Abstract
Purpose
Self-expandable metallic stent (SEMS) placement is widely used as a bridge to surgery (BTS) procedure for obstructive colorectal cancer. However, evidence regarding the optimal interval between SEMS placement and elective surgery is lacking.
Methods
We retrospectively collected data from patients with BTS between January 2013 and October 2021. Inverse probability treatment-weighted propensity score analyses were used to compare short- and long-term outcomes between the short-interval (SI) and long-interval (LI) groups, using a cutoff of 20 days.
Results
In total, 138 patients were enrolled in this study (SI group, n = 63; LI group, n = 75). In the matched cohort, the patients’ backgrounds were well balanced. The incidence of Clavien–Dindo grade ≥ II postoperative complications was not significantly different between the SI and LI groups (19.0% vs. 14.0%, P = 0.47). There were no significant differences between the SI and LI groups in the 3-year recurrence-free survival (68.0% vs. 76.4%, P = 0.73) or 3-year overall survival rates (86.0% vs. 90.6%, P = 0.72).
Conclusions
A longer interval did not deteriorate the oncological outcomes. Individual perioperative management with an appropriate interval to improve the patient’s condition is required to ensure safe surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obstructive colorectal cancer (OCRC) accounts for 8–34% of colorectal cancer (CRC) [1,2,3]. Although these conditions require immediate intervention, emergency one-stage resection for OCRC is associated with significantly higher mortality and morbidity rates than elective surgery [4, 5].
The placement of a self-expandable metallic stent (SEMS) as a bridge to surgery (BTS) procedure, followed by elective surgery, has been introduced as an alternative to emergency surgery [6]. While this BTS procedure has demonstrated improved short-term outcomes, such as primary anastomosis rates, stoma construction rates, and postoperative morbidity rates, its effects on long-term outcomes remain unclarified [7,8,9,10,11].
The BTS strategy for left-sided OCRC was recently recommended in the updated version of the guidelines from the European Society of Gastrointestinal Endoscopy (ESGE) in 2020 [12]. The ESGE guidelines recommend an interval of approximately 2 weeks between SEMS placement and surgery; however, the level of evidence for this recommendation is low [12]. A longer interval is reported to contribute to decreased postoperative complications owing to better intestinal decompression and improvement of the patients’ general condition [13,14,15,16]. In contrast, some studies have warned that a longer interval is associated with the deterioration of long-term outcomes [17,18,19]. However, few studies have investigated the optimal BTS interval in terms of short- and long-term outcomes, and no definitive conclusions have been reached.
The present study investigated the association between the interval between SEMS placement and surgery and long-term outcomes in patients with OCRC. The primary endpoints were the 3-year recurrence-free survival (RFS) and 3-year overall survival (OS) rates, and the secondary endpoint was the postoperative complication rate.
Materials and methods
This multicenter, retrospective study was conducted in accordance with the principles of the Declaration of Helsinki. The study protocol was approved by the Ethics Review Committee of Nippon Medical School (approval number: R1-07-1107). The requirement for written informed consent was waived owing to the retrospective nature of this study.
Patients and protocol
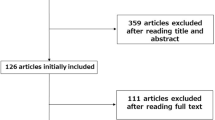
We retrospectively collected and analyzed the data of patients who underwent curative tumor resection for OCRC after SEMS placement between January 2013 and October 2021 at three Departments of Surgery of Nippon Medical School Hospitals (Main, Chiba Hokusoh, and Musashikosugi). Patients were excluded if they showed signs of peritonitis, perforation, or other serious complications necessitating emergency surgery. Patients with benign disease, positive surgical margins, invasive malignancies other than primary CRC, or SEMS placement with a palliative intent were also excluded. None of the patients received neoadjuvant therapy, including chemotherapy or radiotherapy. SEMS placement was considered contraindicated in patients with rectal cancer < 5 cm from the anal verge, extremely long or multiple stenoses, or intestinal perforation. OCRC was diagnosed based on the following symptoms and findings: (1) abdominal pain, fullness, vomiting, and constipation; (2) contrast-enhanced computed tomography findings of colonic dilatation caused by obstructive primary CRC; and (3) endoscopic findings of obstructive primary colorectal tumor. The severity of obstruction was classified using the colorectal obstruction scoring system (CROSS) [20, 21]. A total of 138 patients with pathological stage (pStage) II/III/IV disease were enrolled in this study. Tumors were classified according to the American Joint Committee on Cancer TNM staging system. Nine patients diagnosed with pStage IV disease had synchronous liver metastasis or peritoneal dissemination, which was resected simultaneously with the primary lesion.
Endoscopic stenting procedure and perioperative management
SEMS placement was performed by endoscopists under fluoroscopic and endoscopic guidance in accordance with the mini-guidelines published on the website of the Japan Colonic Stent Safe Procedure Research Group (http://colon-stent.com/). The implanted SEMS were the WallFlex™ colonic stent (Boston Scientific, Marlborough, MA, USA), Niti-S™ colonic stent (Taewoong Medical Inc., Gimpo-si, Korea), JENTLLY colonic stent (Japan Lifeline, Chiba, Japan), and HANAROSTENT® Naturfit colonic stent (Boston Scientific, Marlborough, MA, USA), all of which were uncovered. A guidewire was then passed through the malignant stenosis. The SEMS was deployed over the wire and through the scope without preprocedural balloon dilatation of colonic obstruction. The type, size, and diameter of the SEMS were selected by the endoscopist performing the procedure.
We defined technical and clinical success as proper placement of the SEMS and relief of obstruction without complications until elective surgery, respectively. In this study, we excluded cases of technical failure.
After SEMS insertion, improvement in the obstruction was monitored based on abdominal symptoms and abdominal radiography. If improvement of the obstruction was confirmed, oral intake of magnesium oxide agents was initiated. The baseline clinical and surgical variables and short- and long-term outcomes were retrospectively collected. The timing of elective surgery after SEMS placement was determined by a physician. The surgical approach (open or laparoscopic) and extent of resection were determined based on patient factors. The surgeon attempted to perform single-stage surgery with primary anastomosis. However, if this was not feasible, a diversion method with primary anastomosis was implemented.
Postoperative follow-up
All patients were followed-up every 3 months during the first 2 years postoperatively and every 6 months thereafter. Tumor markers, including carcinoembryonic antigen and carbohydrate antigen 19-9, were examined at each follow-up visit, and computed tomography of the chest and abdomen was performed every 6 months. In accordance with Japanese guidelines, total colonoscopy was performed in the first and third postoperative years [22]. The basic policy of the participating institutions was that adjuvant chemotherapy should be offered to patients treated with a BTS procedure, regardless of their pStage. However, adjuvant chemotherapy was not administered when treatment of other comorbidities was considered a priority or when chemotherapy was considered difficult owing to patient background characteristics, such as recovery from postoperative complications, a poor performance status, a poor organ function, and advanced age.
Determination of cutoff values for the BTS interval
To compare the outcomes of short and long intervals between SEMS placement and surgery, the cutoff was defined by the operating characteristic (ROC) curve, which is the plot of sensitivity versus 1-specificity and the area under the curve [23]. Various opinions exist regarding the interval cutoff, and determining the optimal cutoff is currently difficult. Almost all previous studies investigating this clinical question set the cutoff without a scientific basis, such as the median value and division into three parts. Given that the oncological outcome was defined as the primary outcome in this study, we determined the cutoff based on the long-term outcome, defining the value using the ROC curve based on the RFS in this study. The ROC curve demonstrated that the optimal cutoff interval for RFS events was 20 days, with an area under the curve of 0.512. Although this ROC curve was close to a 45° straight line and was not statistically significant, we conducted analyses to determine the cutoff based on the RFS.
Data analyses
Continuous data are expressed as the mean or median ± standard deviation. Background and perioperative variables were collected from medical charts. Postoperative complications were defined as those that occurred within 30 days after surgery and were evaluated using the Clavien–Dindo (CD) classification system [24]. All statistical analyses were performed using R Commander and EZR [25], which is a modified version of R Commander. The χ2 test was performed to compare frequencies between the groups. For continuous variables, differences between groups were compared using Student’s t test. If the data did not show a normal distribution, the Mann–Whitney U test was used. The Kaplan–Meier method was used to estimate the OS and RFS, and the log-rank test was used to compare survival curves. Statistical significance was set at P < 0.05. Owing to the infeasibility of performing a randomized controlled trial due to the small sample size, inverse probability treatment-weighted (IPTW) propensity score analyses that mimic pseudo-randomized cohorts were used to improve the degree of comparability and reduce bias due to confounding variables between the long-interval (LI) and short-interval (SI) groups [26]. The IPTW method was used to generate a pseudo-population with well-adjusted covariate combinations between the groups, stabilizing the weights between the participants without losing participant strength during matching [27]. This method can alleviate the overadjustment of bias toward the control group without decreasing the sample size. Considering the limited sample size of this study, we applied the IPTW method rather than conventional propensity score matching, which has the disadvantage of a considerable decrease in the sample size.
To estimate propensity scores, a multivariable logistic regression model was fitted using a generalized estimating equation model adjusted for age, sex, body mass index, American Society of Anesthesiologists physical status, Charlson Comorbidity Index score, blood transfusion before surgery, CROSS score before SEMS insertion, SEMS size, tumor location, synchronous CRC, pStage, and pathological features of the tumor, namely poor differentiation, T4 depth of invasion, lymphatic invasion, perineural invasion, and venous invasion.
Univariate and multivariate analyses using Cox proportional hazards models of variables with a P value of < 0.05 in the univariate analysis and those previously reported to affect oncological outcomes were performed to examine the association between the selected variables and the RFS or OS.
Results
A total of 138 patients were enrolled in this study. The distribution of the interval between SEMS placement and surgery for 7 days in all included patients is shown in Fig. 1. The median interval was 15–22 (range: 1–148) days. The patients were divided into two groups: the SI group, who underwent curative surgery ≤ 20 days after SEMS insertion (n = 63), and the LI group, who underwent curative surgery ≥ 21 days after SEMS placement (n = 75).
The preoperative characteristics of the two groups before and after matching are presented in Table 1. There were no marked differences in the patient characteristics between the two groups after matching. The interval between SEMS placement and surgery was longer in the LI group than in the SI group (34.6 ± 20.3 days vs. 14.4 ± 4.0 days, P < 0.01). The clinical success rates of SEMS placement were 97.0% and 89.0% in the SI and LI groups, respectively (P = 0.15). The SEMS-related complication rate was higher in the LI group than in the SI group (4.2% vs. 13.3%, P = 0.04). Emergency surgery was performed for two patients in the SI group due to perforation and fecal impaction and for one patient in the LI group due to bleeding.
The perioperative and pathological outcomes are summarized in Table 2. All patients underwent curative resection. In the matched cohort, surgical outcomes were not significantly different between the SI and LI groups. The incidence of CD grade ≥ II postoperative complications was not significantly different between the SI and LI groups (19.0% vs. 14.0%, P = 0.47). No 30-day mortality was observed in either group. The length of the overall hospital stay was not significantly different between the SI and LI groups. The median follow-up period was significantly longer in the SI group than the LI group (1123.2 ± 643.0 vs. 879.6 ± 524.5 days, P = 0.02).
The SI and LI groups in the unadjusted cohort showed no significant differences in the 3-year RFS (69.6% vs. 78.0%, P = 0.71) and 3-year OS rates (88.6% vs. 90.2%, P = 0.60) (Fig. 2). There were also no significant differences between the SI and LI groups in the adjusted cohort regarding the 3-year RFS (68.0% vs. 76.4%, P = 0.73) and 3-year OS (86.0% vs. 90.6%, P = 0.72) (Fig. 3).
Prognostic factors for the RFS were evaluated using univariate and multivariate Cox proportional hazards models in the adjusted cohort using IPTW with propensity scores. Among the variables with a P value of < 0.05, in the univariate analyses of outcome-related factors, the independent risk factors for a poor RFS were a Charlson Comorbidity Index (hazard ratio [HR] 1.25, 95% confidence interval [CI], 1.029–1.508, P = 0.02), postoperative complications of CD grade ≥ II (HR 3.10, 95% CI, 1.379–6.986, P < 0.01), pT4 positivity (HR 4.90, 95% CI, 2.006–11.96, P < 0.01), venous invasion (HR 4.47, 95% CI, 1.279–15.65, P = 0.02), and the absence of adjuvant chemotherapy (HR 2.87, 95% CI, 1.350–6.093; P < 0.01) (Table 3). Similarly, prognostic factors for the OS were evaluated. The variables identified as independent risk factors for a poor OS in the multivariate analysis were a Charlson Comorbidity Index (HR 1.36, 95% CI, 1.041–1.766; P = 0.02), an interval from SEMS placement to the first meal (HR 1.06, 95% CI, 1.014–1.117, P = 0.01), pT4 positivity (HR 5.91, 95% CI, 1.734–20.14, P < 0.01), and the absence of adjuvant chemotherapy (HR 8.88, 95% CI, 2.851–27.63, P < 0.01) (Table 4).
Discussion
This study aimed to investigate the association between the interval from SEMS placement to elective surgery and long-term outcomes in patients with OCRC. These results demonstrated that a longer BTS interval did not worsen the RFS or OS.
SEMS placement for OCRC was first introduced in 1990 for palliation and has been used as a BTS procedure since 1993 [6, 28]. Since then, SEMS placement as a BTS procedure has rapidly spread as an alternative to emergency surgery for OCRC, and numerous studies on the subject have been published [8,9,10, 29]. There is some concern that SEMS expansion could induce mechanical compression, which alters the biological malignant potential and subsequently worsens the prognosis [30]. However, a summary of the reports to date showed that BTS using SEMS placement improved short-term outcomes and did not worsen long-term outcomes compared to emergency surgery [31]. Thus, the abovementioned findings were reflected in the updated version of the ESGE guidelines for 2020 [12].
There is no consensus regarding the optimal BTS interval for OCRC. SEMS placement with an appropriate BTS interval is a potentially effective treatment strategy for OCRC. However, a longer interval might increase the complications associated with SEMS and worsen the oncological outcomes. Previous studies have applied various cutoff values for the BTS interval (Table 5). Broholm et al. [17] divided patients into two groups using a cutoff of 18 days, which was the median interval from SEMS placement to surgery in their study. Kye et al. [18] divided patients into weekly segments, and Veld et al. [16] divided patients according to the 2014 and 2020 ESGE guidelines [12]. Broholm et al. [17] reported a significantly increased risk of recurrence in the group with an interval of > 18 days from SEMS placement to elective surgery (odds ratio 5.1, 95% CI, 1.6–15.8, P = 0.005). Furthermore, Sato et al. [19] suggested that elective surgery within 16 days after SEMS placement might balance short-term benefits and long-term oncologic risks. In contrast, Ho et al. [32] reported no marked differences in the OS and recurrence between the early and delayed surgery groups (> 4 weeks) of patients managed with BTS SEMS placement for OCRC. Other reports demonstrated that surgical conditions for elective resection improve at intervals of up to 4 weeks after SEMS placement, with an optimal interval of approximately 2–4 weeks [16, 33]. In the present study, there were no significant differences in short- and long-term outcomes between patients who underwent curative surgery within 20 days versus 21 or more days after SEMS placement.
In the present study, a Charlson Comorbidity Index, postoperative complications, pT4 stage, and the absence of adjuvant chemotherapy were independent risk factors for a poor RFS and OS. A great benefit of a longer interval from SEMS placement to elective surgery is a reduction in short-term postoperative complications, which is attributed to an improvement in the patients’ general and intestinal conditions. Although the difference was not statistically significant, the LI group had a lower rate of postoperative complications than the SI group in the present study. In addition, our previous study demonstrated that an interval of > 15 days is recommended to minimize postoperative complications [15]. The negative oncological impact of postoperative complications following CRC surgery has been consistently reported. The potential mechanisms are as follows: 1) local and systemic activation of proinflammatory cytokines and mediators, which promote micrometastasis; 2) delayed and canceled adjuvant chemotherapy; and 3) abdominal implantation of intraluminal cancer cells in patients with anastomotic leakage [34,35,36,37,38]. Recently, we demonstrated a consistent and more powerful negative oncological impact of postoperative complications in a cohort treated using the BTS strategy than in a cohort without SEMS placement [39]. Taken together, these findings suggest that perioperative management should be conducted to avoid postoperative complications and administer adjuvant chemotherapy appropriately, rather than attempting to perform high-risk surgery within a short interval after SEMS placement.
The interval between SEMS placement and curative tumor resection may be extended because of the patients’ condition. In our cohort, the LI group included patients with emergency admission due to heart failure, pneumonia, or cerebrovascular disease who were diagnosed with OCRC and underwent SEMS insertion. These patients required time to sufficiently improve their condition to enable them to tolerate surgery. Interestingly, in the present study, it was not the interval between SEMS placement and elective surgery, but the interval between SEMS placement and the first meal was found to be an independent risk factor for a poor OS. Patients who have difficulty eating despite adequate intestinal decompression probably do not eat due to other factors, such as poor respiratory conditions and dysphagia. Clinicians should aim to improve the preoperative nutritional status of patients with OCRC during an adequate interval between SEMS placement and surgery, as preoperative malnutrition worsens the prognosis of patients with cancer [40, 41].
However, the need for adjuvant chemotherapy for OCRC remains controversial. In the present study protocol, because of the high rate of pathological T4 invasion in patients with OCRC, adjuvant chemotherapy was administered in all cases, except when treatment of other comorbidities was a priority or when the patient’s background characteristics made it difficult [42]. However, chemotherapy was administered to only 58% of patients. Han et al. [43] reported that SEMS placement followed by neoadjuvant chemotherapy prior to elective surgery appears to be safe and well tolerated in patients with OCRC. New strategies are emerging that guarantee oncologic safety while taking advantage of the long interval from SEMS placement to elective surgery.
Several limitations associated with the present study warrant mention. First, it was a retrospective study with a small number of patients. Therefore, the statistical power might have been insufficient, especially for survival analyses. To minimize this limitation, we applied the IPTW matching method instead of conventional propensity score matching because the IPTW data are closer to the clinical data. The IPTW matching method alleviated the overadjustment of bias toward the control group without decreasing the sample size. To our knowledge, this is the first study of the optimal interval of SEMS placement as a BTS procedure in OCRC to be conducted using an IPTW propensity score-matched multicenter design. Second, the follow-up period was relatively short.
In conclusion, a longer interval between SEMS placement and elective surgery did not deteriorate oncological outcomes. Therefore, individual perioperative management with neither an excessive nor an insufficient interval between SEMS placement and surgery should be conducted to improve the condition of each patient with OCRC and to increase the safety of surgery.
References
Carraro PGS, Segala M, Cesana BM, Tiberio G. Obstructing colonic cancer: failure and survival patterns over a 10 year follow up after one stage curative surgery. Dis Colon Rectum. 2001;44:243–50.
De Salvo GL, Gava C, Pucciarelli M. Curative surgery for obstruction from primary left colorectal carcinoma: primary or staged resection? Cochrane Database Syst Rev. 2004;2015(2):CD002101.
Manceau G, Voron T, Mege D, Bridoux V, Lakkis Z, Venara A, AFC (French Surgical Association) Working Group, et al. Prognostic factors and patterns of recurrence after emergency management for obstructing colon cancer: multivariate analysis from a series of 2120 patients. Langenbecks Arch Surg. 2019;404:717–29.
Saida Y, Sumiyama Y, Nagao J, Uramatsu M. Long-term prognosis of preoperative “bridge to surgery” expandable metallic stent insertion for obstructive colorectal cancer: comparison with emergency operation. Dis Colon Rectum. 2003;46:S44–9.
Amelung FJ, Draaisma A, Consten ECJ, Siersema PD, ter Borg F. Self-expandable metal stent placement versus emergency resection for malignant proximal colon obstructions. Surg Endosc. 2017;31:4532–41.
Dohmoto M, Rupp KD, Hohlbach G. Endoscopicallyimplanted prosthesis in rectal carcinoma. Dtsch Med Wochenschr. 1990;115:915.
Yang SY, Park YY, Han YD, Cho MS, Hur H, Min BS, et al. Oncologic outcomes of self-expandable metallic stent as a bridge to surgery and safety and feasibility of minimally invasive surgery for acute malignant colonic obstruction. Ann Surg Oncol. 2019;26:2787–96.
Matsuda A, Miyashita M, Matsumoto S, Matsutani T, Sakurazawa N, Takahashi G, et al. Comparison of long-term outcomes of colonic stent as “bridge to surgery” and emergency surgery for malignant large-bowel obstruction: a meta-analysis. Ann Surg Oncol. 2015;22:497–504.
Erichsen R, Horváth-Puhó E, Jacobsen JB, Nilsson T, Baron JA, Sørensen HT. Long-term mortality and recurrence after colorectal cancer surgery with preoperative stenting: a Danish nationwide cohort study. Endoscopy. 2015;47(6):517–24.
Allievi N, Ceresoli M, Fugazzola P, Montori G, Coccolini F, Ansaloni L. Endoscopic stenting as bridge to surgery versus emergency resection for left-sided malignant colorectal obstruction: an updated meta-analysis. Int J Surg Oncol. 2017;2017:2863272.
Matsuda A, Yamada T, Ohta R, Sonoda H, Shinji S, Iwai T, et al. Surgical site infections in gastroenterological surgery. J Nippon Med Sch. 2023;90:2–10.
van Hooft JE, Veld JV, Arnold D, Beets-Tan RGH, Everett S, Götz M, et al. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Guideline-Update 2020. Endoscopy. 2020;52:389–407.
Lee GJ, Kim HJ, Baek JH, Lee WS, Kwon KA. Comparison of short-term outcomes after elective surgery following endoscopic stent insertion and emergency surgery for obstructive colorectal cancer. Int J Surg. 2013;11(6):442–6.
Gianotti L, Tamini N, Nespoli L, Rota M, Bolzonaro E, Frego R, et al. A prospective evaluation of short-term and long-term results from colonic stenting for palliation or as a bridge to elective operation versus immediate surgery for large-bowel obstruction. Surg Endosc. 2013;27:832–42.
Matsuda A, Miyashita M, Matsumoto S, Sakurazawa N, Kawano Y, Yamada T, et al. Optimal interval from placement of a self-expandable metallic stent to surgery in patients with malignant large bowel obstruction: a preliminary study. Surg Laparosc Endosc Percutan Tech. 2018;28:239–44.
Veld JV, Kumcu A, Amelung FJ, Borstlap WAA, Consten ECJ, Dekker JWT, Dutch Snapshot Research Group, et al. Time interval between self-expandable metal stent placement or creation of a decompressing stoma and elective resection of left-sided obstructive colon cancer. Endoscopy. 2021;53:905–13.
Broholm M, Kobborg M, Frostberg E, Jeppesen M, Gögenür I. Delay of surgery after stent placement for resectable malignant colorectal obstruction is associated with higher risk of recurrence. Int J Colorectal Dis. 2017;32:513–6.
Kye BH, Kim JH, Kim HJ, Lee YS, Lee IK, Kang WK, et al. The optimal time interval between the placement of self-expandable metallic stent and elective surgery in patients with obstructive colon cancer. Sci Rep. 2020;10:9502.
Sato R, Oikawa M, Kakita T, Okada T, Abe T, Yazawa T, et al. A longer interval after stenting compromises the short- and long-term outcomes after curative surgery for obstructive colorectal cancer. Surg Today. 2022;52:681–9.
Saito S, Yoshida S, Isayama H, Matsuzawa T, Kuwai T, Maetani I, et al. A prospective multicenter study on self-expandable metallic stents as a bridge to surgery for malignant colorectal obstruction in Japan: efficacy and safety in 312 patients. Surg Endosc. 2016;30:3976–86.
Suzuki M, Okada K, Koyama N, Yamashita N, Yamagishi A, Yamada T, et al. Usefulness of a colonic stent for colonic obstruction caused by lung cancer metastasis. J Nippon Med Sch. 2021;88:556–60.
Hashiguchi Y, Muro K, Saito Y, Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2020;25:1–42.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36.
Dindo D. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013;48:452–8.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–79.
Cole SR, Hernán MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed. 2004;75:45–9.
Cwikiel W, Andrén-Sandberg A. Malignant stricture with colovesical fistula: stent insertion in the colon. Radiology. 1993;186:563–4.
Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, et al. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol. 2013;22:14–21.
Takahashi G, Yamada T, Iwai T, Takeda K, Koizumi M, Shinji S, et al. Oncological assessment of stent placement for obstructive colorectal cancer from circulating cell-free DNA and circulating tumor DNA dynamics. Ann Surg Oncol. 2018;25:737–44.
Saida Y. Current status of colonic stent for obstructive colorectal cancer in Japan; a review of the literature. J Anus Rectum Colon. 2019;3:99–105.
Ho MF, Futaba K, Chu S, Hon SSF, Ng SSM. Delaying surgery for optimization after colonic stent bridging is safe for left-sided malignant large bowel obstruction: result from 10-year experience and risks factor analysis. Surg Oncol. 2023;47: 101918.
de Roos MAJ, Hugen N, Hazebroek EJ, Bilgen EJS. Delayed surgical resection of primary left-sided obstructing colon cancer is associated with improved short- and long-term outcomes. J Surg Oncol. 2021;124:1146–53.
Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg. 2015;261:497–505.
Law WL, Choi HK, Lee YM, Ho JW. The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol. 2007;14:2559–66.
Alonso S, Pascual M, Salvans S, Mayol X, Mojal S, Gil MJ, et al. Postoperative intra-abdominal infection and colorectal cancer recurrence: a prospective matched cohort study of inflammatory and angiogenic responses as mechanisms involved in this association. Eur J Surg Oncol. 2015;41:208–14.
Salvans S, Mayol X, Alonso S, Messeguer R, Pascual M, Mojal S, et al. Postoperative peritoneal infection enhances migration and invasion capacities of tumor cells in vitro: an insight into the association between anastomotic leak and recurrence after surgery for colorectal cancer. Ann Surg. 2014;260:939–43; discussion 943–4.
Tevis SE, Kohlnhofer BM, Stringfield S, Foley EF, Harms BA, Heise CP, et al. Postoperative complications in patients with rectal cancer are associated with delays in chemotherapy that lead to worse disease-free and overall survival. Dis Colon Rectum. 2013;56:1339–48.
Matsuda A, Yamada T, Takahashi G, Matsumoto S, Yokoyama Y, Sonoda H, et al. Postoperative infectious complications have a negative oncological impact in patients after stent placement with malignant large bowel obstruction. Int J Colorectal Dis. 2023;38:2.
Choi MH, Oh SN, Lee IK, Oh ST, Won DD. Sarcopenia is negatively associated with long-term outcomes in locally advanced rectal cancer. J Cachexia Sarcopenia Muscle. 2018;9:53–9.
Kanazawa Y, Yamada T, Kakinuma D, Matsuno K, Ando F, Fujita I, et al. Skeletal muscle mass depletion after gastrectomy negatively affects the prognosis of patients with gastric cancer. Anticancer Res. 2020;40:4271–9.
Sonoda H, Yamada T, Matsuda A, Yokoyama Y, Ohta R, Shinji S, et al. The T-CEA score: a useful prognostic indicator based on postoperative CEA and pathological T4 levels for patients with stage II-III colorectal cancer. Surg Today. 2023;53:890–8.
Han JG, Wang ZJ, Zeng WG, Wang YB, Wei GH, Zhai ZW, et al. Efficacy and safety of self-expanding metallic stent placement followed by neoadjuvant chemotherapy and scheduled surgery for treatment of obstructing left-sided colonic cancer. BMC Cancer. 2020;20:57.
Acknowledgements
We thank Kelly Zammit, BVSc, from Edanz (https://jp.edanz.com/ac) for editing this manuscript.
Funding
This study received no funding.
Author information
Authors and Affiliations
Contributions
All authors agree with the content of the manuscript. Authors’ contributions are study concept and design; AM and TY, acquisition of data; YY, SM, GT, HS, RO, KU, SS, TI, KT, KS, SK, and TM; analysis and interpretation of data; KS, AM, and YT, drafting of the manuscript; KS and AM, study supervision; TY and HY.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest for this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kanaka, S., Matsuda, A., Yamada, T. et al. Oncologic investigation of the interval from stent placement to surgery in patients with obstructive colorectal cancer. Surg Today 54, 1093–1103 (2024). https://doi.org/10.1007/s00595-024-02818-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-024-02818-w