Abstract
Background
Endoscopic stenting with a self-expandable metallic stent (SEMS) is a widely accepted procedure for malignant colonic obstruction. The Colonic Stent Safe Procedure Research Group conducted the present prospective feasibility study.
Methods
Our objectives were to estimate the safety and feasibility of SEMS placement as a bridge to surgery (BTS) for malignant colorectal obstruction. We conducted a prospective, observational, single-arm, multicenter clinical trial from March 2012 to October 2013. Each patient was treated with an uncovered WallFlex enteral colonic stent. Patients were followed up until discharge after surgery.
Results
A total of 518 consecutive patients were enrolled in this study. The cohort intended for BTS consisted of 312 patients (61 %), and the stent could be released in 305 patients. Technical and clinical success rates were 98 and 92 %, respectively. Elective surgery was performed in 297 patients, and emergency surgery was performed in eight patients for the treatment of complications. The overall preoperative complication rate was 7.2 %. Major complications, including perforation, occurred in 1.6 %, persistent colonic obstruction occurred in 1.0 %, and stent migration occurred in 1.3 % patients. The median time from SEMS to surgery was 16 days. Silent perforations were observed in 1.3 %. Open and laparoscopic surgery was performed in 121 and 184 patients, respectively. The tumor could be resected in 297 patients. The primary anastomosis rate was 92 %. The rate of anastomotic leakage was 4 %, and the overall stoma creation rate was 10 %. The median duration of hospitalization following surgery was 12 days. Overall postoperative morbidity and mortality rates were 16 and 0.7 %, respectively.
Conclusions
This largest, multicenter, prospective study demonstrates the feasibility of SEMS placement as a BTS for malignant colorectal obstruction. SEMS serves as a safe and effective BTS with acceptable stoma creation and complication rates in patients with acute malignant colonic obstruction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Colorectal cancer (CRC) is one of the most common cancers worldwide [1] and the second most common cancer in Japan [2]. At the time of diagnosis, 8–13 % of patients with CRC present with acute colonic obstruction [3–5]. Conventionally, patients with malignant large bowel obstruction receive emergency surgery. These patients have a poor outcome as morbidity and postoperative mortality rates are higher [6], rates of resection and curative resection are lower, and long-term survival is poorer than that in patients admitted electively [7, 8].
Tejero et al. reported using a self-expandable metallic stent (SEMS) as a bridge to surgery (BTS) in patients with colonic obstruction in 1994 [9]. In Japan, in 1996, Saida et al. [10] reported using SEMS as BTS in patients with obstructing CRC. Thereafter, a number of studies have shown that endoscopic stent procedures with SEMS prior to elective surgery represent relatively simple and safe alternatives to conventional emergency surgery [11–16]. Preoperative SEMS placement can prevent high-risk emergency surgery and makes it possible to increase primary anastomosis and decrease stoma creation [17]. Additionally, SEMS gives the physician an opportunity to perform medical resuscitation, optimization of comorbid disorders, bowel preparation, tumor staging, and preoperative total colonic examination for synchronous proximal lesions [15, 16]. Although preoperative stent insertion has such advantages, some stent-related complications, such as perforation, stent migration, and re-obstruction, have been reported [18]. Perforation may lead to an increased risk of peritoneal carcinomatosis and septic and life-threatening conditions [19–22]. If the perforation rate is low, the risk of sepsis or peritoneal carcinomatosis is not increased. Colonic SEMS placement as a bridge to elective surgery is useful as a standard treatment for malignant colonic obstruction because SEMS intervention resulted in more favorable rates of permanent stoma, primary anastomosis, and overall complications [23, 24].
To accurately evaluate the advantages and risks of SEMS, proper stent placement is required. However, to date, SEMS placement studies with quality control information have not been performed. Furthermore, in Japan, the safety and efficacy of SEMS placement are unclear because until 2011, colonic SEMS was used only in clinical research [10, 25, 26]. In 2012, this procedure was covered by the National Health Insurance in Japan. Even under these circumstances, the Colonic Stent Safe Procedure Research Group affiliated with the Japan Gastroenterological Endoscopy Society was organized to provide instructions regarding safety procedures for placement of colonic stents [27].
The present study is the largest prospective, multicenter feasibility study of colonic SEMS as a BTS for acute obstructive CRC. The aim of this study was to investigate the outcomes of SEMS as a BTS for obstructive CRC in terms of treatment details, short-term adverse events, and proportion of patients with stoma.
Materials and methods
Patients
This prospective, observational, single-arm, multicenter clinical trial was conducted from March 2012 to October 2013 [28]. The study was registered with the University Hospital Medical Information Network Clinical Trial Registry (UMIN000007953). Before study start-up, a Web site [27] was launched and the standard methods of SEMS placement based on previously published data were proclaimed [10, 15, 25]. The Colonic Stent Safe Procedure Research Group held a workshop to discuss the tips and tricks of SEMS placement. More than 140 doctors participated in the meeting, and several experienced doctors presented their experience in developing safe SEMS placement procedures. A summary was subsequently uploaded on the Web site [27]. Before the introduction of SEMS in each institute, we requested each member to promote cooperation among endoscopists and surgeons to prepare for the possibility of adverse events.
Patients with acute colorectal obstruction or symptomatic strictures secondary to malignant neoplasms were enrolled in the study. The goal was not to change the usual treatment practice of the investigator or the center. Patients were treated as per usual medical practices.
Forty-six facilities (14 academic centers and 32 community hospitals) participated in the study. Institutional review board approval was obtained for patient enrollment prior to the start of the study. Each patient gave consent to undergo the procedure and registration of patient clinical data. Participating institutions registered all patients with acute colorectal obstructions managed using the WallFlex enteral colonic stent (Boston Scientific Corporation, Natick, MA, USA) until completion of the study. Registration was completed online through the Web site before or immediately after the procedure. Patients with primary tumors receiving operation for resection were classified as “BTS,” whereas patients for whom surgery was not scheduled were classified as “palliative.” All clinical data were prospectively collected. Patients undergoing stenting as a BTS were followed up until discharge after surgery.
Inclusion criteria
A criterion for enrollment was large bowel obstruction as diagnosed by abdominal X-ray, colonoscopy, or computed tomography (CT) scan. Patients with colorectal obstruction secondary to malignant neoplasms were included in the registry. Only patients with no previous colonic stenting were included in the registry.
Exclusion criteria
Exclusion criteria included previous colonic stent placement, enteral ischemia, suspected or impending perforation, intraabdominal abscess/perforation, severe inflammatory changes around the tumor, contraindication to endoscopic treatment, and any use of the stent other than those specifically outlined in indications for use.
Evaluation of obstruction symptoms
To assess oral intake levels and abdominal symptoms before and after the procedure, we constructed a scoring system similar to the one used for eating state assessment in patients with malignant gastric outlet obstruction [29]. The ColoRectal Obstruction Scoring System (CROSS) assigns a point score based on the patient’s oral intake level (Table 1): CROSS 0, requiring continuous decompression; CROSS 1, no oral intake; CROSS 2, liquid or enteral nutrient intake; CROSS 3, soft solids, low residue, and full diet with symptoms of stricture; and CROSS 4, soft solids, low residue, and full diet without symptoms of stricture.
Stent device and procedure
Each patient was treated with an uncovered WallFlex enteral colonic stent (Boston Scientific Corporation, Natick, MA, USA) with midbody and proximal flange diameters of 22/27 and 25/30 mm, respectively, and lengths of 6, 9, and 12 cm. SEMS placement was performed as presented in the pre-introduction publicity announcement. The details of standard procedures for SEMS placement were described on the Web site as a brief guideline [27]. Access across the stricture was established using a guidewire, and a contrast tube was inserted into the proximal lumen. The length of the stricture was fluoroscopically measured using a contrast agent, and the number of stents required to cross the stricture was determined. To maintain good visualization of the tumor orifice, biopsy immediately before SEMS placement was not recommended because the tumor orifice becomes obscure due to bleeding. To identify the stricture location, intraluminal or extraluminal marking with an endoscopic clip, lipiodol, or a radiopaque marker was performed at the discretion of endoscopists. Stricture dilatation before stent placement was generally forbidden.
Outcome measures
We examined the patients undergoing surgery for resection after SEMS placement as a BTS. BTS was defined as scheduled elective surgery, independent of the time between SEMS insertion and surgery. Technical success was defined as accurate SEMS placement with adequate stricture coverage on the first attempt without any adverse events. Clinical success of a BTS was defined as the decompression and relief of obstructive symptoms until surgery without any stent-related complications and without the need for endoscopic re-intervention or emergency surgery. The following conditions were considered to be procedure-related adverse events: perforation, re-obstruction, stent migration, infection/fever, abdominal pain, and tenesmus. Perforation was diagnosed by clinical symptoms and radiological examination or intraoperative findings.
Postprocedural adverse events were distinguished by the onset time: up to 7 days of stent placement, from 7 days after stent placement up to 14 days, from 14 days after stent placement up to 21 days, and from 21 days after stent placement. Silent perforation, in which the stent was intraoperatively exposed in the abdominal cavity without preoperative symptoms, was not regarded as a complication. Patients undergoing stenting were followed up until discharge from hospital. Evaluation of surgical outcomes took into account the ability to perform elective surgery as planned with or without the need for a diverting stoma. Complications and length of hospitalization after surgery were also recorded.
Statistical analysis
Continuous variables were presented as medians (range). Statistical calculations were performed using SPSS version 8.0.2 (SPSS, Chicago, IL, USA).
Results
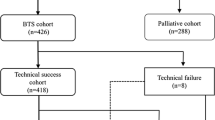
A flowchart of the patient registry is shown in Fig. 1. A total of 518 consecutive patients were enrolled in the study. Five patients were excluded because of loose stenosis identified by colonoscopy (n = 3), adhesive small bowel obstruction (n = 1), and placement of another type of SEMS (n = 1). The remaining 513 patients were the per-protocol cohort. The intention of treatment was a BTS in 312 patients (61 %) and palliative in 201 patients.
Baseline characteristics
Of the 312 BTS patients, 177 (57 %) were males, and the median (range) age was 71 (35–94) years. Of the 312 patients, 296 (95 %) patients presented with acute colonic obstructive symptoms and the remaining 16 patients did not have any stricture-related symptoms. Primary CRC was reported in 98 % of the patients, and the remaining five patients had either locally recurrent CRC (n = 2), extracolonic cancer (n = 1), or benign lesions pathologically proven after surgery (n = 2). The tumor was located in the left colon in 76 % of the patients, in the rectum in 4 % of the patients, and proximal to the splenic flexure in 21 % of the patients. At postoperative staging, 72 % of the patients had localized CRC without metastatic disease, whereas 28 % of the patients had distant metastasis (Table 2).
Technical success
Successful SEMS insertion was achieved in 305 patients (technical success rate 97.8 %). Technical failure occurred in seven patients because of our inability to endoscopically visualize the tumor in one case, inability to pass a guidewire in three cases, and perforation by the guidewire in three cases (Table 3). These patients were included in the assessment of safety but were excluded from the assessment of effectiveness. Seven out of the 305 (2.3 %) patients required two stents in the first attempt. Six of these seven patients had a single stricture, whereas one patient had a double stricture. Of the total of 312 stents, the most commonly used stent length was 6 cm (n = 204, 65.2 %), but in 101 (32.4 %) patients, a 9-cm-long stent was selected. The most commonly used stent diameter was the 22/27-mm body/flare diameter (n = 287, 92.0 %) (Table 4). In only six (2.0 %) patients, the stricture was dilated before stent placement using a balloon.
Clinical success as a BTS
All patients received follow-ups until discharge. In two patients, obstructive colitis that occurred before stenting worsened, and emergency surgery was performed. Adverse events were recorded in 22 (7.2 %) patients (Table 5). A total of 24 patients were categorized as clinical failure. If technical errors were excluded, the clinical success rate was 92.1 %. Emergency surgery was performed in eight patients for the treatment of complications (n = 6) or obstructive colitis (n = 2); the details and outcomes of these patients are summarized in Table 5. In the cases of emergency surgery, Hartmann’s resection was performed in three cases and resection of the tumor with primary anastomosis without diverting stoma was performed in five cases. Major procedural complications related to stent placement included perforation in 1.6 % (n = 5) (Tables 5, 6). One patient presented with abdominal emergency 2 days after stent insertion, and a blowout perforation in the cecum was identified at acute laparotomy. One patient had tumor perforation and underwent acute surgery 2 days after stent insertion. One patient had perforation of the appendix with primary tumor invasion and underwent acute surgery 2 days after stent insertion. Two patients had perforation from the edge of the stent flare and underwent emergency surgery 5 and 19 days after initial stent insertion, respectively. In both these patients, the stents that had been used were 9 cm long and had a 22/27-mm body/flare diameter. Other major complications included persistent colonic obstruction in three (1.0 %) patients, stent migration in four (1.3 %) patients, and sepsis due to obstructive colitis in one (0.3 %) patient who underwent emergency surgery. Minor complications included fever (n = 4), tenesmus (n = 3), and stool impaction (n = 1). Patients with stool impaction underwent endoscopic cleaning next day of stent insertion.
Elective BTS
In total, 297 patients received elective resection. The median time from SEMS insertion to surgery was 16 days (interquartile range 12–24 days). Silent perforation (the stent was intraoperatively exposed in the abdominal cavity without preoperative symptoms) was observed in four (1.3 %) patients (Table 6). The overall perforation rate consisting of technical perforation, procedure-related perforation, and silent perforation was 3.8 % (12/312). The tumor could be resected in 290 patients (97.6 %). Open and laparoscopic surgery was performed in 116 and 181 patients, respectively. The conversion rate from laparoscopic to open surgery was 10.5 % (19/181). There was no correlation between interval from stent insertion to surgery or conversion from laparoscopic to open surgery. Resection of the tumor with primary anastomosis was performed in 276 cases (93 %), which included covering stoma in nine cases and postoperative diverting stoma for anastomotic leakage in two cases. Hartmann’s resection was performed in 13 cases. Palliative colostomy only and palliative bypass only were performed in six and two cases, respectively. The overall stoma creation rate was 10.1 % (30/297), and 89.9 % of the 297 patients were stoma free after surgery. The overall postoperative morbidity rate was 17.7 %. Anastomotic leakage of all grades occurred in 12 (4.3 %) of 279 patients with primary anastomosis. Two of these 12 patients underwent re-operation with stoma creation, and other patients were treated conservatively. There was no correlation between interval from stent insertion to surgery and anastomotic leakage. Wound infection of all grades occurred in 18 patients. Bowel obstruction of all grades occurred in 15 patients, and two of the 15 patients underwent re-operation. Intraperitoneal abscess was observed in three patients (Table 7). The median duration of hospitalization after surgery was 12 days (range 4–73). The overall 30-day mortality rate after technically successful SEMS placement was zero, but the hospital postoperative mortality was 0.7 % (n = 2). These two patients died from cancer progression.
Discussion
In patients with acute colonic obstruction, emergency surgical decompression has become mandatory as the traditional treatment option. It involves a defunctioning stoma with or without primary resection of the obstructing tumor; however, emergency operation is associated with high morbidity and mortality rates, and a colostomy has an impact on the quality of life [30]. Endoscopic placement of SEMS is an effective alternative to surgical decompression for colonic obstruction without emergency surgery. Furthermore, SEMS placement allows the treating physician to perform medical resuscitation, optimization of comorbid disorders, bowel preparation, accurate tumor staging, and preoperative total colonic examination to exclude synchronous proximal lesions [15, 16, 23, 24].
The present study is the largest prospective, multicenter feasibility study on SEMS placement as a BTS for malignant colorectal obstruction. In Japan, colonic SEMS placement was not covered by the National Health Insurance until January 2012. Appropriate SEMS placement resulted in high technical and clinical success rates through the expertise offered by experienced doctors at the pre-stent placement meeting arranged to introduce the standard procedural details of SEMS placement as described on the Web site [27, 28]. As a result, the technical and clinical success rates of stent placement in the present study were similar to those reported in previous reviews and comparable with those reported in the previous largest multicenter prospective study from the WallFlex Colonic Registry Group (technical success rate 98 % and clinical success rate 94 %) [31, 32]. Previously, three systematic reviews [14, 15, 18] evaluated and presented data on the efficacy and safety of colorectal stents in 598, 1198, and 1785 patients, respectively. The reported rates of technical success were 92, 93.2, and 96.2 % (median), with clinical success rates of 85 % (as a BTS with technical failure not being excluded), 78.1 % (as a BTS), and 92 % (median), respectively. In patients with total obstruction, stent placement is challenging [33]. Because all consecutive patients in whom SEMS insertion was attempted were enrolled in the present study, patients without total obstruction, such as those with CROSS 3 or 4, were enrolled, and this situation may be reflected in the high technical success rate.
In the present study, the clinical perforation rate was 2.7 %. In a previous comprehensive prospective study, the clinical perforation rate within 30 days was reported as 3 % [31, 32]. The previous three systematic reviews [14, 15, 18] reported the perforation rates of 4, 3.8, and 4.5 % (median), respectively. On the other hand, some randomized controlled studies and a comparative study reported high perforation rates, such as 10–12.8 % [19, 34, 35]. In the European Society of Gastrointestinal Endoscopy (ESGE) clinical guidelines, published in 2014 [36], it is mentioned that several studies have shown no differences in outcomes (efficacy and safety) based on different stent designs. However, from both our study and the study from the WallFlex Colonic Registry Group, WallFlex enteral colonic stents were used and the perforation rate is about 3 % [31, 32]. On the other hand, in one RCT with a high perforation rate, four-fifths of the stents were Wallstent (Boston Scientific, Natick, MA, USA) and one-fifth of the stents were WallFlex [19]. Therefore, we speculate that stent design may be related to the perforation rate. Furthermore, we believe that the shorter WallFlex colonic stent, which is still longer than the length of the stricture, is better. In the present study, 65 % of the stents used were 6 cm long, and in both patients who had perforation from the edge of the stent flare, the stents used were 9 cm long. However, with regard to the data of the study from the WallFlex Colonic Registry Group, in 61.3 % of the patients, a 9-cm-long stent was selected and stents of 6 and 12 cm length were less commonly used.
In the present study, four of the five postprocedural perforations occurred within 5 days after stent placement; the remaining patient had perforation from the edge of the stent flare 19 days after stent insertion (Table 6). Iversen et al. reported that one of the four perforations occurred 18 days after SEMS insertion, whereas the remaining three patients experienced perforation within 5 days after SEMS placement [37]. Other adverse events occurring after more than 7 days only included fever in three patients who had elective surgery (Table 5). In the present study, the median time from SEMS insertion to surgery was 16 days. In the ESGE clinical guidelines from 2014 [36], a time interval of 5–10 days till operation is suggested when SEMS is used as a bridge to elective surgery in patients with potentially curable left-sided colon cancer (weak recommendation, low-quality evidence). It was written in the commentary to these guidelines that, theoretically, a longer interval (>1 week) may allow for better recovery and better optimal nutritional status; however, this may increase the risk of stent-related complications and may compromise surgery by more local tumor infiltration and fibrosis. Practically, according to our data, a longer interval did not increase the risk of stent-related complications and did not increase anastomotic leakage or conversion from laparoscopic to open surgery. Therefore, we cannot identify any optimal time interval till operation following stent placement as a BTS.
In the present study, up to 97.4 % of patients with technical success underwent an elective surgery, whereas only 2.6 % required emergency surgery. As demonstrated by several previous systematic reviews based on meta-analyses, SEMS intervention was associated with a higher successful primary anastomosis rate, lower stoma creation rate, and lower overall morbidity after surgery [16, 23, 24]; this was consistent with the observations in the present study. Anastomotic leakage and postoperative mortality were very low compared with several systematic reviews and meta-analyses.
In the ESGE clinical guidelines from 2014 [36], it is mentioned that colonic SEMS placement as a bridge to elective surgery is not recommended as a standard treatment for symptomatic left-sided malignant colonic obstruction with strong recommendation, because of a high risk of stent-related perforation. Follow-up data of the Stent-in 2 trial also showed that the cumulative incidence of overall recurrences in patients with clinical stent-related perforation was significantly increased than in those who underwent emergency surgery or stenting without perforation [38]. The long-term impact of silent perforation, in which the edge of the stent was visible during the scheduled operation, is unknown. Silent perforation was observed in four (1.3 %) patients in the present study. Two randomized clinical trials of SEMS as a BTS versus emergency surgery reported that the silent perforation rate among cases with successfully placed stents was 9 and 57 %, respectively. We speculate that these high silent perforation rates correlated with relatively low technical success rates of stent placement (70 and 47 %, respectively) or selective stent design [19, 34]. In the ESGE guidelines, it is mentioned that potential concerns have been raised about impaired oncological outcomes after SEMS placement in patients with potentially curable colon cancer, particularly following stent perforation.
Our study group discussed some RCTs and cohort studies, which reported long-term outcomes after SEMS insertion as a bridge to elective surgery and referred in the ESGE guidelines [35, 38–41]. In the three studies with high perforation rates, oncological long-term outcomes were unfavorable. Therefore, we speculate that technical failure of the stent placement affects the long-term outcome. With no study having good evidence of setting the primary endpoint of oncological outcomes, the conclusions of the ESGE guidelines appear to be premature. The most recent systematic review and meta-analysis evaluated long-term outcomes of colonic stent as a BTS (n = 704) and emergency surgery (n = 432) for malignant large bowel obstruction, in which main outcome measures were overall survival (OS), disease-free survival (DFS), and recurrence [42]. This study suggests that SEMS insertion followed by surgery has no adverse influence in terms of patient oncological outcomes, including OS, DFS, and recurrence, compared with emergency surgery.
However, theoretically enforced radial dilatation by SEMS suggests the possibility of increased risk of not only perforation but also tumor manipulation that can induce dissemination of cancer cells into the peritoneal cavity, surrounding lymphatic vessels, and bloodstream [43, 44]. On the other hand, long-term outcomes of malignant colorectal obstruction may improve whether cases of postoperative morbidity are fewer by SEMS placement as a bridge to elective surgery.
The current analysis has at least three limitations. First, this was a non-randomized, single-arm study; therefore, a comparison of stent and surgical morbidity and mortality could not be performed. Second, 20 % of the patients without total obstruction categorized as CROSS 3 (13 %) or 4 (7 %) received a preventive stent procedure. Finally, there are no data on long-term outcomes available yet. In order to clarify the long-term outcomes of SEMS, patient survival is currently being monitored.
To reveal the oncological long-term effects of colonic SEMS as bridge to elective surgery, large-size randomized controlled trials are warranted. However, for randomized controlled studies of colonic SEMS as BTS versus emergency surgery, it will be difficult to recruit patients for an emergency setting and to standardize the procedure of stent insertion. In our group, procedures of SEMS placement for malignant colorectal obstruction had been standardized already. In future, our group is going to undertake a large-size randomized controlled trial to reveal the oncological effects of colonic stent placement.
References
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90
Matsuda A, Matsuda T, Shibata A, Katanoda K, Sobue T, Nishimoto H, The Japan Cancer Surveillance Research Group (2013) Cancer incidence and incidence rates in Japan in 2008: a study of 25 population-based cancer registries for the monitoring of cancer incidence in Japan (MCIJ) Project. Jpn J Clin Oncol 44:388–396
Winner M, Mooney SJ, Hershman DL, Feingold DL, Allendorf JD, Wright JD, Neugut AI (2013) Incidence and predictors of bowel obstruction in elderly patients with stage IV colon cancer: a population-based cohort study. JAMA Surg 148:715–722
Jullumstro E, Wibe A, Lydersen S, Edna TH (2011) Colon cancer incidence, presentation, treatment and outcomes over 25 years. Colorectal Dis 13:512–518
Cheynel N, Cortet M, Lepage C, Benoit L, Faivre J, Bouvier AM (2007) Trends in frequency and management of obstructing colorectal cancers in a well-defined population. Dis Colon Rectum 50:1568–1575
Iversen LH, Bülow S, Christensen IJ, Laurberg S, Harling H (2008) Danish Colorectal Cancer Group. Postoperative medical complications are the main cause of early death after emergency surgery for colonic cancer. Br J Surg 95:1012–1019
Cuffy M, Abir F, Audisio RA, Longo WE (2004) Colorectal cancer presenting as surgical emergencies. Surg Oncol 13:149–157
McArdle CS, Hole DJ (2004) Emergency presentation of colorectal cancer is associated with poor 5-year survival. Br J Surg 91:605–609
Tejero E, Mainar A, Fernández L, Tobío R, De Gregorio MA (1994) New procedure for the treatment of colorectal neoplastic obstructions. Dis Colon Rectum 37:1158–1159
Saida Y, Sumiyama Y, Nagao J, Takase M (1996) Stent endoprosthesis for obstructing colorectal cancers. Dis Colon Rectum 39:552–555
Martinez-Santos C, Lobato RF, Fradejas JM, Pinto I, Ortega-Deballon P, Moreno-Azcoita M (2002) Self-expandable stent before elective surgery vs. emergency surgery for the treatment of malignant colorectal obstructions: comparison of primary anastomosis and morbidity rates. Dis Colon Rectum 45:401–406
ASGE Standards of Practice Committee, Harrison ME, Anderson MA, Appalaneni V, Banerjee S, Ben-Menachem T, Cash BD, Fanelli RD, Fisher L, Fukami N, Gan S, Ikenberry SO, Jain R, Khan K, Krinsky ML, Maple JT, Shen B, Guilder TV, Baron TH, Dominitz JA (2010) The role of endoscopy in the management of patients with known and suspected colonic obstruction and pseudo-obstruction. Gastrointest Endosc 71:669–679
Ansaloni L, Andersson RE, Bazzoli F, Catena F, Cennamo V, Di Saverio S, Fuccio L, Jeekel H, Leppäniemi A, Moore E, Pinna AD, Pisano M, Repici A, Sugarbaker PH, Tuech JJ (2010) Guidelines in the management of obstructing cancer of the left colon: consensus conference of the world society of emergency surgery (WSES) and peritoneum and surgery (PnS) society. World J Emerg Surg 5:29
Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M (2004) Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol 99:2051–2057
Khot UP, Lang AW, Murali K, Parker MC (2002) Systematic review of the efficacy and safety of colorectal stents. Br J Surg 89:1096–1102
Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, Parisi A, Noya G, Sagar J (2013) Safety and efficacy of endoscopic colonic stenting as a BTS in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol 22:14–21
Cennamo V, Luigiano C, Coccolini F, Fabbri C, Bassi M, De Caro G, Ceroni L, Maimone A, Ravelli P, Ansaloni L (2013) Meta-analysis of randomized trials comparing endoscopic stenting and surgical decompression for colorectal cancer obstruction. Int J Colorectal Dis 28:855–863
Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ (2007) Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg 246:24–30
van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Lutke Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P (2011) Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol 12:344–352
van Hooft JE, Fockens P, Marinelli AW, Bossuyt PM, Bemelman WA (2006) Dutch stent-in study group. Premature closure of the Dutch stent-in I study. Lancet 368:1573–1574
Tan KK, Zhang J, Liu JZ, Shen SF, Earnest A, Sim R (2009) Right colonic perforation in an Asian population: predictors of morbidity and mortality. J Gastrointest Surg 13:2252–2259
Anwar MA, D’Souza F, Coulter R, Memon B, Khan IM, Memon MA (2006) Outcome of acutely perforated colorectal cancers: experience of a single district general hospital. Surg Oncol 15:91–96
Tan CJ, Dasari BV, Gardiner K (2012) Systematic review and meta-analysis of randomized clinical trials of self-expanding metallic stents as a bridge to surgery versus emergency surgery for malignant left-sided large bowel obstruction. Br J Surg 99:469–476
Huang X, Lv B, Zhang S, Meng L (2014) Preoperative colonic stents versus emergency surgery for acute left-sided malignant colonic obstruction a meta-analysis. J Gastrointest Surg 18:584–591
Saida Y, Sumiyama Y, Nagao J, Uramatsu M (2003) Long-term prognosis of preoperative ‘‘bridge to surgery’’ expandable metallic stent insertion for obstructive colorectal cancer: comparison with emergency operation. Dis Colon Rectum 46:S44–S49
Yoshida S, Watabe H, Isayama H, Kogure H, Nakai Y, Yamamoto N, Sasaki T, Kawakubo K, Hamada T, Ito Y, Yashima Y, Sasahira N, Hirano K, Yamaji Y, Tada M, Omata M, Koike K (2013) Feasibility of a new self-expandable metallic stent for patients with malignant colorectal obstruction. Dig Endosc 25:160–166
Colonic Stent Safe Procedure Research Group. http://www.colon-stent.com/001_mainpage_en.html
Matsuzawa T, Ishida H, Yoshida S, Isayama H, Kuwai T, Maetani I, Shimada M, Yamada T, Saito S, Tomita M, Koizumi K, Hirata N, Sasaki T, Enomoto T, Saida Y (2015) A Japanese prospective multicenter study of self-expandable metallic stent placement for malignant colorectal obstruction: short-term safety and efficacy within 7 days of stent procedure in 513 cases. Gastrointest Endosc 82:697–707
Adler DG, Baron TH (2002) Endoscopic palliation of malignant gastric outlet obstruction using self-expanding metal stents: experience in 36 patients. Am J Gastroenterol 97:72–78
Tekkis PP, Kinsman R, Thompson MR, Stamatakis JD, Association of Coloproctology of Great Britain I (2004) The Association of Coloproctology of Great Britain and Ireland study of large bowel obstruction caused by colorectal cancer. Ann Surg 240:76–81
Jiménez-Pérez J, Casellas J, García-Cano J, Vandervoort J, García-Escribano OR, Barcenilla J, Delgado AA, Goldberg P, Gonzalez-Huix F, Vázquez-Astray E, Meisner S (2011) Colonic stenting as a bridge to surgery in malignant large-bowel obstruction: a report from two large multinational registries. Am J Gastroenterol 106:2174–2218
Meisner S, González-Huix F, Vandervoort JG, Goldberg P, Casellas JA, Roncero O, Grund KE, Alvarez A, García-Cano J, Vázquez-Astray E, Jiménez-Pérez J (2011) Self-expandable metal stents for relieving malignant colorectal obstruction: short-term safety and efficacy within 30 days of stent procedure in 447 patients. Gastrointest Endosc 74:876–884
Baron TH (2010) Colonic stenting: a palliative measure only or a bridge to surgery? Endoscopy 42:163–168
Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL (2011) Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc 25:1814–1821
Sabbagh C, Browet F, Diouf M, Cosse C, Brehant O, Bartoli E, Mauvais F, Chauffert B, Dupas JL, Nguyen-Khac E, Regimbeau JM (2013) Is stenting as “a bridge to surgery” an oncologically safe strategy for the management of acute, left-sided, malignant, colonic obstruction? A comparative study with a propensity score analysis. Ann Surg 258:107–115
van Hooft JE, van Halsema EE, Vanbiervliet G, Beets-Tan RG, DeWitt JM, Donnellan F, Dumonceau JM, Glynne-Jones RG, Hassan C, Jiménez-Perez J, Meisner S, Muthusamy VR, Parker MC, Regimbeau JM, Sabbagh C, Sagar J, Tanis PJ, Vandervoort J, Webster GJ, Manes G, Barthet MA, Repici A (2014) Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Gastrointest Endosc 80:747–761
Iversen LH, Kratmann M, Bøje M, Laurberg S (2011) Self-expanding metallic stents as bridge to surgery in obstructing colorectal cancer. Br J Surg 98:275–281
Sloothaak DAM, van den Berg MW, Dijkgraaf MGW, Fockens P, Tanis PJ, van Hooft JE, Bemelman WA (2014) Oncological outcome of malignant colonic obstruction in Dutch Stent-In 2 trial. Br J Surg 101:1751–1757
Tung KL, Cheung HY, Ng LW, Chung CC, Li MK (2013) Endo-laparoscopic approach versus conventional open surgery in the treatment of obstructing left-sided colon cancer: long-term follow-up of a randomized trial. Asian J Endosc Surg 6:78–81
Alcantara M, Serra-Aracil X, Falco J, Mora L, Bombardo J, Navarro S (2011) Prospective, controlled, randomized study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J Surg 35:1904–1910
Gorissen KJ, Tuynman JB, Fryer E, Wang L, Uberoi R, Jones OM, Cunningham C, Lindsey I (2013) Local recurrence after stenting for obstructing left-sided colonic cancer. Br J Surg 100:1805–1809
Matsuda A, Miyashita M, Matsumoto S, Matsutani T, Sakurazawa N, Takahashi G, Kishi T, Uchida E (2014) Comparison of long-term outcomes of colonic stent as “bridge to surgery” and emergency surgery for malignant large-bowel obstruction: a meta-analysis. Ann Surg Oncol 22:497–504
Koch M, Kienle P, Sauer P, Willeke F, Buhl K, Benner A, Lehnert T, Herfarth C, von Knebel Doeberitz M, Weitz J (2004) Hematogenous tumor cell dissemination during colonoscopy for colorectal cancer. Surg Endosc 18:587–591
Maruthachalam K, Lash GE, Shenton BK, Horgan AF (2007) Tumour cell dissemination following endoscopic stent insertion. Br J Surg 94:1151–1154
Acknowledgments
This study was conducted with Japan Gastroenterological Endoscopy Society funding support and the Colonic Stent Safe Procedure Research Group membership dues.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Shuji Saito has received personal fees from Boston Scientific Japan, Century Medical Inc; Dr. Shuntaro Yoshida has received personal fees from Boston Scientific Japan, Century Medical Inc and ZEON Co, Dr. Hiroyuki Isayama has received donations and personal fees from Boston Scientific Japan, Century Medical Inc and Taewoong Medical Devices Inc. Drs. Takeaki Matsuzawa, Toshio Kuwai, and Mamoru Shimada have received personal fees from Boston Scientific Japan; Dr. Iruru Maetani has received personal fees from Boston Scientific Japan, Century Medical Inc, Piolax Medical Device Inc, and MC Medical Inc; Dr. Koichi Koizumi has received personal fees from Century Medical Inc and Olympus Medical Systems Corp; and Dr. Yoshihisa Saida has received grants and personal fees from Boston Scientific Japan, Century Medical Inc, and Olympus Medical Systems Corp. Drs. Tomonori Yamada, Masafumi Tomita, Nobuto Hirata, Hideki Kanazawa, Toshiyuki Enomoto, and Hitoshi Sekido have no conflicts of interest or financial ties to disclose.
Appendix
Appendix
The Colonic Stent Safe Procedure Research Group includes the following members in addition to the authors.
Tatsuya Osuga, Aijinkai Takatsuki General Hospital, Takatsuki, Japan; Masaki Kikkawa, Akita Red Cross Hospital, Akita, Japan; Mitsuru Goto, Asahikawa Kosei Hospital, Asahikawa, Japan; Shungo Endo, Fukushima Medical University Aizu Medical Center, Aizu-wakamatsu, Japan; Sayo Kobayashi, Fukuyama City Hospital, Fukuyama, Japan; Shigeru Yamagishi, Fujisawa City Hospital, Fujisawa, Japan; Ken Konishi, Higashiosaka City General Hospital, Higashiosaka, Japan; Masanori Yoshimitsu, Hiroshima City Asa Hospital, Hiroshima, Japan; Satoshi Ikeda, Hiroshima Prefectural Hospital, Hiroshima, Japan; Rintaro Moroi, Iwate Prefectural Isawa Hospital, Oshu, Japan; Michiaki Watanabe, Kawaguchi Municipal Medical Center, Kawaguchi, Japan; Hirofumi Kawamoto, Kawasaki Medical School, Okayama, Japan; Hirotoshi Hasegawa, Keio University School of Medicine, Tokyo, Japan; Atsushi Yamauchi, Kitano Hospital, Osaka, Japan; Fuminori Teraishi, Kochi Health Sciences Center, Kochi, Japan; Kohei Takayasu, Kyorin University Hospital, Mitaka, Japan; Takahiro Horimatsu, Kyoto University Hospital, Kyoto, Japan; Yoshinori Kushiyama, Matsue Red Cross Hospital, Matsue, Japan; Hiroaki Naota, Matsusaka Chuo General Hospital, Matsusaka, Japan; Takuya Yamaguchi, Mimihara General Hospital, Sakai, Japan; Shigenori Masaki, Miyanomori Memorial Hospital, Sapporo, Japan; Taku Sakamoto, National Cancer Center Hospital, Tokyo, Japan; Kazuhiro Watanabe, National Center for Global Health and Medicine, Tokyo, Japan; Masafumi Inomata, Oita University Faculty of Medicine, Oita, Japan; Nobuya Obana, Osaki Citizen Hospital, Osaki, Japan; Masayoshi Horimoto, Osaka Saiseikai Senri Hospital, Suita, Japan; Rika Kyo, Saiseikai Yokohamashi-Nanbu Hospital,Yokohama, Japan; Shinei Kudo, Showa University Northern Yokohama Hospital, Yokohama, Japan; Yasushi Nakamura, Takano Hospital, Kumamoto, Japan; Mitsunori Ushigome, Toho University Omori Medical Center, Tokyo, Japan; Takeshi Ohki, Tokyo Women’s Medical University, Tokyo, Japan; Hiroyuki Kato, Tokyo Women’s Medical University Medical Center East, Tokyo, Japan; Shiro Hayashi, Toyonaka Municipal Hospital, Toyonaka, Japan; Tomio Hirakawa, Yao Tokushukai General Hospital, Yao, Japan; and Kensuke Kubota, Yokohama City University Hospital, Yokohama, Japan.
Rights and permissions
About this article
Cite this article
Saito, S., Yoshida, S., Isayama, H. et al. A prospective multicenter study on self-expandable metallic stents as a bridge to surgery for malignant colorectal obstruction in Japan: efficacy and safety in 312 patients. Surg Endosc 30, 3976–3986 (2016). https://doi.org/10.1007/s00464-015-4709-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-015-4709-5