Abstract
Purpose
To investigate a prognostic score for stage II–III colorectal cancer (CRC) based on post-CEA and pT4 levels.
Methods
Two cohorts of stage II–III CRC patients who underwent curative surgery between 2011 and 2017 were included. The prognostic score (T-CEA score) was calculated as follows: T-CEA-0, post-CEA ≤ 5 ng/mL and pT1–3; T-CEA-1, post-CEA > 5 ng/mL or pT4; T-CEA-2, post-CEA > 5 ng/mL and pT4.
Results
The T-CEA scores of the 587 patients were as follows: T-CEA-0 (n = 436; 74%), T-CEA-1 (n = 129; 22%), and T-CEA-2 (n = 10; 2%). The 5-year recurrence-free survival (RFS) rates of the T-CEA-0, 1, and 2 groups were 80.3%, 54.8%, and 0%, respectively (P < 0.01), and the 5-year overall survival (OS) rates were 90.9%, 74.2%, and 0%, respectively (T-CEA-0 vs T-CEA-1: P < 0.01, T-CEA-1 vs T-CEA-2: P = 0.04). Multivariate analysis revealed that an elevated T-CEA score of 1 or 2 was a significant risk factor for poor RFS (HR: 2.89, P < 0.01) and OS (HR: 2.85, P < 0.01).
Conclusion
The T-CEA score is a reliable and convenient prognostic score for stage II–III CRC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Recurrence is likely to develop in patients with stage II or III colorectal cancer (CRC) (15% and 30%, respectively) even if they have undergone curative surgery followed by adjuvant chemotherapy [1]. This means staging based on tumor–node–metastasis (TNM) classification is not sufficient for a risk assessment of recurrence of CRC.
Various prognostic markers using molecular techniques such as VCAN [2] and KRT17 [3] have been reported; however, these markers are not readily available in daily practice. In Western countries [4, 5] and in Japan [6], nomograms using common clinicopathologic factors for predicting survival and recurrence after curative colonic cancer resection have been developed. These nomograms used preoperative CEA (pre-CEA); however, we subsequently reported that postoperative CEA (post-CEA) is more useful for predicting the prognosis of stage II–III CRC than pre-CEA [7]. In that article, we also demonstrated that pathological T4 (pT4) is associated with the poor prognosis of patients with stage II–III CRC [7]. Others concur that pT4 is a poor prognostic factor for stage II–III CRC [8]. Post-CEA and pathological depth of tumor invasion are readily available in normal clinical settings. Thus, an accurate prognosis for CRC using these factors would be useful in clinical practice.
We conducted the present study to evaluate a prognostic score we developed using post-CEA and pT4 for stage II–III CRC patients who have undergone curative surgery.
Patients and methods
Patients
Consecutive patients with pathological stage II–III CRC from two cohorts (Nippon Medical School Hospital and Nippon Medical School Musashikosugi Hospital) diagnosed between 2011 and 2017 were enrolled in this study. The following patients were excluded from the analysis: those who underwent non-curative operations, those who underwent emergency operations, those who received neoadjuvant chemotherapy, those with double CRC, those who underwent concomitant surgery for other types of cancer, those who had not had CEA measurements taken either pre- or post-surgery, and those who were not followed up for more than 6 months after surgery.
Data were collected for 16 variables that can affect the prognosis of stage II–III CRC, including age, sex, smoking history, pre- and post-CEA levels, tumor location, obstructive CRC, tumor size, tumor histology, pathological depth of the tumor invasion, number of harvested lymph nodes (LNs), surgical procedures, intraoperative transfusion, anastomotic leakage, adjuvant chemotherapy, and the presence or absence of LN metastasis. Tumor location was categorized as right-side CRC (cecum, ascending colon, and transverse colon) or left-side CRC (descending colon, sigmoid colon, and rectum). Obstructive CRC was defined as that requiring preoperative fasting, placement of a trans-nasal or trans-anal decompression tube, or placement of a trans-anal, self-expanding, metallic stent. Pre-CEA was defined as the serum CEA level obtained closest to the time of surgery, and post-CEA was defined as the serum CEA level obtained at the first visit to the outpatient clinic after surgery. We scheduled the patient’s first postoperative visit to the outpatient clinic 4–8 weeks after surgery. Patients with a serum CEA > 5 ng/mL were classified as having elevated CEA levels.
T-CEA score
The T-CEA score was calculated from the postoperative serum CEA level and pathological depth of tumor invasion. Non-elevated postoperative serum CEA (≤ 5 ng/mL) and pathological T1–3 levels were categorized as T-CEA-0. Elevated postoperative serum CEA (> 5 ng/mL) or pathological T4 levels were considered T-CEA-1, and elevated postoperative serum CEA (> 5 ng/mL) and pathological T4 levels were classified as T-CEA-2.
Study design
We verified the T-CEA score as a prognostic score for stage II–III curative CRC in the two cohorts (Nippon Medical School Hospital and Nippon Medical School Musashikosugi Hospital). This study complied with the Declaration of Helsinki and current ethical guidelines, and the Ethics Committee of Nippon Medical School Hospital approved the study protocol (Tokyo, Japan) (Approval No. B-2020–144).
Staging and surveillance protocol
Postoperative surveillance of stage II–III CRC followed the JSCCR 2019 guidelines (1): CEA testing was performed at 3-month intervals for the first 2–3 years; then at 6-month intervals thereafter for 5 years. Chest, abdomen, and pelvic computed tomography scans were performed every 6 months for 5 years. Colonoscopy was performed 1 year after surgery and then repeated every 2–3 years.
Statistical analysis
The primary endpoint of this study was 5-year recurrence-free survival (RFS), and the secondary endpoint was 5-year overall survival (OS). RFS and OS were estimated using the Kaplan–Meier method. RFS was calculated from the date of surgery to the date of recurrence, death, or last follow-up. OS was calculated from the date of surgery to the date of death. Differences in RFS and OS were assessed using the log-rank test (univariate analysis). Hazard ratios (HRs) and 95% confidence intervals (CIs) were estimated using Cox regression models and assessed with the Wald test. Association with RFS and OS was evaluated using multivariable Cox regression. Variables with P values < 0.10 on univariate analysis were included in the final multivariable model. P values < 0.05 were considered significant. The Mann–Whitney U test was used to analyze categorical and continuous variables. All statistical analyses were performed using R, version 4.0.2 (https://www.r-project.org).
Results
A total of 725 consecutive patients from two cohorts, comprised of 580 patients from Nippon Medical School Hospital (Cohort 1) and 145 patients from Nippon Medical School Musashikosugi Hospital (Cohort 2), underwent surgery for CRC during the study period. After the exclusion of 138 patients based on the study exclusion criteria, 587 patients (482 from Cohort 1 and 105 from Cohort 2) were included in the final analysis.
T-CEA score and patient clinicopathological characteristics (Table 1)
Of the 587 patients, 436 (74%) were classified as having T-CEA-0, 129 (22%) as having T-CEA-1, and 10 (2%) as having T-CEA-2. Table 1 summarizes the relationship between the T-CEA score and clinicopathological characteristics. Female gender, right-side CRC, a large tumor, intraoperative transfusion, lymph node metastasis, and histology other than tub1-2 were significantly associated with a higher T-CEA score (each P < 0.05).
Survival analysis
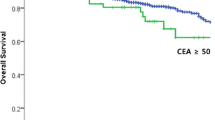
The median follow-up period was 51 months (range 6–115 months). The 5-year RFS rate was 72.9% (95% CI: 68.8–76.7%) and the 5-year OS rate was 86.1% (95% CI: 82.3–89.1%) for all patients. The 5-year RFS rates for the T-CEA-0, 1, and 2 groups were 80.3%, 54.8%, and 0%, respectively (T-CEA-0 vs T-CEA-1: P < 0.01, T-CEA-1 vs T-CEA-2: P < 0.01) (Fig. 1A), and the 5-year OS rates were 90.9%, 74.2%, and 0%, respectively (T-CEA-0 vs T-CEA-1: P < 0.01, T-CEA-1 vs T-CEA-2: P = 0.04) (Fig. 1B). Univariate analysis revealed that an elevated T-CEA score (1 and 2) (P < 0.01), pN + (P < 0.01), and intraoperative transfusion (P = 0.05) were significant risk factors for RFS, and an elevated T-CEA score (1 and 2) (P < 0.01), pN + (P = 0.02), intraoperative transfusion (P = 0.02), and smoking (P = 0.03) were significant risk factors for OS (Table 2). Multivariate analysis revealed that an elevated T-CEA score (1 and 2) (HR: 2.89; 95% CI: 2.07–4.04, P < 0.01), pN+ (HR: 1.88; 95% CI: 1.34–2.64, P < 0.01), and smoking (HR: 1.54; 95% CI: 1.08–2.21, P = 0.02) were significant risk factors for RFS, and that an elevated T-CEA score (1 and 2) (HR: 2.85; 95% CI: 1.78–4.56, P < 0.01), smoking (HR: 1.96; 95% CI: 1.20–3.19, P < 0.01), and age over 70 (HR: 1.66; 95% CI: 1.03–2.67, P = 0.04) were significant risk factors for OS (Table 2).
Impact of the T-CEA score on RFS (Fig. 2) and OS (Fig. 3)
For RFS in Cohort 1, the hazard ratio of both the T-CEA-1 (2.7, 95% confidence interval [95% CI] 1.8–3.8, P < 0.001) and T-CEA-2 groups (11.6, 95% CI 5.7–23.6, P < 0.001) was significantly higher than that of the T-CEA-0 group. For RFS in Cohort 2, the hazard ratio of T-CEA-1 (5.3, 95% CI 1.9–14.5, P = 0.001) was significantly higher than that of the T-CEA-0 group. For RFS of all patients, the hazard ratio of both the T-CEA-1 (2.8, 95% CI 2.0–4.0, P < 0.001) and T-CEA-2 groups (3.6, 95% CI 2.5–5.1, P < 0.001) was significantly higher than that of the T-CEA-0 group. For OS in Cohort 1, the hazard ratio of both the T-CEA-1 (2.7, 95% CI 1.5–4.4, P < 0.001) and T-CEA-2 groups (8.6, 95% CI 3.0–24.9, P < 0.001) was significantly higher than that of the T-CEA-0 group. For OS in Cohort 2, the hazard ratio of T-CEA-1 (3.3, 95% CI 0.9–12.1, P = 0.08) was higher than that of the T-CEA-0 group; however, the difference was not significant. For the OS of all patients, the hazard ratio of both the T-CEA-1 (2.7, 95% CI 1.7–4.3, P < 0.001) and T-CEA-2 groups (3.0, 95% CI 1.8–5.1, P < 0.001) was significantly higher than that of the T-CEA-0 group.
Discussion
The findings of the current study confirmed that a novel prognostic score combining elevated post-CEA and pT4 was useful for our two cohorts of stage II–III CRC patients who underwent curative surgery. Our previous study [7] also found that post-CEA elevation and pT4 were powerful prognostic indicators of unfavorable outcomes for patients with stage II–III CRC. In the current study, the prognosis was worse for patients with both post-CEA elevation and pT4 than for patients with post-CEA elevation or pT4 alone in both cohorts. Another strength of this study is that it shows how the T-CEA score is useful in daily practice as the serum CEA level is measured routinely with laboratory tests. We did not set the cutoff value for CEA using the ROC curve analysis, but rather used a cutoff value of 5 ng/ml, which is the cutoff value in routine clinical practice. Previous multivariate analyses have demonstrated that VCAN (HR:8.98) [2] and KRT17 (HR:7.18) [3] are strongly associated with RFS for stage II–III CRC. These HRs were higher than our T-CEA score, although these predictors require immunohistochemical staining and are difficult to use in clinical settings.
Malnutrition and inflammation (mGPS [9], CONUT score [10], LMR [11], NLR [12], PLR [12], and LCR [13]) have been reported as poor prognostic factors for CRC. These markers are also measured easily in the daily clinical setting, but are highly dependent on the patient's general condition on the day of measurement, as well as on disease progression. Therefore, these scores are not objective. On the other hand, the half-life of serum CEA is approximately 7 days [14]; thus, post-CEA measured 1 month after surgery may reflect minimal residual disease (MRD). A score combining post-CEA with depth of tumor invasion is an objective and reliable indicator, which is another strength of this study.
CEA is a widely used biomarker that has utility for CRC patients. Worldwide guidelines, including those of the National Comprehensive Cancer Network (NCCN) [15] and the European Society for Medical Oncology (ESMO) [16], recommend pre-CEA testing in patients with CRC. In our previous report [7], multivariate analysis showed that an elevated post-CEA level was an independent poor prognostic for RFS (HR: 3.14) and OS (HR: 3.13), but that an elevated pre-CEA was not. Furthermore, the 5-year RFS and OS of patients with an elevated pre-CEA, but normal post-CEA were similar to those of patients with normal pre- and post-CEAs. Thus, we concluded that post-CEA elevation, not pre-CEA elevation, is a strong poor prognostic for patients with stage II–III CRC.
In our previous study [7], using multivariate analysis, we demonstrated that a high pT4 level was an independent poor prognostic indicator for stage II–III CRC. Other studies [8, 17, 18] have shown that pT4 has high prognostic importance in stage II–III CRC. The importance of pT4 is also reflected in the fact that pT4 stage II CRC has a worse prognosis than low risk (pT1-2N1-2) stage III CRC [19]. Our previous results demonstrated that pT4 is as important as the post-CEA value and pathological lymph node metastatic status for predicting prognoses.
In this study, only ten of the total 587 patients (2%) were classified as having T-CEA-2, representing the worst prognosis. These cases are summarized in Table 3. Characteristically, eight of these ten patients had a pre-CEA of 40 ng/mL or higher, which did not decrease to within normal limits after surgery in six. Furthermore, in three of the ten patients, the post-CEA value was higher than the pre-CEA despite curative surgery. These findings tell us that postoperative minimal residual disease (MRD), which indicates the presence of a minimal number of residual cancer cells after surgery, is closely related to prognosis. MRD is a novel concept and can be used to identify patients with a high risk of recurrence after curative surgery. We, as well as other authors, have shown that MRD is a risk factor for recurrence after curative surgery for stage I–III [20,21,22] and IV [23] CRC. However, the method of ctDNA detection is still in its developmental stages and the high cost of detecting ctDNA could impede its clinical use. Conversely, the strength of our method is that it requires no new technology or additional cost. Based on these findings, several clinical trials [24,25,26] are being conducted and planned around the world to evaluate the efficacy of adjuvant chemotherapy in MRD-positive patients without definite distant metastases. These trials include not only FOLFOX and CAPOX [24], the current standard of care for adjuvant chemotherapy for CRC, but also novel therapies such as trifluridine/tipiracil hydrochloride therapy [25] and FOLFOXIRI [26]. The results of these studies have the potential to create new treatment strategies for highly recurrent cases, such as MRD-positive and T-CEA-2.
This study had some limitations. First, our cohort 2 was much smaller than our Cohort 1. Second, the number of T-CEA-2 patients was small, and there were none in Cohort 2, which probably accounts for the difference in hazard ratios between T-CEA-1 and T-CEA-2 being smaller when cohort 1 and cohort 2 were analyzed together. Third, this analysis was not adjusted for the presence or absence of adjuvant chemotherapy. Thus, a larger, multicenter study is needed to ensure the utility of this score.
Conclusions
The T-CEA score is a reliable and convenient prognostic tool for stage II–III CRC patients who have undergone curative surgery.
Data availability
All authors are available for the raw data of this study.
References
Hashiguchi Y, Muro K, Saito Y, Ito Y, Ajioka Y, Hamaguchi T, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int J Clin Oncol. 2000;25(1):1–42.
Chiba S, Okayama H, Noda M, Saito K, Nakajima T, Aoto K, et al. Stromal VCAN expression as a potential prognostic biomarker for disease recurrence in stage II–III colon cancer. Carcinogenesis. 2016;37(9):878–87.
Ujiie D, Okayama H, Saito K, Ashizawa M, Min AKT, Endo E, et al. KRT17 as a prognostic biomarker for stage II colorectal cancer. Carcinogenesis. 2020;41(5):591–9.
Weiser MR, Landmann RG, Kattan MW, Gonen M, Shia J, Chou J, et al. Individualized prediction of colon cancer recurrence using a nomogram. J Clin Oncol. 2008;26:380–5.
Weiser MR, Gönen M, Chou JF, Kattan MW, Schrag D. Predicting survival after curative colectomy for cancer: individualizing colon cancer staging. J Clin Oncol. 2011;29:4796–802.
Kanemitsu Y, Shida D, Tsukamoto S, Ueno H, Ishiguro M, Ishihara S, et al. Nomograms predicting survival and recurrence in colonic cancer in the era of complete mesocolic excision. BJS Open. 2019;3:539–48.
Sonoda H, Yamada T, Matsuda A, Ohta R, Shinji S, Yokoyama Y, et al. Elevated serum carcinoembryonic antigen level after curative surgery is a prognostic biomarker of stage II–III colorectal cancer. Eur J Surg Oncol. 2021;47(11):2880–7.
Snaebjornsson P, Coupe VMH, Jonasson L, Meijer GA, van Grieken NC, Jonasson JG. pT4 stage II and III colon cancers carry the worst prognosis in a nationwide survival analysis. Shepherd’s local peritoneal involvement revisited. Int J Cancer. 2014;135:467–78.
Park JH, Fuglestad AJ, Køstner AH, Oliwa A, Graham J, Horgan PG, et al. Systemic inflammation and outcome in 2295 patients with stage I-III colorectal cancer from Scotland and Norway: first results from the Scotscan colorectal cancer group. Ann Surg Oncol. 2020;27:2784–94.
Takagi K, Buettner S, Ijzermans JNM. Prognostic significance of the controlling nutritional status (CONUT) score in patients with colorectal cancer: a systematic review and meta-analysis. Int J Surg. 2020;78:91–6.
Song W, Wang K, Zhang RJ, Zou SB. Prognostic value of the lymphocyte monocyte ratio in patients with colorectal cancer: a meta-analysis. Medicine (Baltimore). 2016;95(49): e5540.
Zhang J, Zhang HY, Li J, Shao XU, Zhang CX. The elevated NLR, PLR and PLT may predict the prognosis of patients with colorectal cancer: a systematic review and meta-analysis. Oncotarget. 2017;8(40):68837–46.
Okugawa Y, Toiyama Y, Yamamoto A, Shigemori T, Ide S, Kitajima T, et al. Lymphocyte-C-reactive protein ratio as promising new marker for predicting surgical and oncological outcomes in colorectal cancer. Ann Surg. 2020;272:342–51.
Ito K, Hibi K, Ando H, Hidemura K, Yamazaki T, Akiyama S, et al. Usefulness of analytical CEA doubling time and half-life time for overlooked synchronous metastases in colorectal carcinoma. Jpn J Clin Oncol. 2002;32(2):54–8.
National Comprehensive Cancer Network (NCCN). Clinical practice guidelines in oncology: colon cancer. Version 4.2020. Fort Washington: NCCN; 2020.
Argiles G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, et al. Localized colon cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(10):1291–305.
Gill S, Loprinzi CL, Sargent DJ, Thomé SD, Alberts SR, Haller DG, et al. Pooled analysis of fluorouracil-based adjuvant therapy for stage II and III colon cancer: who benefits and by how much? J Clin Oncol. 2004;22(10):1797–806.
Merkel S, Wein A, Günther K, Papadopoulos T, Hohenberger W, Hermanek P. High-risk groups of patients with Stage II colon carcinoma. Cancer. 2001;92:1435–43.
Gunderson LL, Jessup JM, Sargent DJ. Revised TN categorization for colon cancer based on national survival outcome data. J Clin Oncol. 2010;28(2):264–71.
Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8(346):346ra92.
Tie J, Cohen JD, Wang Y, Christie M, Simons K, Lee M, et al. Circulating tumor DNA analyses as markers of recurrence risk and benefit of adjuvant therapy for stage III colon cancer. JAMA Oncol. 2019;5(12):1710–7.
Reinert T, Henriksen TV, Christensen E, Sharma S, Salari R, Sethi H, et al. Analysis of plasma cell-free DNA by ultradeep sequencing in patients with stages I to III colorectal cancer. JAMA Oncol. 2019;5(8):1124–31.
Iwai T, Yamada T, Takahashi G, Takeda K, Koizumi M, Shinji S, et al. Circulating cell-free long DNA fragments predict post-hepatectomy recurrence of colorectal liver metastases. Eur J Surg Oncol. 2020;46(1):108–14.
Lonardi S, Montagut C, Pietrantonio F, Elez E, Sartore-Bianchi A, Tarazona N, et al. The PEGASUS trial: Post-surgical liquid biopsy-guided treatment of stage III and high-risk stage II colon cancer patients. J Clin Oncol. 2020;38(15):suppl.TPS124.
Shirasu H, Taniguchi H, Matsuhashi N, Kotaka M, Nakamura Y, Oki E, et al. A randomized, double-blind, phase III study comparing trifluridine/tipiracil hydrochloride therapy versus placebo in resected colorectal cancer patients who are positive for blood circulating tumor DNA after standard adjuvant therapy (EPOC 1905): ALTAIR trial in CIRCULATE-Japan (trial in progress). J Clin Oncol. 2022;40(4):suppl.TPS215.
Moretto R. Exploiting circulating tumour DNA to intensify the postoperative treatment resected colon cancer patients (ERASE-CRC). ClinicalTrials.gov NCT05062889.
Acknowledgements
We thank Mr. Steven D. Aird for preliminary English language editing.
Funding
This research did not receive any grants from funding agencies in the public, commercial, or non-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
We have no conflicts of interest or financial ties to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sonoda, H., Yamada, T., Matsuda, A. et al. The T-CEA score: a useful prognostic indicator based on postoperative CEA and pathological T4 levels for patients with stage II–III colorectal cancer. Surg Today 53, 890–898 (2023). https://doi.org/10.1007/s00595-023-02644-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-023-02644-6