaneous determination of lead and cadmium. Sensors Actuators B 207:526–534
Kuila T, Bose S, Khanra P, Mishra AK, Kim NH, Lee JH (2011) Recent advances in graphene-based biosensors. Biosens Bioelectron 26:4637–4648
Sookhakian M, Amin Y, Baradaran S, Tajabadi M, Golsheikh AM, Basirun W (2014) A layer-by-layer assembled graphene/zinc sulfide/polypyrrole thin-film electrode via electrophoretic deposition for solar cells. Thin Solid Films 552:204–211
Xu HY, Xiao JJ, Liu BH, Griveau S, Bedioui F (2015) Enhanced electrochemical sensing of thiols based on cobalt phthalo-cyanine immobilized on nitrogen-doped grapheme. Biosens Bioelectron 66:438–444
Tian Y, Wang FL, Liu YX, Pang F, Zhang X (2014) Green synthesis of silver nanoparticles on nitrogen-doped graphene for hydrogen peroxide detection. Electrochim Acta 146:646–653
Shi LB, Niu XH, Liu TT, Zhao HL, Lan MB (2015) Electrocatalytic sensing of hydrogen peroxide using a screen printed carbon electrode modified with nitrogen-doped graphene nanoribbons. Microchim Acta 182:2485–2493
Giribabu K, Suresh R, Manigandan R, Kumar SP, Muthamizh S, Munusamy S, Narayanan V (2014) Preparation of nitrogen-doped reduced graphene oxide and its use in a glassy carbon electrode for sensing 4-nitrophenol at nanomolar levels. Microchim Acta 181:1863–1870
Tsierkezos NG, Szroeder P, Ritter U (2014) Voltammetric study on pristine and nitrogen-doped multi-walled carbon nanotubes decorated with gold nanoparticles. Microchim Acta 181:329–337
Li YH, Zhai XR, Wang HB, Liu XS, Guo L, Ji XL, Wang L, Qiu HY, Liu XY (2015) Non-enzymatic sensing of uric acid using a carbon nanotube ionic-liquid paste electrode modified with poly(β-cyclodextrin). Microchim Acta 182:1877–1884
Lee PM, Chen Z, Li L, Liu E (2015) Reduced graphene oxide decorated with tin nanoparticles through electrodeposition for simultaneous determination of trace heavy metals. Electrochim Acta 174:207–214
Liu M, Feng YH, Wang GF, Fang B (2012) Determination of cadmium(II) using glassy carbon electrodes modified with cupferron, ß-naphthol, and multiwalled carbon nanotubes. Microchim Acta 177:221–228
Rutyna I, Korolczuk M (2014) Determination of lead and cadmium by anodic stripping voltammetryat bismuth film electrodes following double deposition andstripping steps. Sensors Actuators B 204:136–141
Tang L, Chen J, Zeng GM, Zhu Y, Zhang Y, Zhou YY, Xie X, Yang GD, Zhang S (2014) Ordered mesoporous carbon and thiolated polyaniline modified electrode for simultaneous determination of cadmium(II) and lead(II) by anodic stripping voltammetry. Electroanalysis 26:2283–2291
Buica GO, Ungureanu EM, Birzan L, Razus AC, Mandoc (Popescu) LR (2013) Voltammetric sensing of lead and cadmium using poly(4-azulen-1-yl-2,6-bis(2-thienyl)pyridine) complexing films. J Electroanal Chem 693:67–72
Ping JF, Wang YX, Wu J, Ying YB (2014) Development of an electrochemically reduced graphene oxide modified disposable bismuth film electrode and its application for stripping analysis of heavy metals in milk. Food Chem 151:65–71
Li YH, Liu XY, Zeng XD, Liu Y, Liu XT, Wei WZ, Luo SL (2009) Simultaneous determination of ultra-trace lead and cadmium at a hydroxyapatite-modified carbon ionic liquid electrode by square-wave stripping voltammetry. Sensors Actuators B 139:604–610
Acknowledgments
This research was supported by Ningxia Medical University Scientific Research Project (No. XM201415 and No. XZ2015002).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 76 kb)
Rights and permissions
About this article
Cite this article
Liu, X., Li, Z., Ding, R. et al. A nanocarbon paste electrode modified with nitrogen-doped graphene for square wave anodic stripping voltammetric determination of trace lead and cadmium. Microchim Acta 183, 709–714 (2016). https://doi.org/10.1007/s00604-015-1713-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-015-1713-3