Abstract
Purpose
We assessed the cerebrovascular CO2 reactivity (CO2R) in chronic renal failure (CRF) patients without diabetes mellitus (DM), uncontrolled hypertension, peripheral vascular disease, or neurological disease under isoflurane-nitrous oxide anesthesia.
Methods
Forty-nine patients undergoing surgery, including 36 CRF patients (30 receiving dialysis and six pre-dialysis patients) and 13 patients without CRF (controls). Middle cerebral artery flow velocity (VMCA) was measured by transcranial Doppler ultrasonography at an end-tidal CO2 of 35 to 45 mmHg. CO2R was calculated as an absolute value (change in VMCA per mmHg PaCO2) and a relative value (absolute CO2R/baseline VMCA × 100). Factors associated with CO2R were evaluated simultaneously.
Results
Despite no significant differences in the absolute and relative values of CO2R between the CRF (mean 2.5 cm/s/mmHg; median 5.0%/mmHg) and control (2.4 cm/s/mmHg; 5.0%/mmHg) groups, blood urea nitrogen (BUN) concentrations in the CRF group correlated inversely with both absolute and relative CO2R. BUN concentration was higher (mean 72 versus 53 mg/dl, p = 0.006) and relative CO2R was lower (mean 2.6 versus 5.7%/mmHg, p = 0.011) in patients with pre-dialysis CRF (n = 6) versus CRF patients receiving dialysis (n = 30).
Conclusions
CO2R in CRF patients was not significantly different from that in controls. However, in CRF patients with high BUN concentrations, CO2R might be impaired, leading to reduced cerebrovascular reserve capacity. Because DM is a major cause of CRF and we excluded DM patients, our results might not be applicable to patients with DM-induced CRF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic renal failure (CRF) is a critical condition that requires careful perioperative management to prevent cerebral insult and protect cerebral function. CRF patients have an increased risk of ischemic stroke [1] and cognitive dysfunction [2,3,4] and morbidity and mortality is particularly high during the perioperative period [5,6,7,8,9]. Although several factors are involved, impaired cerebral blood flow (CBF) in CRF patients may be one of the major causes of ischemic stroke [1] and cognitive dysfunction [3].
Evaluating cerebrovascular reactivity to CO2 partial pressure (CO2R) is an established method for assessing cerebrovascular reserve capacity [10]. Impaired CO2R has been linked to increased risk of cerebral ischemia [11], postoperative confusion [12], and postoperative cognitive dysfunction [13]. However, limited information is available regarding cerebrovascular reactivity in CRF patients. To our knowledge, only two studies have reported CO2R in CRF patients in the awake state [14, 15]. Using positron emission tomography (PET), Kuwabara et al. [14] demonstrated that CBF was higher in CRF patients with severe anemia versus normal controls and that CO2R was attenuated in CRF patients with mild and severe anemia. In contrast, Skinner et al. [15], using transcranial Doppler ultrasonography (TCD), found no CO2R impairment in CRF patients who had not obvious anemia. These findings suggest that CO2R is unaffected in CRF patients without obvious anemia.
However, CRF patients tend to have several other comorbidities, apart from anemia, that might also affect CO2R. These include DM [13, 16,17,18], uncontrolled hypertension [19], peripheral vascular disease [16], and cerebrovascular disease [20]. Based on these factors, the interpretation of investigations on CO2R in CRF patients could be complicated if selection of patients is not carefully done.
We presumed that accumulation of humoral factors such as creatinine and BUN in particular could affect CO2R in CRF patients, because both elevated serum creatinine or BUN concentrations interfere with CBF [21]. Therefore, we sought to evaluate CO2R in CRF patients under conditions where comorbidities that could affect CO2R were excluded as much as possible.
As mentioned above, previous studies of CO2R in CRF patients were conducted with patients in the awake state [14, 15], and no studies to date have evaluated CO2R in CRF patients under general anesthesia. Evaluating CO2R under general anesthesia could be important for the management of CRF patients in the perioperative period. For example, Kadoi et al. [13] found that impaired CO2R during anesthesia was associated with postoperative cognitive dysfunction in patients with DM.
In the present study, we examined CO2R using TCD to measure mean VMCA in CRF patients under isoflurane-nitrous oxide anesthesia. These values were compared to those in patients without CRF (controls). We also evaluated CRF factors that may explain possible CO2R changes including blood urea nitrogen (BUN), creatinine and hemoglobin (Hb) concentrations.
Methods
Patients and preoperative assessment
The Yamaguchi University Graduate School of Medicine Ethics Committee for Human Study approved the study protocol (25th April 2012:H23-172-5). Our institutional Medicine Ethics Committee for Human Study judged that the present study was observational prospective study and no application for clinical trial registration was necessary. Written informed consent was obtained from all participants. Forty-two CRF patients (CRF group) and 17 contemporaneous patients without CRF (control group) were enrolled in the study. All patients in the CRF group had glomerulonephritis, blood creatinine concentration ≥ 4 mg/dl, and were scheduled for living-related renal transplantation. Patients with DM, uncontrolled hypertension (systolic blood pressure, and diastolic blood pressure exceeding 140 and 90 mmHg, respectively during the 3 days before surgery), peripheral vascular disease, or neurological disease including cerebral vascular disease, as well as those with a Hb concentration < 8 g/dl were excluded from the study. The 17 patients in the control group were classed as American Society of Anesthesiologists (ASA) physical status (PS) 1 and were scheduled for elective resection of tumor in the gastrointestinal tract or kidney, or for uterus or orthopedic surgery. No laparoscopic procedures were involved in either of the groups. Blood sampling for complete blood cell count and biochemical tests were performed, and heart rate and blood pressure were evaluated at rest the evening prior to the day of surgery.
Anesthesia and monitoring
Anesthesia was induced using 4 mg/kg thiopental and 0.1–0.15 mg/kg vecuronium. The trachea was intubated to mechanically ventilate the lungs. Anesthesia was maintained using 0.5–1.5% isoflurane in 50% nitrous oxide and oxygen. Fentanyl was administered as required. The radial artery was cannulated to monitor arterial blood pressure and sample blood for measurement of arterial blood gases, Hb, and electrolytes. Bladder or nasopharyngeal temperature was monitored and the patient was maintained at approximately 36 °C using a warming blanket (Medi-Therm II; Gaymar, New York, NY).
Pulse oximetry was monitored, and end-tidal CO2 (ETCO2) tension, isoflurane concentration, and nitrous oxide concentration were assessed continuously using an infrared analyzer (Capnomac Ultima; Datex, Helsinki, Finland). A central venous catheter was inserted via the right internal jugular vein in all CRF patients and in five controls. A potassium-free electrolyte solution with 2.6% glucose (Solita-T1; Ajinomoto, Tokyo, Japan; or Soldem-1; Terumo, Tokyo, Japan) was infused intravenously at 2–5 ml/kg/h in the CRF group, while acetate Ringer’s solution (Veen F; Nikken Kagaku, Tokyo, Japan) was infused intravenously at 5–10 ml/kg/h in the control group.
Determination of VMCA and cerebrovascular CO2R during anesthesia
VMCA and CO2R were determined using previously described methods [16]. A 2-MHz TCD probe (Neurogard; Medasonics, Fremont, CA) was attached to the patient’s head at the left temporal window after inducing anesthesia and VMCA was measured continuously. The right temporal window was used if signals on the left side could not be appropriately detected. The probe was attached to the head using a probe holder to maintain the insonating angle after the appropriate signals were identified at a depth of 45–60 mm. The values were recorded at end-expiration because VMCA fluctuated with the respiratory cycle.
Baseline VMCA measurements were performed more than 2–3 h after induction of anesthesia at an ETCO2 of approximately 35 mmHg, while anesthesia was maintained with 1% isoflurane (end-tidal) and 50% nitrous oxide in oxygen. Baseline VMCA and blood samples were obtained during a hiatus in the surgical procedures. ETCO2 was then increased to 45 mmHg by adding a dead-space tube of approximately 100 ml to the endotracheal tube and reducing the ventilatory frequency by two or three breaths per minute. These alterations increased the partial pressure of CO2 in arterial blood (PaCO2) by approximately 10 mmHg (i.e., hypercapnia) within a few minutes. VMCA values were recorded when ETCO2 was elevated and remained stable for 10 min. Blood samples were obtained at the same time. Small doses of vecuronium were administered intravenously to prevent spontaneous respiration during hypercapnia.
The CO2R in each patient was calculated as both the absolute change in VMCA per mmHg change in PaCO2 and as the relative change that is percentage change in VMCA (percentage of baseline VMCA), using the following formula:
Here, ΔVMCA is the difference between the flow velocity of hypercapnia and baseline, and ΔPaCO2 is the difference in PaCO2 between hypercapnia and baseline.
Statistical analysis
A previous CO2R study at our institution reported VMCA in ASA PS 1 patients to be 40 ± 6 cm/s (mean ± standard deviation) at the same baseline condition as the present study [16]. We calculated that at least 13 patients would be needed to detect differences in VMCA of 10 cm/s at an α level of 0.05 with 80% power. However, because a large variation in VMCA values was observed in the CRF patients during the study proceeded, we calculated and tried to enroll at least 36 cases of CRF patients.
Data were analyzed using SPSS version 11 (SPSS Inc., Chicago, IL, USA). Data expressed as number of cases, mean ± standard deviation if data distributed normally, or median and interquartile range if data did not distributed normally. Statistical analysis was performed using the Chi-square test, unpaired t test (Welch’s t test was applied if no homogeneity of variance was observed) and the Wilcoxon rank-sum test to compare patient characteristics and CO2R between the groups. According to the distribution of the data, the unpaired t test or Wilcoxon rank-sum test were used to evaluate differences in physiological variables and VMCA between the groups, and paired t test or Wilcoxon signed-rank test were used for comparing the baseline and hypercapnia in each group.
Based on the findings of previous studies, we hypothesized that the following predictive factors may affect CO2R: age [22, 23], Hb concentration [14, 24], BUN, creatinine concentration [21, 25], pH, base excess (BE) [26], and the duration of dialysis [22, 27].
To assess associations between these predictive factors and CO2R, we performed a simple linear regression analysis based on univariate analysis. Pearson’s product-moment correlation coefficients (r) were calculated and factors related to CO2R on univariate analysis at p < 0.1 were entered into a multivariate linear regression analysis.
Because our group of CRF patients included both patients who had been receiving dialysis or were with pre-dialysis, we performed sub-group analyses between these two groups using the same statistics used to compare the CRF and control groups.
Statistical significance was set at p < 0.05. Statistical analysis was performed and was counterchecked by Kazuyoshi Ishida and Mishiya Matsumoto.
Results
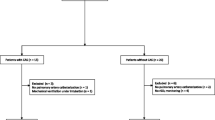
Of the 59 CRF patients and control, five patients were excluded before surgery due to no acquisition of informed consent. Of the remaining 39 CRF and 15 control patients, three patients in CRF and two patients in control were excluded because of inability to access middle cerebral artery for measuring VMCA. Finally, 36 CRF patients and 13 control patients were enrolled for analysis (Fig. 1).
Twenty patients had been receiving hemodialysis (HD). Eight patients were receiving continuous ambulatory peritoneal dialysis (CAPD). Two patients received both HD and CAPD as a hybrid treatment, while the remaining six CRF patients were not receiving dialysis treatment (pre-dialysis). HD was performed three times weekly and continued until the day before surgery. CAPD was performed overnight and continued until the morning of surgery. The duration of dialysis treatment varied from no dialysis at all (pre-dialysis) to 180 months. Patient demographics and preoperative data are presented in Table 1. No significant differences were found for age, sex, height, weight, or heart rate between the CRF and control groups. The number of patients with hypertension and preoperative mean blood pressure (MBP) was higher in the CRF versus control group. Preoperative Hb was lower in the CRF group than in the control group. BUN and creatinine in concentrations in the CRF group were higher than those in the control group.
Intraoperative physiological and CO2R values are presented in Table 2. PaCO2 values were similar between groups. pH, BE, and Hb were lower in the CRF group compared with the control group. VMCA increased in both groups during hypercapnia compared with baseline values (Table 2). Differences between groups could not be detected for VMCA or absolute and relative CO2R (Table 2).
Table 3 shows the results of simple linear regression analysis of factors associated with CO2R. Only age correlated inversely and significantly with absolute CO2R (r = − 0.62, p = 0.025) in the control group (Table 3). In the CRF group, only BUN correlated inversely and significantly with absolute CO2R (r = − 0.33, p = 0.049) (Table 3, Fig. 2). BUN(r = − 0.45, p = 0.006), pH (r = 0.29, p = 0.086), BE (r = 0.3, p = 0.08) and duration of dialysis (r = − 0.31, p = 0.097) correlated with relative CO2R (p < 0.1) (Table 3). However, in multiple linear regression analysis, only BUN concentration correlated inversely with relative CO2R (r = − 0.45, p = 0.016) (Fig. 2).
Correlations between blood urea nitrogen (BUN) concentration and absolute (a) and relative (b) CO2 reactivity (CO2R) in the chronic renal failure (CRF) group (n = 36). The BUN concentration correlates inversely with both absolute (r = − 0.33, p = 0.049) and relative CO2R (r = − 0.45, p = 0.006). Open circles indicate CRF patients receiving dialysis (n = 30) and closed circles indicate CRF patients with pre-dialysis (n = 6)
Table 4 shows patient demographics and preoperative data of sub-group analysis. BUN was higher in the patients with pre-dialysis (mean BUN 72 mg/dl, n = 6) than those receiving dialysis (mean BUN 53 mg/dl, n = 30). Creatinine concentration was lower in the former patients than the latter.
Table 5 shows the physiological variables and CO2R in sub-group analysis. Lower pH and BE values and higher baseline VMCA values (median 67 vs. 49 cm/s, p = 0.017) were observed in pre-dialysis CRF group compared with those in the dialysis group. No significant differences were observed in absolute CO2R between the groups but relative CO2R was significantly lower in pre-dialysis CRF group than in the dialysis group (mean 2.6 vs. 5.7%/mmHg, p = 0.011).
Discussion
To our knowledge, this study is the first trial to evaluate CO2R in CRF patients under general anesthesia. No significant difference was noted in baseline VMCA or absolute and relative CO2R between CRF patients and controls under isoflurane-nitrous oxide anesthesia. However, we found a significant inverse correlation between BUN and absolute and relative CO2R in the CRF group. Furthermore, in patients with pre-dialysis CRF, relative CO2R was significantly lower and BUN concentrations were significantly higher than those in patients who had been receiving dialysis. Observed impairment of CO2R in CRF patents with high BUN concentrations may suggest that humoral factors that accumulate in CRF patients could reduce cerebrovascular reserve capacity.
Kuwabara et al. [14] reported that attenuated CO2R has been reported in severe anemia (mean Hb 6.8 g/dl) in CRF in association with high basal CBF and even in mild anemia (mean Hb 10.8 g/dl) with no associated elevation in basal CBF in CRF patients receiving dialysis. Because significant correlation between hematocrit and CO2R was found by evaluation with a wide range of hematocrit values in all the CRF patients together, they speculated that the cause of the decrease in CO2R and increase in basal CBF during CRF is maximal dilation of resistance vessels to allow supply of sufficient O2 to the brain under anemic conditions. Skinner et al. [15] demonstrated that there was no CO2R impairment in CRF patients without obvious anemia (mean Hb 11.7 g/dl) when examined either before or after hemodialysis or when compared with normal control references values. These results suggest that anemia might be the cause of CO2R impairment in CRF patients. In the present study, on the other hand, the mean value of Hb (mean Hb 10.4 g/dl) in the CRF group corresponded to mild anemia, but no CO2R impairment was observed in this group. We speculate that this could be because of the exclusion of patients with comorbidities in the present study, differences in measurement conditions (under general anesthesia or awake), or measurement procedures (TCD or PET).
An interesting finding in this study was that there was a significant inverse correlation between BUN (but not creatinine) and absolute and relative CO2R in CRF patients. Furthermore, in the CRF patients who had not yet started dialysis, CO2R was impaired in association with higher BUN compared with that of patients receiving dialysis. We speculate that high BUN concentration results in cerebral vasodilation and hence attenuates CO2R because it has been reported that CBF is positively correlated with BUN concentration in CRF patients [21]. In fact, in the present study, higher BUN and baseline VMCA were observed in patients with pre-dialysis CRF, and the concomitant CO2R was significantly decreased. However, Skinner et al. [15] assessed changes in CO2R, comparing the values before and after hemodialysis. They observed no significant changes even though BUN concentrations significantly decreased [23.9 (66.9)–8.7 (24.3) mmol/l (mg/dl)] occurred after hemodialysis, suggesting that there was no prominent effect of BUN on CO2R. This was in contrast to our results. Their results suggest that the acute change of BUN concentrations did not affect CO2R. Conversely, our present study might demonstrate the long-term effects of BUN on CO2R.
Regarding creatinine, a controversial effect of increased creatinine on CBF was reported [21, 28]. Furthermore, creatinine has been shown to exert less influence than BUN on electroencephalogram findings [25] and latency of visual event-related potentials [29]. Our results that indicate creatinine does not alter CO2R may be consistent with these neurophysiologic observations.
This study has some limitations. First, the number of patients with pre-dialysis CRF was small. Second, unknown accumulated substances in CRF that could possibly affect CO2R were not evaluated. Third, we did not perform perioperative neuropsychological examinations, which might have provided insight as to the precise impact of elevated BUN on cerebral function in patients with impaired CO2R. Fourth, DM is one of the most common causes of CRF [30], but we excluded CRF patients with DM. Because DM is reported to affect CO2R in patients under general anesthesia [13, 16, 17], our results may not be applicable to patients with DM-induced CRF.
Further study is warranted to evaluate contributory factors to CO2R including unknown accumulated substances and to evaluate the effect of impaired CO2R on postoperative cognition in CRF patients.
Conclusions
CO2R in a group of CRF patients was not significantly different from that in controls. However, we found a significant inverse correlation between BUN and absolute and relative CO2R in the CRF group. In addition, subgroup analysis revealed impairment of CO2R in patients with pre-dialysis CRF with high BUN concentrations. These findings indicate that cerebrovascular reserve capacity is not impaired in CRF patients if dialysis is adequately performed, but is impaired in CRF patients with elevated BUN concentrations.
Change history
23 December 2017
In the original publication of the article, the first sentence was published incorrectly under the section “Patients and preoperative assessment”. The correct sentence should read as, “The Yamaguchi University Graduate School of Medicine Ethics Committee for Human Study approved the study protocol (18th August 2004: H16-71)”.
References
Koren-Morag N, Goldbourt U, Tanne D. Renal dysfunction and risk of ischemic stroke or TIA in patients with cardiovascular disease. Neurology. 2006;67:224–8.
Fazekas G, Fazekas F, Schmidt R, Kapeller P, Offenbacher H, Krejs GJ. Brain MRI findings and cognitive impairment in patients undergoing chronic hemodialysis treatment. J Neurol Sci. 1995;134:83–8.
Fazekas G, Fazekas F, Schmidt R, Flooh E, Valetitsch H, Kapeller P, Krejs GJ. Pattern of cerebral blood flow and cognition in patients undergoing chronic haemodialysis treatment. Nucl Med Commun. 1996;17:603–8.
Wolfgram DF, Sunio L, Vogt E, Smith HM, Visotcky A, Laud P, Whittle J. Haemodynamics during dialysis and cognitive performance. Nephrology (Carlton). 2014;19:771–6.
Plecha EJ, King TA, Pitluk HC, Rubin JR. Risk assessment in patients undergoing carotid endarterectomy. Cardiovasc Surg. 1993;1:30–2.
Rigdon EE, Monajjem N, Rhodes RS. Is carotid endarterectomy justified in patients with severe chronic renal insufficiency? Ann Vasc Surg. 1997;11:115–9.
Toh Y, Yano K, Takesue F, Korenaga D, Maekawa S, Muto Y, Ikeda T, Sugimachi K. Abdominal surgery for patients on maintenance hemodialysis. Surg Today. 1998;28:268–72.
Penta de Peppo A, Nardi P, De Paulis R, Pellegrino A, Forlani S, Scafuri A, Chiariello L. Cardiac surgery in moderate to end-stage renal failure: analysis of risk factors. Ann Thorac Surg. 2002;74:378–83.
Wang F, Dupuis JY, Nathan H, Williams K. An analysis of the association between preoperative renal dysfunction and outcome in cardiac surgery: estimated creatinine clearance or plasma creatinine level as measures of renal function. Chest. 2003;124:1852–62.
Ulrich PT, Becker T, Kempski OS. Correlation of cerebral blood flow and MCA flow velocity measured in healthy volunteers during acetazolamide and CO2 stimulation. J Neurol Sci. 1995;129:120–30.
Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain. 2001;124:457–67.
Kessler C, Junge HM, Walker ML, Busack R, Albrecht DM, von Ackeren K. Reduced cerebral vasomotor reactivity as an indicator of postoperative confusion. Anaesthesia. 1997;52:433–7.
Kadoi Y, Kawauchi C, Kuroda M, Takahashi K, Saito S, Fujita N, Mizutani A. Association between cerebrovascular carbon dioxide reactivity and postoperative short-term and long-term cognitive dysfunction in patients with diabetes mellitus. J Anesth. 2011;25:641–7.
Kuwabara Y, Sasaki M, Hirakata H, Koga H, Nakagawa M, Chen T, Kaneko K, Masuda K, Fujishima M. Cerebral blood flow and vasodilatory capacity in anemia secondary to chronic renal failure. Kidney Int. 2002;61:564–9.
Skinner H, Mackaness C, Bedforth N, Mahajan R. Cerebral haemodynamics in patients with chronic renal failure: effects of haemodialysis. Br J Anaesth. 2005;94:203–5.
Kawata R, Nakakimura K, Matsumoto M, Kawai K, Kunihiro M, Sakabe T. Cerebrovascular CO2 reactivity during anesthesia in patients with diabetes mellitus and peripheral vascular disease. Anesthesiology. 1998;89:887–93.
Kado Y, Hinohara H, Kunimoto F, Saito S, Ide M, Hiraoka H, Kawahara F, Goto F. Diabetic patients have an impaired cerebral vasodilatory response to hypercapnia under propofol anesthesia. Stroke. 2003;34:2399–403.
Lasek-Bal A, Kazibutowska Z, Golba A, Motta E. Cerebral vasoreactivity in hypocapnia and hypercapnia in patients with diabetes mellitus type 2 with or without arterial hypertension. Neurol Neurochir Pol. 2012;46:529–35.
Serrador JM, Sorond FA, Vyas M, Gagnon M, Iloputaife ID, Lipsitz LA. Cerebral pressure-flow relations in hypertensive elderly humans: transfer gain in different frequency domains. J Appl Physiol. 2005;98:151–9.
Wijnhoud AD, Koudstaal PJ, Dippel DW. Relationships of transcranial blood flow Doppler parameters with major vascular risk factors: TCD study in patients with a recent TIA or nondisabling ischemic stroke. J Clin Ultrasound. 2006;34:70–6.
Kanai H, Hirakata H, Nakane H, Fujii K, Hirakata E, Ibayashi S, Kuwabara Y. Depressed cerebral oxygen metabolism in patients with chronic renal failure: a positron emission tomography study. Am J Kidney Dis. 2001;38:S129–33.
Holzer H, Marguc K, Pogglitsch H, Ott E, Katschnig H. The effects of haemodialysis on cerebral blood flow. Proc Eur Dial Transpl Assoc. 1981;18:126–32.
Lipsitz LA, Mukai S, Hamner J, Gagnon M, Babikian V. Dynamic regulation of middle cerebral artery blood flow velocity in aging and hypertension. Stroke. 2000;31:1897–903.
Hata R, Matsumoto M, Handa N, Terakawa H, Sugitani Y, Kamada T. Effects of hemodialysis on cerebral circulation evaluated by transcranial Doppler ultrasonography. Stroke. 1994;25:408–12.
Hughes JR. Correlations between EEG and chemical changes in uremia. Electroencephalogr Clin Neurophysiol. 1980;48:583–94.
van de Ven M, Colier WN, Kersten BT, Oeseburg B, Folgering H. Cerebrovascular response to acute metabolic acidosis in humans. Adv Exp Med Biol. 2003;530:707–15.
Szprynger K, Kwiecinski J, Szczepanska M, Pierzchala K. Evaluation of cerebrovascular reactivity in children (corrected) with chronic renal failure. Pediatr Nephrol. 2000;14:993–6.
Kamano C, Komaba Y, Sakayori O, Iino Y, Katayama Y. Decreased cerebral blood flow in renal transplant recipients. Intern Med. 2002;41:677–83.
Evers S, Tepel M, Obladen M, Suhr B, Husstedt IW, Grotemeyer KH, Zidek W. Influence of end-stage renal failure and hemodialysis on event-related potentials. J Clin Neurophysiol. 1998;15:58–63.
Iseki K. Predictors of diabetic end-stage renal disease in Japan. Nephrology. 2005;10:S2–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
This work was supported by a grant from the Ministry of Education, Science, Sports and Culture of Japan (no. 22591734 for Kazuyoshi Ishida).
Additional information
A correction to this article is available online at https://doi.org/10.1007/s00540-017-2443-y.
About this article
Cite this article
Ishida, K., Uchida, M., Utada, K. et al. Cerebrovascular CO2 reactivity during isoflurane-nitrous oxide anesthesia in patients with chronic renal failure. J Anesth 32, 15–22 (2018). https://doi.org/10.1007/s00540-017-2422-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00540-017-2422-3